Abstract
Background
There are sparse and conflicting data regarding the long‐term clinical course of atopic dermatitis (AD). Although often described as a childhood disease, newer population‐based estimates suggest the prevalence of pediatric and adult disease may be similar.
Methods
Our objective was to determine whether there is a decline in the prevalence of AD in population‐based cohorts of patients followed longitudinally beyond childhood. We conducted a systematic review and meta‐analysis including studies assessing AD prevalence across 3 or more points in time. The primary outcome was weighted overall risk difference (percentage decrease in AD prevalence).
Results
Of 2080 references reviewed, 7 studies with 13 515 participants were included. Participants were assessed at 3‐6 time points, ranging from age 3 months to 26 years. The percentage decrease in prevalence after age 12 was 1%, which was not significantly different from zero (95% confidence interval −2%‐5%). Similar results were found with other age cut‐offs.
Conclusion
The prevalence of AD in longitudinal birth cohort studies is similar in childhood and adolescence/early adulthood.
Keywords: atopic dermatitis, atopic eczema, eczema, natural history, prevalence
1. INTRODUCTION
Atopic dermatitis (AD), also known as atopic eczema or simply eczema,1 is one of the most common and burdensome diseases of childhood, yet little is known about the long‐term clinical course of the disease.2 Textbooks and review articles suggest that most individuals develop disease within the first 2 years of life, experience episodic symptoms throughout childhood, and improve by adolescence; yet, sparse and conflicting data exist regarding the proportion of individuals whose disease resolves and little is known about predictors of disease persistence or adult‐onset disease.3, 4, 5 Challenging the traditional notion of AD as a predominantly childhood disease, a growing body of research on the pathophysiology of AD points to genetic causes of altered skin barrier and immune dysfunction that could predispose to episodic disease throughout life.6, 7 Additionally, population‐based estimates from cross‐sectional surveys suggest that AD may be as common in adults as in children.8 If AD prevalence does not decline over time, there are important implications for patient counseling, management, and future research.
The clinical course of AD has been particularly challenging to study because the condition is heterogeneous and intermittent. Individuals have different clinical presentations, and many will have periods without symptoms or skin lesions. Clinical trials generally focus on short‐term disease control, and cross‐sectional studies offer a snapshot of the population and hence cannot be used to generate prognostic information for individuals. Thanks to growing interest in atopic diseases, a number of longitudinal cohort studies included measures of AD disease activity at multiple time points in the same individuals, enabling the estimation of changes in AD prevalence as a given population ages. The primary objective of this study was to systematically review and analyze longitudinal studies of AD that include repeated measurements in the same cohort during and after childhood to test the hypothesis that the prevalence of AD does not decrease with age.
2. METHODS
2.1. Data source and searches
This review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) and Meta‐analysis of Observational studies in Epidemiology (MOOSE) guidance.9, 10 Our research protocol was registered on PROSPERO and was publically available prior to the study start date (registration ID: 42016033553). PubMed and EMBASE were searched from database inception to October 2015 using prespecified search terms, MeSH (medical subject heading) headings and keywords, developed in consultation with a professional medical librarian. Search terms are described in Table S1. Reference lists of included studies were also screened for additional potentially relevant articles.
This study was exempt from IRB review because it included only published data and is not considered Human Subjects Research by the UCSF IRB (http://irb.ucsf.edu/not-human-subjects-research).
2.2. Study selection
We included studies of patients with AD as diagnosed by a physician or using a standardized definition such as the UK working party diagnostic criteria for AD or the International Study of Asthma and Allergies in Children ISAAC criteria.11, 12 Because our objective was to examine changes in AD prevalence within a given cohort as populations aged, we included only longitudinal cohort studies that assessed AD activity in the same patients at 3 or more distinct time points, to account for the episodic nature of AD and allow for observation of any nonlinear patterns of change. Further, to capture AD prevalence beyond childhood, studies were required to include at least 1 assessment with participants over the age of 12 years. We chose this cutoff because prior studies suggested high rates of “remission” in adolescence and a longitudinal prospective study suggested that prevalence declines most rapidly between ages 8 and 11.13, 14 We excluded studies that were clinic‐based, or focused on patients with localized forms of AD (eg, hand eczema). We did not select studies on the basis of interventions and our search included studies in all available languages.
