Abstract
Endoplasmic reticulum (ER) stress, a dysfunction in protein-folding capacity, is involved in many pathological and physiological responses, including embryonic development. This study aims to determine the developmental competence, apoptosis, and stress-induced gene expression in mouse preimplantation embryos grown in an in vitro culture medium supplemented with different concentrations of the ER stress inducer tunicamycin (TM) and the antioxidant glutathione (GSH). Treatment of zygotes with 0.5 µg/ml TM significantly decreased (P < 0.05) the rate of blastocyst formation, whereas 1 mM GSH supplementation improved the developmental rate of blastocysts. Furthermore, TM treatment significantly increased (P < 0.05) the apoptotic index and reduced the total number of cells, whereas GSH significantly increased the total number of cells and decreased the apoptotic index. The expression levels of ER chaperones, including immunoglobulin-binding protein, activating transcription factor 6, double-stranded activated protein kinase-like ER kinase, activating transcription factor 4, and C/EBP homologous protein were significantly increased (P < 0.05) by TM, but significantly decreased (P < 0.05) by GSH treatment. A similar pattern was observed in the case of the pro-apoptotic gene, B cell lymphoma-associated X protein. The expression level of the anti-apoptotic gene B cell lymphoma 2, was decreased by TM, but significantly increased after co-treatment with GSH. In conclusion, GSH improves the developmental potential of mouse embryos and significantly alleviates ER stress.
Keywords: Apoptosis, Developmental potential, Endoplasmic reticulum stress, Glutathione, In vitro culture medium, Tunicamycin
Preimplantation embryos are vulnerable to various cellular stresses when grown in an in vitro environment [1]. In vitro embryo production requires a culture medium of in vitro environment similar to that of the oviduct and uterine tube [2]. Embryos in an in vivo environment can resist oxidative stress through antioxidants that are present in the follicular fluid [3], or produced by the embryos themselves and the oviduct [4]. In vitro culture is performed to produce embryos for the establishment of animal and disease models [5]. However, developmental anomalies are major obstacles in the production of high-quality in vitro-produced embryos [6]. The coupling of reactive oxygen species (ROS) and endoplasmic reticulum (ER) stress initiation negatively affects in vitro cultures [7]. In eukaryotes, the ER plays a major role in protein structural modification; however, unfolded protein accumulation in the ER lumen triggers ER stress, which leads to cellular damage and apoptosis [8, 9].
Preimplantation embryo development requires a transition from maternal to embryonic RNA, which requires extensive new protein synthesis [10]. These new proteins must be folded properly in the ER lumen to maintain preimplantation embryo development. The ER is the first compartment in the secretory pathway in eukaryotic cells. This organelle is responsible for the synthesis of proteins and their modification and delivery to target sites within the secretory pathway and the extracellular spaces [11]. Secretory proteins enter the secretory pathway through the ER. Apart from protein folding, the ER also participates in helping proteins achieve correct conformation during post-transitional modifications [12]. Furthermore, the ER is a major intracellular organelle responsible for Ca2+ homeostasis [13] and plays an important role in the structural modification of proteins via balanced Ca2+ signaling [11]. However, under pathological conditions, the accumulation of misfolded proteins in the ER lumen leads to ER stress [8], which ultimately leads to cellular damage and apoptosis [9, 14].
Reduced glutathione (GSH) is an antioxidant composed of three amino acids, namely, glutamate, cysteine, and glycine. GSH serves as an antioxidant, a cellular protectant, a regulatory signaling molecule, and also maintains intracellular redox state [15, 16]. GSH protects the cells from toxicants and ROS [17]. Due to its high cellular abundance and low redox potential, GSH is utilized as a redox buffer for reducing oxidized cysteine residues, providing protection against irreversible oxidation, and scavenging various endogenous and exogenous electrophilic compounds [18]. GSH plays a major role in the formation of oocytes, fertilization, and initiation of embryo development [19, 20]. Decreased GSH concentration before in vitro fertilization prevents pronuclear formation and arrests embryonic development [21]. During the development of bovine embryos, inhibition of GSH synthesis reduces development at the eight-cell and blastocyst stages [22], whereas GSH supplementation in the in vitro culture medium promotes pronuclear formation [23]. GSH is involved in multiple molecular processes, such as enzyme activation, protein folding, cellular proliferation, differentiation, and apoptosis [24]. Moreover, GSH is a free thiol compound involved in cellular protection, amino acid transport, and DNA and protein synthesis [25]. GSH plays a major role in the refolding of misfolded proteins by converting non-native disulfide bonds into native disulfide bonds [26]. However, tunicamycin (TM) inhibits N-linked glycosylation in newly synthesized polypeptides and elicits ER stress [27]. TM supplementation in the culture medium negatively affects embryonic development by inducing ER stress [28, 29]. In the current study, we investigated the effects of GSH on TM-induced ER stress. To the best of our knowledge, this study is the first to elucidate the effect of GSH on ER stress during in vitro mouse preimplantation embryo.
Materials and Methods
Animal ethics statement
Healthy 8–10-week-old male and female Kunming mice (Mus musculus), an outbred mouse strain originating from the Swiss albino mouse [30], were purchased from the Experimental Animal Center of Yanbian University. All mice were housed during the experimental study in a 12:12 h light/dark cycle at approximately 24°C. The experimental procedure was approved by the Institutional Animal Care and Use Committee of Yanbian University.
Chemicals and reagents
All chemicals and reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise indicated.
Superovulation, embryo collection, and culture
Each female mouse first received an intraperitoneal injection of 10 IU pregnant mare serum gonadotropin (Ninbo Hormone Product, China; Catalog Number 140825), and then 10 IU human chorionic gonadotropin (hCG; Ninbo Hormone Product, China; Catalog Number 140913) 48 h later. Immediately after hCG administration, each female mouse was mated with one male mouse and checked for vaginal plugs 12 h later. After 22 h of hCG injection, the mice with plugs were sacrificed.