2.3. Data extraction and quality assessment
Title and abstract screening followed by full‐text screening was performed independently and in duplicate to ascertain whether studies met eligibility criteria. Extracted variables included study information (authors, year of publication); cohort information (country of study, cohort name, method of participant recruitment); study size (number of patients at study start/end, and alternate measures of loss to follow‐up); patient characteristics (sex, race/ethnicity, other demographic information); AD diagnostic criteria; and finally, method and age of AD prevalence assessment.
Methodological quality was assessed using the Newcastle‐Ottawa Scale for cohort studies.15 The risk of bias and scoring criteria used in the present review are described in Table S2. Screening, data extraction, and risk of bias assessment were all performed in duplicate by 2 authors (AY and JO), and discrepancies were resolved by group consensus (AY, JO, KA, and SML).
2.4. Data synthesis and analysis
The primary outcome was percentage change in AD prevalence by age, and all measured time points were included. Secondary outcomes included the prevalence of other atopic conditions (asthma, rhinitis/hayfever), treatment information, and severity data. Corresponding authors of included studies were contacted via e‐mail to verify extracted study prevalence data used for the analyses.
We tabulated and plotted the AD prevalence by age for each study. We then calculated the standardized difference in mean prevalence before and after the age of 12 years (using all time points from each study), and report individual study differences and the mean weighted risk difference. We tested for heterogeneity with the chi‐square test and measured inconsistency using the I2 statistic, which represents the percentage of total variation across studies.16 Because of the methodologic and clinical heterogeneity inherent in the data, we used random‐effects models for all meta‐analyses.
Preplanned sensitivity analyses were conducted to assess the impact of varying the age cutoffs. The primary analysis was repeated using different age cutoffs: <10 years vs ≥10 years; <8 years vs ≥8 years. These analyses were performed to test whether our choice of an age 12 cutoff affected the results. We also repeated the analysis comparing those aged <2 years to 3‐11 years and those aged 2‐12 years vs >12 years to test whether including very young children (when the diagnosis of AD can be more challenging) affected our results. Additional stratified analyses were performed to explore sources of heterogeneity, including analyses by age, by country, by loss to follow‐up, by the number of measurements, and a “jackknife” analysis that eliminated 1 study at a time. Egger's test was used to evaluate for publication bias.17 All analyses were performed with Stata (version 14, Stata Corporation, College Station, TX, USA).
3. RESULTS
The search yielded 2080 records. After full‐text review of 28 manuscripts, 7 studies were selected for inclusion.14, 18, 19, 20, 21, 22, 23 The study flow diagram (Figure 1) lists the reasons why 21 studies were excluded.
Figure 1.

PRISMA flow diagram. Results of search strategy [Colour figure can be viewed at wileyonlinelibrary.com]
3.1. Study characteristics
Table 1 outlines the characteristics of each study in detail. The included studies followed 13 515 individuals. All geographical locations could be broadly classified as Northern European: Studies were conducted in Sweden, Iceland, Germany, Denmark, and the UK (3 studies). All studies began follow‐up at birth, and most were initiated the 1980s, with the exception of Williams et al's study which began follow‐up in 1958, and Ballardini et al, which began follow‐up in 1994‐1996. Each study measured prevalence at 3‐6 separate time points, which ranged between patient ages of 3 months and 26 years. The annual period prevalence of AD was assessed via questionnaire in all studies, with the addition of physical examination at some time points in 3 studies. The diagnostic criteria varied slightly by study and are outlined in detail in Table S3. Data were largely unavailable for the secondary outcomes: Only 4 studies reported data on the prevalence of other atopic outcomes, and the methods for assessment and reporting differed. None of the studies reported data on treatment or severity of AD (Table 1).
Table 1.