Their oviducts were extracted and placed in M2 medium (M7167; Sigma-Aldrich). Zygotes were released from the oviducts as observed under a stereo microscope. Cumulus cells were removed with 0.03% hyalunronidase (H3506; Sigma-Aldrich), and the zygotes were then washed with fresh M2 medium. Different concentrations (0.5, 1, and 1.5 mM) of GSH were used. For this purpose, a stock solution (100 mM) of GSH was prepared by dissolving 0.0307 g of reduced GSH in 1 ml M16 medium. For the above-mentioned concentrations, 5 μl (0.5 mM), 10 μl (1 mM), and 15 μl (1.5 mM) of this solution was added to 995 μl, 990 μl, and 985 μl of M16 medium, respectively. The zygotes were divided into four groups (n = 12 each) for further analyses. The zygotes in the first group were cultured in 35-µl drops of M16 medium (M7292; Sigma-Aldrich). The zygotes in the second, third, and fourth groups were cultured in 35-µl drops of M16 medium containing 0.5 µg/ml TM, 0.5 µg/ml TM plus 1 mM GSH, and 1 mM GSH covered with mineral oil, respectively. All embryos were incubated at 37°C under a water-saturated atmosphere of 5% CO2. Embryonic development was observed every 24 h. Each experiment was repeated at least three times.
Measurement of ROS content
Twenty blastocysts and four-cell embryos from each group were washed twice in poly vinyl alcohol- phosphate buffered saline PVA-PBS (1 mg/ml), placed in 50-µl droplets of 10 µM 2,7-dichlorodihydro-fluoroscein diacetate (DCFH-DA) (D6883; Sigma-Aldrich), and then incubated at 37°C in an atmosphere of 5% CO2 for 15 min. After incubation, the embryos were washed three times in PVA-PBS and then observed under a fluorescent microscope (1X71; Olympus, Tokyo, Japan). The fluorescence of each blastocyst was measured and analyzed with Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA). All images were captured under same conditions. The excitation wavelength was 480 nm and the emission wavelength was 510 nm. The results are shown as the relative intensity of fluorescence.
Assessment of intracellular GSH level
Mouse embryos were assessed for intracellular GSH level on day 4 using 10 µM Cell Tracker Blue 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (CMF2HC; Catalog Number C12881, Life Technologies Corporation Carlsbad USA). The embryos were washed with PVA-PBS and then observed under a fluorescent microscope (1X71; Olympus). The fluorescence of each blastocyst was measured and analyzed with Image-Pro Plus 6.0 (Media Cybernetics). All images were captured under the same conditions. The excitation wavelength was 371 nm and the emission wavelength was 464 nm. The results are shown as the relative intensity of fluorescence.
TUNEL assay
The apoptotic blastomeres in blastocysts were evaluated by performing TUNEL assay using an cell death detection kit (Calbiochem Roche Diagnostics, San Diego USA; Catalog Number QIA 39). The blastocysts (n = 30) were briefly fixed, washed them three times in 4% paraformaldehyde (PFA; pH 7.4), and then fixed in 4% PFA for 1 h at 30°C. The fixed blastocysts were washed in tris buffered saline (TBS) and then permeabilized by incubation in 0.5% Triton X-100 for 1 h. The blastocysts were washed three times in TBS and then placed in 1 × terminal deoxynucleotidyl transferase (1 × TdT) for 20 min at 30°C. After 20 min, the blastocysts were transferred to 50 µl of TdT labeling reaction mixture (57 µl of fluorescin-Frag FL TdT labeling reaction + 3 µl TdT enzyme) and then incubated for 1 h. After washing three times, the blastocysts were mounted on a microscope slide in a drop of fluorescent media, and then pressed gently with a glass cover slip. The blastocysts were observed immediately using a Nikon microscope (ECLIPSE Ti-S 634268 Nikon, Melville, USA) at 400 × magnification. Photographs were taken with 1600 ASA color film. The excitation wavelength was 380 nm and the emission wavelength was 495 nm. The apoptotic cells appeared green. The results are shown as the relative intensity of fluorescence.
RNA isolation and cDNA synthesis
Three biological replicates, each containing 100 blastocysts from each group, were used for RNA isolation. Total RNA was isolated from whole embryos by using Qiagen RNeasy Mini Kit (Qiagen, Hilden, Germany; Catalog Number 74104) in accordance with the manufacturer’s instructions. RNA concentration and purity were measured as previously described [31]. cDNA was synthesized using the Prime Script™ RT Reagent Kit (Takara Biotechnology, Dalian, China; Catalog Number RR036A).
Semi quantitative real-time polymerase chain reaction (RT-PCR)
Primer sequences and annealing temperatures are listed in Table 1. PCR was performed in 25-µl reaction volume containing 13 µl of 2 × Taq PCR Master Mix (Tiangen Biotech, Beijing, China; Catalog Number KT201), 4 µl of cDNA, 6 µl of H2O, and 1 µl of forward and reverse primers. The PCR program was as follows: initial pre-incubation at 95°C for 3 min; 35 cycles of denaturation at 95°C for 30 sec, annealing at 59–62°C for 30 sec, and extension at 72°C for 30 sec; and a final extension at 72°C for 5 min. The PCR products were separated by gel-electrophoresis on 2% agarose gels containing ethidium bromide, and then visualized under UV illumination [32].
Table 1. Information on the primers used for amplification.
| Gene | Access Number | Sequence | Tmperrature (°C) |
Length (bp) |
| BIP | NM_001163434 | forward, 5’ACTTGGGGACCACCTATTCCT-3’ | 59 | 134 |
| reverse, 5’ATCGCCAATCAGACGCTCC-3’ | ||||
| PERK | NM_010121 | forward, 5’GGGAAAACGGTTCTGAGACA-3’ | 59 | 332 |
| reverse, 5’GCTGACCAGCTAGTCTTGGG-3’ | ||||
| ATF4 | NM_009716 | forward, 5’TCGATGCTCTGTTTCGAATG-3’ | 59 | 312 |
| reverse, 5’AAGCAGCAGAGTCAGGCTTC-3’ | ||||
| ATF6 | NM_001081304 | forward, 5’TGGGCAGGACTATGAAGTAATG-3’ | 62 | 190 |
| reverse, 5’CAACGACTCAGGGATGGTGCTG-3’ | ||||
| CHOP | NM_007837 | forward, 5’ACAGAGGTCACACGCACATC-3’ | 62 | 336 |
| reverse, 5’CTTCCGGAGAGACAGACAGG-3’ | ||||
| BAX | NM_007527 | forward, 5’CCAGGATGCGTCCACCAA-3’ | 60 | 195 |
| reverse, 5’AAGTAGAAGAGGGCAACCAC-3’ | ||||
| BCL2 | NM_007527 | forward, 5’ACCTCTTCAGGGATGGGG-3’ | 60 | 144 |
| reverse, 5’GCCGGTTCAGGTACTCAG-3’ | ||||
| GAPDH | BC023196 | forward, 5’CATCACCATCTTCCAGGAGCG-3’ | 59 | 357 |
| reverse, 5’GAGGGGCCATCCACAGTCTTC-3’ |
Statistical analysis
All assays were performed on three separate occasions. All comparisons for parametric data were performed using multiple comparisons in SPSS 17.0 and one-way analysis of variance (ANOVA) followed by post-hoc Turkey’s multiple comparison test using GraphPad Prism 5 software (La Jolla, CA, USA). Statistical significance was considered at P < 0.05.
Results
Effect of TM and GSH on the developmental ability of preimplantation embryos
TM was initially used alone to induce stress; then, TM and GSH were used in combination and their effect on ER stress and embryo developmental competence was observed (Table 2). The optimum dose of TM and GSH were used as mention in Tables 3 and 4, respectively. No significant difference in the two- and four-cell stages was observed between the groups (TM alone, or in combination with GSH). However, the proportion of embryos reaching the eight-cell stage was significantly lower after exposure to 0.5 µg/ml TM than after exposure to 0.5 µg/ml TM + 1 mM GSH or control treatment or 1 mM GSH alone (36.00 ± 3.14 vs. 72.50 ± 3.74 vs. 70.50 ± 3.53 and 82.00 ± 3.95, respectively). Meanwhile, a higher blastocyst rate was found in the embryos treated with control or 0.5 µg/ml TM + 1 mM GSH or 1 mM GSH alone than those treated with TM (50.00 ± 3.16 vs. 53.50 ± 3.50 vs. 69.5 ± 4.91 and 18.50 ± 1.30, respectively). Table 2 shows the beneficial effects of GSH on embryonic development in the presence of TM.
Table 2. Effect of tunicamycin and GSH on the developmental potential of preimplantation embryos.
| Treated group | Zygote No | 2 Cell | 4 Cell | 8 Cell | Blastocyst |
| Control | 185 | 92.50 ± 3.27 a | 82.00 ± 5.83 a | 70.50 ± 3.53 a | 50.00 ± 3.16 a |
| TM | 170 | 87.00 ± 4.16 a | 74.50 ± 5.74 a | 36.00 ± 3.14 b | 18.50 ± 1.30 b |
| TM +GSH | 171 | 94.00 ± 3.14 a | 85.00 ± 5.00 a | 72.50 ± 3.74 a | 53.50 ± 3.50 a |
| Control+GSH | 180 | 97.50 ± 1.70 a | 86.50 ± 3.80 a | 82.00 ± 3.95 c | 69.50 ± 4.91 c |
Data are the proportions of zygotes reaching the indicated stages within each treatment. Data are expressed as a mean value ± standard error of the mean (SEM) of three independent experiments. Different letters in the same column mean significant difference between the treatments (P < 0.05).
Table 3. Effect of different concentrations of tunicamycin on the developmental potential of mouse embryos in vitro.
| Treated group | Zygote No | 2 Cell | 4 Cell | 8 Cell | Blastocyst |
| Control | 185 | 92.50 ± 3.27 a | 82.00 ± 5.83 a | 70.50 ± 3.53 a | 50.00 ± 3.16 a |
| TM (0.25) | 190 | 90.00 ± 2.58 a | 78.50 ± 3.16 a | 65.50 ± 2.73 a | 44.50 ± 3.90 a |
| TM (0.5) | 170 | 87.00 ± 4.16 a | 74.50 ± 5.74 a | 36.00 ± 3.14 b | 18.50 ± 1.30 b |
| TM (0.75) | 180 | 85.50 ± 3.20 a | 73.00 ± 2.80 a | 26.00 ± 3.39 c | 10.00 ± 2.23 c |
Data are the proportions of zygotes reaching the indicated stages within each treatment. Data are expressed as a mean value ± standard error of the mean (SEM) of three independent experiments. Different letters in the same column mean significant difference between the treatments (P < 0.05).
Table 4. Effect of different concentrations of GSH on the developmental potential of mouse embryos in vitro.
| Treated group | Zygote No | 2 Cell | 4 Cell | 8 Cell | Blastocyst |
| Control | 185 | 92.50 ± 3.27 a | 82.00 ± 5.83 a | 70.50 ± 3.53 a | 50.00 ± 3.16 a |
| TM | 170 | 87.00 ± 4.16 a | 74.50 ± 5.74 a | 36.00 ± 3.14 b | 18.50 ± 1.30 b |
| TM + 0.5 mM GSH | 181 | 90.00 ± 3.16 a | 81.00 ± 3.85 a | 50.50 ± 3.83 c | 32.00 ± 4.66 c |
| TM + 1 mM GSH | 171 | 94.00 ± 3.14 a | 85.00 ± 5.00 a | 72.50 ± 3.74 a | 53.50 ± 3.50 a |
| TM + 1.5 mM GSH | 170 | 95.00 ± 2.58 a | 87.00 ± 3.43 a | 75.00 ± 4.15 a | 56.00 ± 5.51 a |
Data are the proportions of zygotes reaching the indicated stages within each treatment. Data are expressed as a mean value ± standard error of the mean (SEM) of three independent experiments. Different letters in the same column mean significant difference between the treatments (P < 0.05).