Summary of study characteristics of included studies
| Study | Country | Longitudinal cohort | Number of patients (N) | Sex (% male) | Prevalence | Other atopic outcomes reported (Y/N) | Treatment reported (Y/N) | Severity reported (Y/N) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study cohort name | Year of birth | Study start | Study end | Loss to follow‐up | Time points (years) | Method of assessment | Period prevalence | ||||||
| Ballardini et al18 | Sweden | BAMSE birth cohort | 1994‐1996 | 2916 | 2916 | 29%b | N/R | 1, 2, 4, 8, 12 | Questionnaire | 1 y | Y | N | N |
| Burr et al19 | UK | Not reported | 1982‐1984 | 497 | 304 | 39% | N/R | 0.25, 0.5, 1, 7, 15, 23 | Clinical examination (until the age of 7), Questionnaire | 1 y | N | N | N |
| Finnbogadóttir et al.20 | Iceland | Not reported | 1987 | 179 | 120 | 33% | N/R | 2, 4, 8, 16, 21 | Clinical examination, Questionnaire | 1 y | Y | N | N |
| Gough et al21 | Germany | MAS‐90 birth cohort | 1990 | 1314 | 942 | 28% | 52% | 3, 6, 9, 12, 15, 20 | Questionnaire | 1 y | Y | N | N |
| Nissen et al22 | Denmark | Not reported | 1985 | 276 | 193 | 30% | 47% | 2, 5, 10, 15, 26 | Clinical examination | 1 y | Y | N | N |
| Williams et al14 | UK | National Child Development Study | 1958 | 6877 | 6877 | 53%b | N/R | 1‐7, 11, 16, 23 | Clinical examination, Questionnaire | 1 yb | N | N | N |
| Ziyab et al23 | UK | 1989 Isle of Wight birth cohort | 1989 | 1456 | 1307 | 10% | N/R | 1, 2, 4, 10, 18 | Questionnaire | 1 y | N | N | N |
UK: United Kingdom; Y: yes; N: no; y: year.
The publication only included those with complete data on all health outcomes at every follow‐up, which represented 71% of the original cohort.
The publication only included those with complete data on all health outcomes at every follow‐up, which represented 47% of the original cohort.
At the age of 7, participants were asked about any AD up to the age of 7, at subsequent ages they were asked about AD over the past year.
3.2. Prevalence estimates
The annual period prevalence of AD ranged from 6% at the age of 26 years22 to 34% at the age of 12 years.21 Study‐specific figures are presented graphically in Figure 2, and the exact numbers used in the meta‐analysis are included in Table S4.
Figure 2.
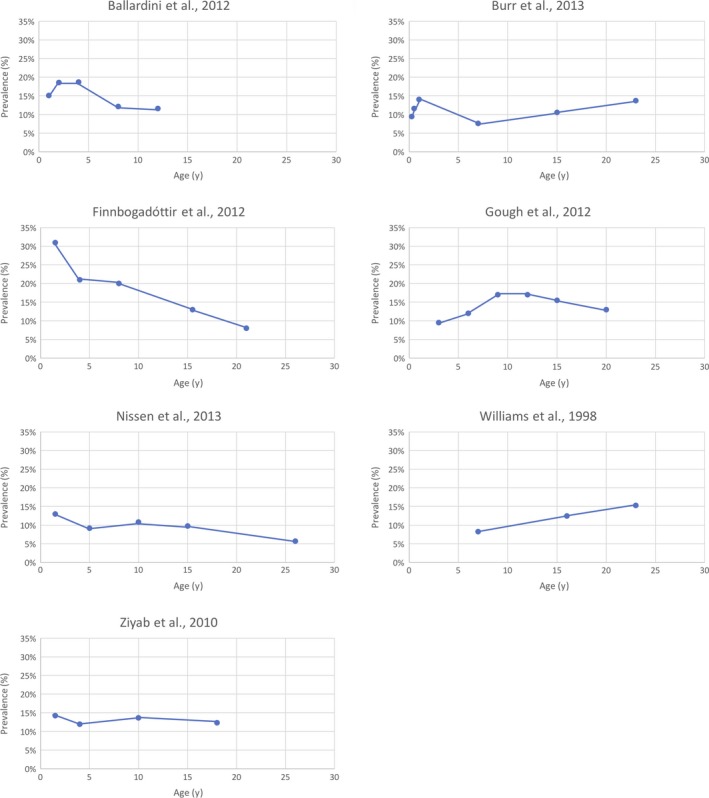
Longitudinal prevalence estimates of included studies. Proportion of the population with AD at each age [Colour figure can be viewed at wileyonlinelibrary.com]
Data on gender were inconsistent. Gough et al observed that AD prevalence tended to increase with age in the female population, and decrease among males. Similarly, in Burr et al and Ziyab et al, AD prevalence in females rose from age of 15 to 23 years and 10 to 18 years, respectively, while declining in males over the same time period. In contrast, Finnobogadottir et al, and Ballardini et al, did not find any difference between AD prevalence between males or females across all time points.
3.3. Risk difference
The primary outcome was the percentage change in prevalence after the age of 12 years. This ranged from −0.05 (meaning there was a 5% increase in the prevalence after the age of 12 years, 95% CI: −0.03, −0.04)14 to 0.10 (a 10% decrease in prevalence after the age of 12 years, 95% CI: 0.06, 0.15) (Figure 3).20 The overall mean weighted percentage difference in prevalence was not significantly different from 0 (0.01, a 1% decrease, 95% CI: 0.02, −0.050). We found similar results when we explored multiple age cutoffs in sensitivity analyses (Table S5), and Eggers test showed no significant publication bias (P = .314). Because of the small number of included studies, funnel plots were not created.
Figure 3.
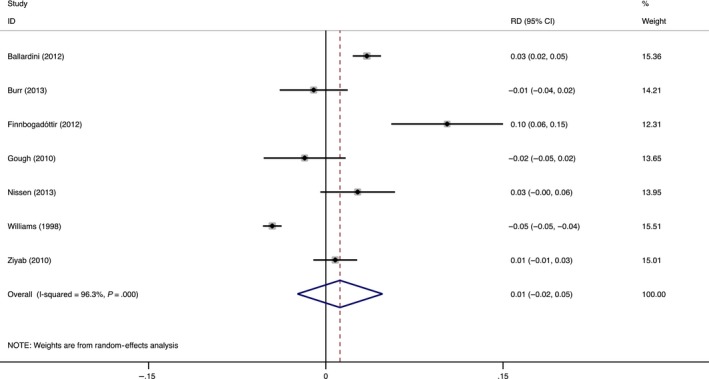
Results of meta‐analysis of risk difference in AD prevalence. Risk difference (RD) in prevalence in children age <12 years of age compared with children age ≥12 years [Colour figure can be viewed at wileyonlinelibrary.com]
3.4. Heterogeneity
There was significant heterogeneity in the primary analysis (I2 96.3%). Multiple sensitivity analyses were performed to explore the impact of study design features on the degree of heterogeneity and the outcome. First, we considered differences in the age structure of the cohorts: Ballardini et al's study only followed individuals through the age of 12 years, while all the other studies followed individuals through ages of 18‐26 years. Excluding this single study only reduced the I2 to 93.4%. Secondly, we considered the possibility of chronological trends: Williams et al's study included individuals who were born in 1958 (2‐3 decades prior to the other cohorts); excluding this study reduced the I2 to 81.9%. Next, we examined whether the disease definition impacted the heterogeneity: Finnobogadottir et al was the only study to require eczematous symptoms to have begun in early childhood, which would have excluded individuals with older‐onset disease from their AD classification and could have made the risk difference seem especially large. Excluding this study from the meta‐analysis did not change the I2; it remained at 96.3%. In all of these aforementioned analyses, our overall conclusion that there was no significant change in the prevalence of AD after the age of 12 years remained robust. Finally, we tested whether the origin of the study (UK vs non‐UK), and the number of times AD was measured (3‐4, 4‐5, or 5‐6) affected our estimates. In both cases, there were only mild reductions in heterogeneity (see Table S5 and Figures S1 and S2).
3.5. Risk of bias
The included studies had variable risk of bias with Newcastle‐Ottawa scores ranging from 3 to 7 of a total maximum score of 8. One of the criteria, demonstration that the outcome of interest (ie, AD diagnosis) was not present at the start of the study, was not relevant given that all of the included studies were birth cohorts. In terms of selection, most (7/9) cohorts were representative of the general population. The Gough et al and Burr et al studies included only healthy newborn children with family histories of atopic disease and therefore may overestimate AD prevalence. Moreover, the Burr cohort was followed after a randomized controlled trial of milk protein over the first 4 months of life, which may introduce a bias as families willing to participate in a RCT may be different from those who decline. The ascertainment of AD was sometimes self‐reported, but always based on a standardized questionnaire or examination applied to all participants (Table S6). In Burr et al's study, the definition of AD used varied over time and could have introduced bias: They measured the point prevalence of AD through age of 7 years, then annual period prevalence from ages 15 and 23 years, which could make AD rates appear higher during adulthood. A sensitivity analysis excluding this study did not change the results (Table S5). All studies had good comparability due to equivalent measurements between AD and non‐AD controls, and all studies had an adequate length of follow‐up. Loss to follow‐up was >30% for 3 of 7 studies; excluding these studies did not affect our results (Table S5 and Figure S3).