Effect of TM and GSH on internal ROS level
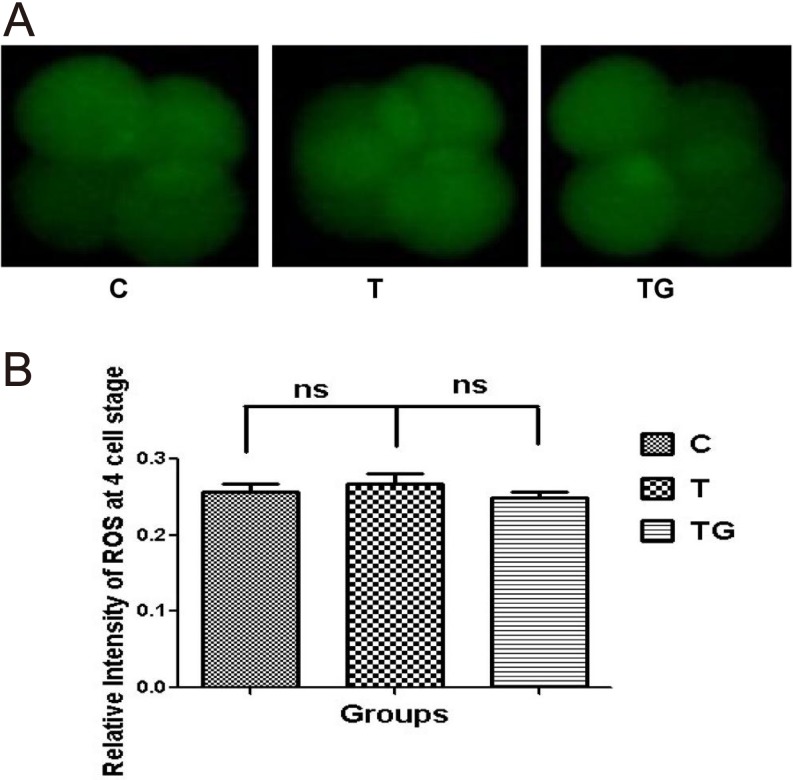
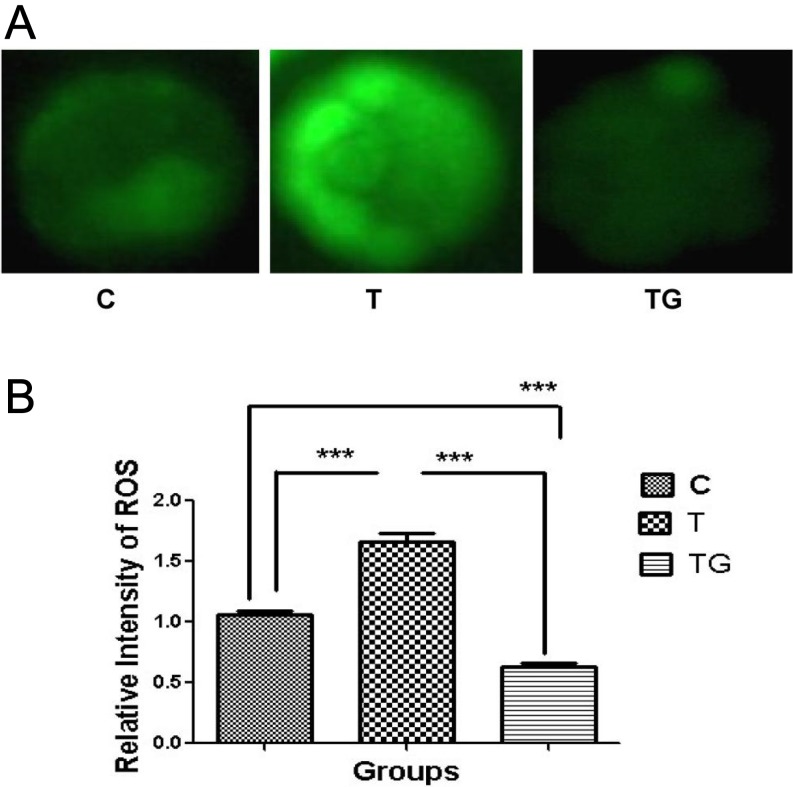
As shown in Table 2, GSH influenced embryonic development at the blastocyst stage. We investigated whether this effect was due to reduced ROS production. We assessed the ROS level at the four-cell and blastocyst stages. Our results showed no significant difference in ROS levels at the four-cell stage between the different groups (Figs. 1A and 1B). However, ROS level at the blastocyst stage was significantly higher in the group supplemented with 0.5 µg/ml TM as compared with the control group, whereas addition of 1 mM GSH significantly decreased ROS level (Figs. 2A and 2B).
Fig. 1.
Evaluation of ROS content in embryos at 4-cell stage in different culture media (control = C, tunicamycin = T, and tunicamycin + GSH = TG) by staining with DCFH-DA. (A) Fluorescence microscopy of embryos cultured in different media. (B) Quantification of ROS levels in the indicated groups. Fluorescence intensity analysis demonstrates no significant difference in ROS level at the 4-cell stage between the different groups. Images are presented at 400 × magnification. Data are expressed as mean ± standard error of the mean (SEM) of three independent experiments. Asterisks represent statistically significant differences (P < 0.05).
Fig. 2.
Evaluation of ROS content in embryos at blastocyst stage in different culture media (control = C, tunicamycin = T, and tunicamycin + GSH = TG) by staining with DCFH-DA. (A) Fluorescence microscopy of embryos cultured in different media. (B) Quantification of ROS levels in the indicated groups. Fluorescence intensity analysis demonstrates a higher significance (P < 0.05) for blastocyst-stage embryo exposed to TM. Images are presented at 400 × magnification. Data are expressed as mean ± SEM of three independent experiments. Asterisks represent statistically significant differences (P < 0.05).
Effect of TM and/or reduced GSH on intracellular GSH level
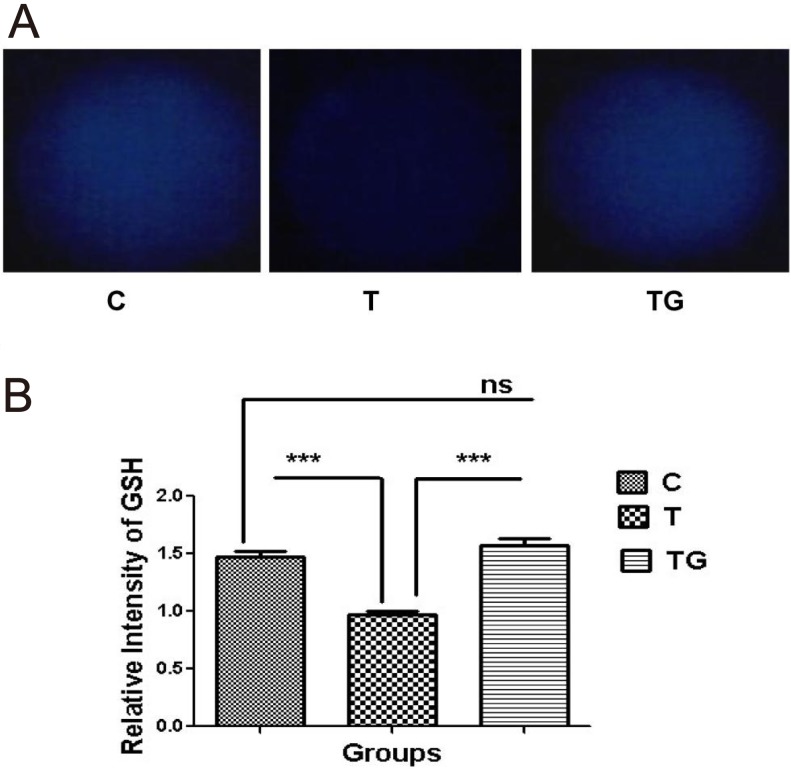
As shown in Table 2 and Fig. 3, TM negatively affected the embryos, and GSH reversed the negative effects of TM. Further investigation showed that TM significantly decreased the level of intracellular GSH (Figs. 3A and 3B).
Fig. 3.
Evaluation of GSH content in embryos at blastocyst stage in different culture media (control = C, tunicamycin = T, and tunicamycin + GSH = TG) by staining with CMF2HC (A) Fluorescence microscopy of embryos cultured in different media. (B) Quantification of GSH levels in the indicated groups. Fluorescence intensity analysis demonstrates a higher significance (P < 0.05) for blastocyst-stage embryo exposed to TM. Images are presented at 400 × magnification. Data are expressed as mean ± SEM of three independent experiments. Asterisks represent statistically significant differences (P < 0.05).
Effect of GSH on the quality of blastocysts in in vitro environment
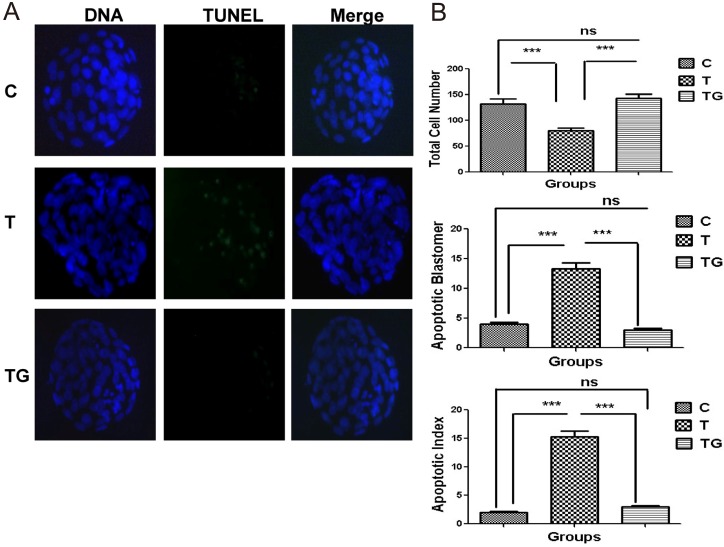
We investigated whether GSH only reduces ROS level or also affects the quality of blastocysts in in vitro environment. TUNEL assay was performed to determine the apoptotic index in the various treatment groups. Mouse embryos were cultured in a medium supplemented with TM, the ER stress inducer, or GSH, the ER stress inhibitor. Embryos cultured in medium without any supplementation served as the control group. The results of TUNEL assay showed that blastocysts in the TM group underwent apoptosis and possessed lower total cell number compared with those in the control and GSH groups (Figs. 4A and 4B).
Fig. 4.
Effect of tunicamycin and/or GSH on apoptosis in mouse preimplantation embryos. (A) Apoptotic cells in the mouse blastocyst were evaluated by TUNEL assay in different treatment groups (control = C, tunicamycin = T, and tunicamycin + GSH = TG) counterstained with DAP1. (B) The total cell numbers, apoptotic blastomeres, and apoptotic index. Apoptotic index = apoptotic cell/total cell in a blastocyst. Data are expressed as mean ± SEM of three independent experiments. Asterisks represent statistically significant differences (P < 0.05).
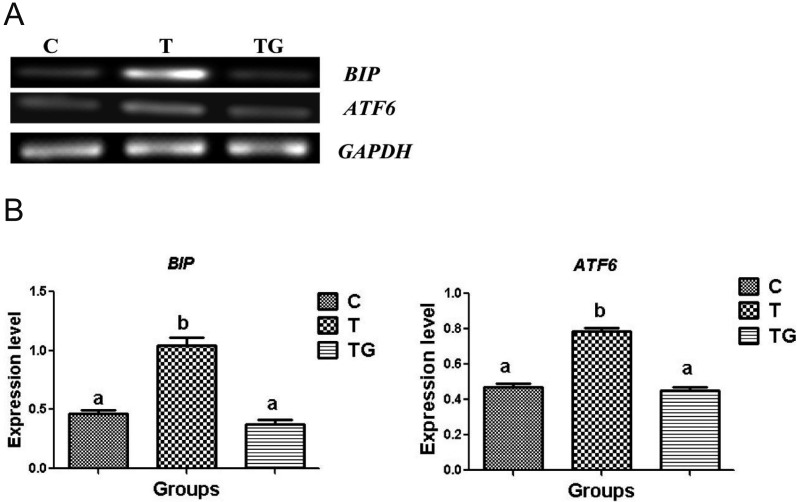
Effect of GSH on ATF6 localization in mouse preimplantation embryos
Considering that GSH benefits embryonic development by alleviating ROS generation, we further investigated whether this effect may involve ER stress pathways during unfolded protein response (UPR). The glucose regulatory protein, immunoglobulin-binding protein (BiP), has been used as an indicator of the onset of ER stress [33]. During ER stress, activating transcription factor 6 (ATF6) dissociates from BiP, and the activated form of ATF6 upregulates C/EBP homologous protein (CHOP) expression [33]. Thus, we determined whether GSH affects the BiP-ATF6 pathway in mouse preimplantation embryos. Results showed that the expression levels of BiP and ATF6 were significantly reduced in the GSH group as compared with the TM group (Figs. 5A and 5B). This result suggests that GSH significantly reduces TM-induced ER stress.