3.6. Analyses of repeated measures among individuals
Four studies examined whether individuals reporting AD symptoms at each age had a history of prior disease. All found that there was substantial turnover with many new and remitting cases throughout the study period. However, studies were inconsistent in providing study definitions of disease “remission” and “persistence” (Table S3). In 1 study, persistence was defined as AD symptoms at 2 or more observation points at any time within the study18; another required subjects to have AD symptoms over the course of all study assessments, and across consecutive observation points.23 This inconsistency in the definition of AD persistence and reporting makes it difficult to compare results across studies.
Multiple studies reported the proportion of patients with new‐onset disease at each age of measurement. Incidence rates were highest in early childhood, but remained above zero throughout the teens and early 20s. Williams et al found that of the 870 cases with examined or reported AD onset by the age of 16 years, 66% had onset by the age of 7 years. Nissen et al, found the highest proportions of new‐onset AD onset before the age of 1.5, and Ballardini et al reported that over all time periods, the total proportion of new (vs repeated cases) was 53%.
Multiple studies found that individuals with early‐onset AD were more likely to have symptoms at older ages. In Williams et al, patients with early‐onset AD by the age of 1 year were more likely to have AD at the age of 23 years (P < .001). Similar findings were presented by Burr et al, whereby AD onset by the age of 1 year was associated with AD at the age of 7 and 15 years, and for those with AD at 7 or 15 years, there was an association with AD at the age of 23 years. In Ziyab et al, of the patients that developed AD by the age of 2 years, 16.9% of patients had AD present at all time points up to the age of 18 years; by contrast, in children who developed AD by the age of 4 years, the proportion with AD at all time points was only 10.9%.
4. DISCUSSION
In this systematic review and meta‐analysis of 7 birth cohort studies including over 13 000 individuals, we found no significant difference in AD prevalence before and after childhood. Our finding fills a gap in the literature about AD in adolescence and early adulthood. It also highlights the importance of longitudinal analyses to understand the natural history of an episodic condition. In the studies included in our review that report on the consistency of individual responses over time, the presence of AD symptoms was variable. Individuals reported intermittent periods without symptoms followed by periods with symptoms again. Because studies did not consistently present repeated measures of disease activity or severity among individuals, we are unable to conclude whether AD tends to get better or worse over time. Nonetheless, the available data suggest that the reason for steady prevalence estimates across ages is due to a combination of active disease in both childhood and early adulthood among some individuals, remission or clearance of disease among others, and later‐onset disease among others.
4.1. Comparison with other studies
A recent review focused on “AD persistence” concluded that “80% did not persist by 8 years.”24 It measured the change in the average proportion of a population reporting AD symptoms upon second measurement at any age. Upon first glance, these findings may seem contrary to ours. However, the authors caveat that “it is possible that some of the patients reported to have AD remittance had unobserved recurrences later in life.” Thus, although their results are not directly comparable to ours because they included studies with variable populations, definitions of AD, and timing of follow‐up, their findings also highlight the episodic nature of the condition.
Our results concur with previous work showing high rates of AD persistence in a longitudinal cohort of children and young adults treated with pimecrolimus, which may be biased toward more persistent disease.25 This study did not meet inclusion criteria for this review because enrollment was clinic‐based and it included only patients with AD which did not allow for estimates of disease prevalence. The population‐based ISAAC studies calculated AD prevalence at 2 age points in sites around the world, but were cross‐sectional in nature and did not follow the same individuals over time. There was variation in prevalence by site and by age, but as with our findings, the average prevalence at both time points was similar (7.9% at the age of 6‐7 and 7.3% at the age of 13‐14).26
It is important to highlight that diagnostic definitions may impact prevalence estimates, possibly biasing toward smaller numbers at older ages. Notably, the 1 study that required symptom onset occur in “early childhood” for the definition of AD found the sharpest decline in prevalence,20 possibly because new‐onset cases at later ages were not included. Other, more commonly used definitions of AD, such as the classic Hanifin and Rajka criteria and the widely used UK Working Party Criteria, assign extra points if the AD diagnosis is made in early childhood.11, 27 Subsequently, studies using these criteria may report smaller numbers of individuals with later‐onset disease, which should be taken into account when considering estimates of adult AD.