Fig. 5.
The expression of BiP and ATF6 in mouse embryos cultured in different media (control = C, tunicamycin = T, and tunicamycin + GSH = TG). GAPDH was used as an internal control. (A) PCR results of BiP and ATF6 in mouse embryos. (B) Relative expression of BiP and ATF6 normalized with the internal marker GAPDH (P < 0.05). Data are expressed as mean ± SEM of three independent experiments. Asterisks represent statistically significant differences (P < 0.05).
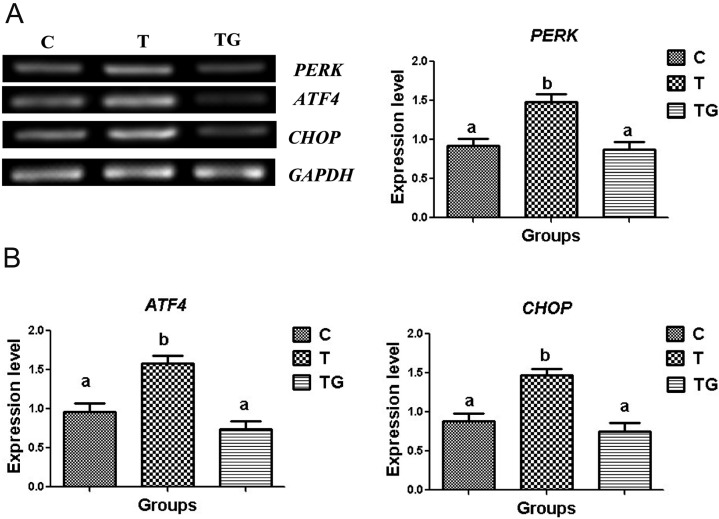
Effect of GSH on the expression of protein kinase-like ER kinase (PERK) pathway-related genes
We evaluated the expression of PERK, which plays a key role in ER stress, to further investigate the ER stress response pathways. PERK is released from BiP during ER stress and then activates activating transcription factor 4 (ATF4), leading to the upregulation of CHOP expression [34, 35]. Thus, we determined whether GSH affects the PERK-ATF4-CHOP pathway. Real-time polymerase chain reaction (RT-PCR) analysis demonstrated that PERK expression was significantly downregulated in the GSH group as compared with the TM group. In addition, co-treatment with GSH significantly reduced the expression of ATF4 and CHOP as compared with TM group (Figs. 6A and 6B). Hence, these results confirm that GSH alleviates ER stress.
Fig. 6.
The expression of PERK, ATF4, and CHOP in mouse embryos cultured in different media (control = C, tunicamycin = T, and tunicamycin + GSH = TG). GAPDH was used as an internal control. (A) PCR results of PERK, ATF4, and CHOP expression in mouse embryos. (B) Relative expression of PERK, ATF4, and CHOP normalized with the internal marker GAPDH (P < 0.05). Data are expressed as mean ± SEM of three independent experiments. Asterisks represent statistically significant differences (P < 0.05).
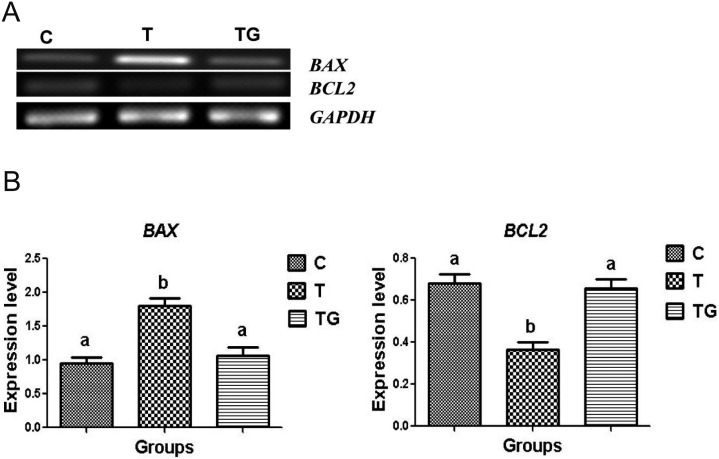
Effect of GSH on blastocyst apoptosis
The rate of apoptosis is determined by DNA fragmentation in blastomeres, which occurs during preimplantation embryonic development under stress conditions [36]. Thus, we investigated whether GSH is involved in the prevention of ER stress-induced apoptosis. The expression levels of anti- and pro-apoptotic genes, including B cell lymphoma 2 (BCL2) and B cell-associated X protein (BAX), in blastocysts cultured in the presence and absence of GSH were measured using RT-PCR. The RNA expression of BCL2 significantly increased, whereas that of BAX significantly decreased in blastocysts grown with 1 mM GSH compared to that in blastocysts grown with 0.5 µg/ml TM (Figs. 7A and 7B). These findings indicate that GSH significantly reduces TM-induced apoptosis.
Fig. 7.
The expression of BAX and BCL2 in mouse embryos cultured in different media (control = C, tunicamycin = T, and tunicamycin + GSH = TG). GAPDH was used as an internal control. (A) PCR results of BAX and BCL2 expression in mouse embryos. (B) Relative expression of BAX and BCL2 normalized with the internal marker GAPDH (P < 0.05). Data are expressed as mean ± SEM of three independent experiments. Asterisks represent statistically significant differences (P < 0.05).
Discussion
To the best of our knowledge, this study is the first to demonstrate GSH as an ER stress inhibitor in mouse preimplantation embryo development. GSH reduces oxidative stress and enhances embryonic development [4, 23]. Exposure of embryos to in vitro environment can cause developmental defects [37] due to several types of stresses, such as growth and survival factors, deprivation, metabolic and substrate imbalance, and oxidative and osmotic stress [38]. In addition, ER stress plays an important role in embryonic development [13]. In the ER, UPR is an adaptive mechanism that attempts to restore the balance between newly synthesized unfolded protein and folding capacity. Various physiological stress conditions may interrupt protein folding and induce protein misfolding, ultimately leading to ER stress and triggering apoptosis during in vitro embryo development [13, 39, 40].
GSH reduces ROS and oxidative stress during embryo development [41]. Exposure of cleavage-stage embryos to low GSH concentration reduces embryo development from the two-cell to the blastocyst stage [42, 43]. However, the precise functional role of GSH as an ER stress inhibitor in preimplantation embryos is unclear. We hypothesized that GSH plays a similar role in the protection of preimplantation embryos against ER stress. Embryos were developed in ER stress environment with 0.5 µg/ml TM as a stress inducer. TM inhibits protein synthesis at the N-linked glycosylation step in the ER [27]. TM significantly reduced the developmental potential of embryos at the eight-cell and blastocyst stages. Co-treatment with TM and 1 mM GSH significantly increased the developmental potential of embryos at the eight-cell and blastocyst stages. Similarly, previous studies have shown that TM reduces mouse embryo development at the blastocyst stage, while the ER stress inhibitor tauroursdexoycholate (TUDCA) enhances this process [44]. Furthermore, GSH supplementation increases the blastocyst rate during embryonic development with increasing O2 concentration [7]. GSH assists in protein refolding and reduces the misfolded proteins in the ER lumen by converting non-native disulfide bonds into disulfide bonds [26]. TUDCA is a bile acid that acts as a potent chemical chaperon inhibiting apoptosis associated with ER stress; it enhances the maturation of oocytes and the development of preimplantation embryos by activating the mitogen-activated protein kinase pathway [45].
To further explore the relationship between ER stress and ROS level, we detected the ROS level at the blastocyst stage by using DCFH-DA fluorescence. Results revealed that ROS levels were significantly increased in embryos exposed to TM; however, GSH treatment significantly reduced the ROS levels in these embryos. Our findings are in line with those from previous work by Yoon et al. [7], who reported that ROS level is increased with increasing O2 concentration, while GSH decreases the ROS level in bovine embryos.
GSH plays a critical role in regulating intracellular redox balance and protecting cells against ROS [46]. In the present study, we examined the effect of ER stress on intracellular GSH levels in mouse embryos. Results showed that ER stress reduced intracellular GSH levels, whereas 1 mM GSH increased the levels significantly by inhibiting ER stress (Fig. 3). Our results are supported by the findings of Lin et al. [28], who reported that TM reduces intracellular GSH levels in porcine embryos, while co-treatment with TUDCA increases its levels.
The TUNEL assay is useful in exploring the quality and viability of blastocysts produced under in vitro conditions [47, 48]. Using this method, we analyzed the quality of blastocysts after TM and GSH treatment. Compared with those treated with GSH, blastocysts treated with GSH showed higher expansion level, greater total cell number, and lesser apoptotic cell number. Our results are similar to the findings of Sharma et al., who reported that TM increases the apoptotic index and decreases the total cell number, while TUDCA enhances the total cell number and decreases the apoptotic index in bovine embryos [29]. Yoon et al. [7] reported that GSH reduces the number of apoptotic cells in the blastocysts during bovine early embryonic development under oxidative stress conditions. In porcine embryo development, TUDCA increases the level of expansion and the number of total cells and decreases the number of apoptotic cells compared with TM.
BiP regulates ER stress; under physiological conditions, binding of BiP to the luminal domain of ATF6 in the ER maintains it in an inactive form. However, upon induction of ER stress, BiP dissociates from ATF6 and activates ATF6 [50]. In the present study, the expression levels of BiP and ATF6 were increased in embryos treated with TM, whereas GSH supplementation significantly downregulated the expression of BiP and ATF6. Similarly, Murat et al. [44] reported that TM enhances the expression of BiP, which negatively affects embryonic development. TUDCA supplementation significantly downregulates the expression of BiP in mouse embryos. Rapamycin downregulates the expression of BiP and ATF6 in mouse preimplantation embryos, whereas ER stress inducers upregulate their expression [51]. In bovine somatic cells nuclear transfer embryos, valproic acid downregulates the expression of BiP and improves embryonic development [52].
PERK is an ER transmembrane sensor present in the ER lumen and attached to BiP. In ER stress conditions, dimerization of PERK leads to its release from BiP, and trans-autophosphorylation leads to activation of eukaryotic translational initiation factor 2 alpha kinase [53]. PERK induces the UPR-dependent gene transcription [54]. ATF4 is a transcription factor whose translation is initiated upon PERK-mediated phosphorylation. Phosphorylated PERK and ATF4 are required for inducing apoptosis-inducing factors, such as CHOP [55]. CHOP participates in the ER stress-induced apoptosis pathway and promotes apoptosis under severe ER stress conditions [33]. In the present study, TM treatment significantly upregulated the gene expression of PERK, ATF4, and CHOP, whereas GSH supplementation significantly reduced their expression. Similarly, Yoon et al. found that in bovine embryos, increased oxygen concentrations enhance the expression of the above genes, while TUDCA suppresses it [7]. During ER stress ATF4 is activated, and treatment with histone deacetylase 4 inhibits ATF4 activation and protects the cells from ER stress-induced apoptosis [56]. Rapamycin improves mouse embryonic development by downregulating the expression of PERK, ATF4, and CHOP [51].
ER stress can induce apoptosis [31]. Our results suggest that GSH also plays an important role in protecting mouse embryos against ER stress-induced apoptosis. To investigate the mechanism through which GSH protects mouse embryos against ER stress-induced apoptosis, we examined the expression levels of the pro-apoptotic gene BAX and the anti-apoptotic gene BCL2. BAX is a member of the pro-apoptotic gene family, whereas BCL2 is a prominent inhibitor of apoptosis [54]. We measured the expression levels of BAX and BCL2 after TM and GSH treatments. TM decreased the expression of BCL2, but increased that of BAX. GSH supplementation significantly increased the expression of BCL2, but decreased that of BAX. Our results are supported by the findings of Zhang et al. [45], who reported that TM as an ER stress inducer increases the expression of BAX and decreases that of BCL2, while co-treatment with TUDCA significantly downregulates BAX and upregulates BCL2 expression in mouse embryos. TM increases the expression of BAX and decreases that of BCL2, whereas TUDCA treatment decreases the expression of BAX and upregulates that of BCL2 in bovine embryos [29].