4.2. Strengths and limitations
Major strengths of our review and meta‐analysis include the comprehensive search, careful selection and critical appraisal of studies, and inclusion of large representative cohorts followed from birth over 2‐3 decades. Of note, we did not specify the initial age of follow‐up in our review; it was coincidental that all of the studies that met inclusion criteria were designed as birth cohort studies.
Limitations also warrant discussion. First, there is variability in the terminology used to describe AD: “Atopic dermatitis,” “atopic eczema,” and “eczema” are all used to refer to the condition.28 In some settings, “eczema” is considered a less specific term that may include other types of dermatitis. Therefore, we carefully reviewed the diagnostic criteria used in each study (Table S3) and assessed the impact on our results as described above. Second, we found a high level of statistical heterogeneity between studies. This might be due in part to the use of a risk difference as our outcome measure, as risk differences are often less homogeneous.16, 29 We used a model with random effects to help account for the statistical heterogeneity, and found no appreciable differences in the results after multiple analyses to address differences in the methods for and timing of AD ascertainment between studies (Table S5). Nonetheless, our results should be interpreted with caution and replicated as additional follow‐up data from longitudinal cohorts become available. Third, most studies lacked important information on secondary outcomes including treatment use, disease severity, and other atopic conditions. These data may impact the clinical course of AD and are important to report. Fourth, as is common for studies that follow large cohorts of patients over decades, attrition was substantial, ranging from 10 to 53%. Most of the studies (7 of 9) were not designed to specifically study AD, and none were organized through clinic visits so dropout was likely random with respect to AD and would therefore be unlikely to bias prevalence estimates. However, other factors differentially associated with attrition such as race/ethnicity and socioeconomic status may also be associated with AD resulting in selection bias.30 These data should be reported in future longitudinal studies. Finally, all of the studies identified were from Northern European countries, and patients were only followed into the third decade of life. Additional work is needed to understand the clinical course throughout adulthood and in more diverse settings.
5. CONCLUSIONS AND IMPLICATIONS FOR PRACTICE AND RESEARCH
Our finding of similar AD prevalence before and after childhood supports the emerging paradigm of AD as a lifelong genetic predisposition to episodic skin lesions.3 Studies examining predictors of individual disease course are a high priority for additional research and can help to elucidate the relationship between genetic susceptibility and environmental influences on the epidemiological trends described herein.
CONFLICT OF INTEREST
None reported.
AUTHOR CONTRIBUTIONS
All authors were involved in the study design. Dr. Abuabara had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Abuabara, Langan, and Allen involved in study concept and design. Yu and Okhovat involved in acquisition, analysis, and interpretation of data. Yu and Abuabara drafted the manuscript. All authors critically revised the manuscript for important intellectual content. Abuabara and Allen WERE involved in the statistical analysis. Abuabara obtained funding. None of the authors receive administrative, technical, or material support. Abuabara supervised the study.
Supporting information
ACKNOWLEDGMENT
We are indebted to Ms. Carol Mita for her assistance with developing and validating our study search strategy.
Abuabara K, Yu AM, Okhovat J‐P, Allen IE, Langan SM. The prevalence of atopic dermatitis beyond childhood: A systematic review and meta‐analysis of longitudinal studies. Allergy. 2018;73:696–704. https://doi.org/10.1111/all.13320
Edited by: Stephan Weidinger
Funding information
This study was supported in part by a training grant from the National Institutes of Health National Center for Advancing Translational Sciences UCSF‐CTSI Grant Number KL2 TR000143 (KA) and by a Wellcome senior research fellowship in clinical science to SML (205039/Z/16/Z). Funding/sponsor was involved in design and conduct of the study: no; collection, management, analysis and interpretation of data: no; preparation review or approval of the manuscript: no; decision to submit the manuscript for publication: no.
REFERENCES
- 1. Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832‐836. [DOI] [PubMed] [Google Scholar]
- 2. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109‐1122. [DOI] [PubMed] [Google Scholar]
- 4. Williams HC. Clinical practice atopic dermatitis. N Engl J Med. 2005;352:2314‐2324. [DOI] [PubMed] [Google Scholar]
- 5. Bieber T, Bussmann C. Atopic dermatitis; Chapter 12 In: Bolongia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed 225:97‐192: Elsevier Saunders; 2012. [Google Scholar]
- 6. Abuabara K, Margolis DJ. Do children really outgrow their eczema, or is there more than one eczema? J Allergy Clin Immunol. 2013;132:1139‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population‐based study. J Allergy Clin Immunol. 2013;132:1132‐1138. [DOI] [PubMed] [Google Scholar]
- 9. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008‐2012. [DOI] [PubMed] [Google Scholar]
- 10. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams HC, Burney PG, Hay RJ, et al. The UK Working Party's diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol. 1994;131:383‐396. [DOI] [PubMed] [Google Scholar]
- 12. Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483‐491. [DOI] [PubMed] [Google Scholar]
- 13. Williams HC, Wüthrich B. The Natural History of Atopic Dermatitis Atopic Dermatitis: the Epidemiology, Causes and Prevention of Atopic Eczema. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 14. Williams HC, Strachan DP. The natural history of childhood eczema: observations from the British 1958 birth cohort study. Br J Dermatol. 1998;139:834‐839. [DOI] [PubMed] [Google Scholar]
- 15. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomized studies in meta‐analyses. The Ottawa Hospital. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 1, 2016.
- 16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Egger M, Altman DG, Smith GD (editors). Systematic Reviews in Health Care: Meta‐Analysis in Context. BMJ Books; 2001. 2nd Edition 3‐19 [Google Scholar]
- 18. Ballardini N, Kull I, Lind T, et al. Development and comorbidity of eczema, asthma and rhinitis to age 12: data from the BAMSE birth cohort. Allergy. 2012;67:537‐544. [DOI] [PubMed] [Google Scholar]
- 19. Burr ML, Dunstan FD, Hand S, Ingram JR, Jones KP. The natural history of eczema from birth to adult life: a cohort study. Br J Dermatol. 2013;168:1339‐1342. [DOI] [PubMed] [Google Scholar]
- 20. Finnbogadottir AF, Ardal B, Eiriksson H, et al. A long‐term follow‐up of allergic diseases in Iceland. Pediatr Allergy Immunol. 2012;23:181‐185. [DOI] [PubMed] [Google Scholar]
- 21. Gough H, Grabenhenrich L, Reich A, et al. Allergic multimorbidity of asthma, rhinitis and eczema over 20 years in the German birth cohort MAS. Pediatr Allergy Immunol. 2015;26:431‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nissen SP, Kjaer HF, Host A, Nielsen J, Halken S. The natural course of sensitization and allergic diseases from childhood to adulthood. Pediatr Allergy Immunol. 2013;24:549‐555. [DOI] [PubMed] [Google Scholar]
- 23. Ziyab AH, Raza A, Karmaus W, et al. Trends in eczema in the first 18 years of life: results from the Isle of Wight 1989 birth cohort study. Clin Exp Allergy. 2010;40:1776‐1784. [DOI] [PubMed] [Google Scholar]
- 24. Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): a systematic review and meta‐analysis. J Am Acad Dermatol. 2016;75:681‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abuabara K, Hoffstad O, Troxel A, Gelfand JM, Margolis DJ. Atopic dermatitis disease control and age: a cohort study. J Allergy Clin Immunol. 2015;136:190‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, Group IPTS . Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251‐1258. [DOI] [PubMed] [Google Scholar]
- 27. Hanifin JMRG. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92(suppl):44‐47. [Google Scholar]
- 28. Kantor R, Thyssen JP, Paller AS, Silverberg JI. Atopic dermatitis, atopic eczema, or eczema? A systematic review, meta‐analysis, and recommendation for uniform use of ‘atopic dermatitis’. Allergy. 2016;71:1480‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgins JP. Commentary: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37:1158‐1160. [DOI] [PubMed] [Google Scholar]
- 30. Abuabara K, Hoffstad O, Troxel AB, Gelfand JM, McCulloch CE, Margolis DJ. Patterns and predictors of atopic dermatitis disease control past childhood: an observational cohort study. J Allergy Clin Immunol. 2017: pii: S0091‐6749(17)30985‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