In conclusion, GSH plays a major role in the development of preimplantation mouse embryos under ER stress conditions. TM-induced ER stress causes the arrest of embryo development at the eight-cell and blastocyst stages. GSH supplementation in the medium increases embryo development, decreases the number of apoptotic cells, downregulates pro-apoptotic and ER stress-related gene expression, and upregulates anti-apoptotic gene expression.
Acknowledgments
The authors wish to thank Zhang Ze Qian and He Li Na for their help. This work was supported by the National Natural Science Foundation of China (No. 31360546).
References
- 1.Lane M, Gardner DK. Understanding cellular disruptions during early embryo development that perturb viability and fetal development. Reprod Fertil Dev 2005; 17: 371–378. [DOI] [PubMed] [Google Scholar]
- 2.Yuan YQ, Van Soom A, Coopman FO, Mintiens K, Boerjan ML, Van Zeveren A, de Kruif A, Peelman LJ. Influence of oxygen tension on apoptosis and hatching in bovine embryos cultured in vitro. Theriogenology 2003; 59: 1585–1596. [DOI] [PubMed] [Google Scholar]
- 3.Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update 2001; 7: 175–189. [DOI] [PubMed] [Google Scholar]
- 4.Gardiner CS, Reed DJ. Glutathione redox cycle-driven recovery of reduced glutathione after oxidation by tertiary-butyl hydroperoxide in preimplantation mouse embryos. Arch Biochem Biophys 1995; 321: 6–12. [DOI] [PubMed] [Google Scholar]
- 5.Hall V, Hinrichs K, Lazzari G, Betts DH, Hyttel P. Early embryonic development, assisted reproductive technologies, and pluripotent stem cell biology in domestic mammals. Vet J 2013; 197: 128–142. [DOI] [PubMed] [Google Scholar]
- 6.Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology 2000; 54: 741–756. [DOI] [PubMed] [Google Scholar]
- 7.Yoon SB, Choi SA, Sim BW, Kim JS, Mun SE, Jeong PS, Yang HJ, Lee Y, Park YH, Song BS, Kim YH, Jeong KJ, Huh JW, Lee SR, Kim SU, Chang KT. Developmental competence of bovine early embryos depends on the coupled response between oxidative and endoplasmic reticulum stress. Biol Reprod 2014; 90: 104. [DOI] [PubMed] [Google Scholar]
- 8.Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol 2010; 48: 1105–1110. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Jiang K, Gao D, Kang X, Sun C, Zhang Q, Li Y, Sun L, Zhang S, Guo K, Liu Y. Clusterin protects hepatocellular carcinoma cells from endoplasmic reticulum stress induced apoptosis through GRP78. PLoS ONE 2013; 8: e55981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol 2005; 283: 40–57. [DOI] [PubMed] [Google Scholar]
- 11.Brostrom MA, Brostrom CO. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium 2003; 34: 345–363. [DOI] [PubMed] [Google Scholar]
- 12.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8: 519–529. [DOI] [PubMed] [Google Scholar]
- 13.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 2011; 13: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bánhegyi G, Baumeister P, Benedetti A, Dong D, Fu Y, Lee AS, Li J, Mao C, Margittai E, Ni M, Paschen W, Piccirella S, Senesi S, Sitia R, Wang M, Yang W. Endoplasmic reticulum stress. Ann N Y Acad Sci 2007; 1113: 58–71. [DOI] [PubMed] [Google Scholar]
- 15.Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 2003; 333: 19–39. [DOI] [PubMed] [Google Scholar]
- 16.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med 1999; 27: 916–921. [DOI] [PubMed] [Google Scholar]
- 17.Reed DJ. Chemical mechanisms of drug-induced liver injury. In: Zakim D, Boyer TD (eds.), Hepatology: A Textbook of Liver Disease. Philadelphia: WB Saunders Company; 1990: 737–753.
- 18.Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells 2008; 25: 332–346. [PMC free article] [PubMed] [Google Scholar]
- 19.Perreault SD, Barbee RR, Slott VL. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev Biol 1988; 125: 181–186. [DOI] [PubMed] [Google Scholar]
- 20.Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev 2003; 64: 106–112. [DOI] [PubMed] [Google Scholar]
- 21.Zuelke KA, Jones DP, Perreault SD. Glutathione oxidation is associated with altered microtubule function and disrupted fertilization in mature hamster oocytes. Biol Reprod 1997; 57: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M, Nagai T, Hamano S, Kuwayama M, Okamura N, Okano A. Effect of thiol compounds on in vitro development and intracellular glutathione content of bovine embryos. Biol Reprod 1993; 49: 228–232. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida M, Ishigaki K, Nagai T, Chikyu M, Pursel VG. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol Reprod 1993; 49: 89–94. [DOI] [PubMed] [Google Scholar]
- 24.Hansen JM, Harris C. Glutathione during embryonic development. Biochim Biophys Acta 2015; 1850: 1527–1542. [DOI] [PubMed] [Google Scholar]
- 25.You J, Kim J, Lim J, Lee E. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology 2010; 74: 777–785. [DOI] [PubMed] [Google Scholar]
- 26.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 1992; 257: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 27.Brandish PE, Kimura KI, Inukai M, Southgate R, Lonsdale JT, Bugg TD. Modes of action of tunicamycin, liposidomycin B, and mureidomycin A: inhibition of phospho-N-acetylmuramyl-pentapeptide translocase from Escherichia coli. Antimicrob Agents Chemother 1996; 40: 1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin T, Lee JE, Oqani RK, Kim SY, Cho ES, Jeong YD, Baek JJ, Jin DI. Tauroursodeoxycholic acid improves pre-implantation development of porcine SCNT embryo by endoplasmic reticulum stress inhibition. Reprod Biol 2016; 16: 269–278. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Agrawal H, Mullani N, Sandhu A, Singh MK, Chauhan MS, Singla SK, Palta P, Manik RS. Supplementation of tauroursodeoxycholic acid during IVC did not enhance in vitro development and quality of buffalo IVF embryos but combated endoplasmic reticulum stress. Theriogenology 2015; 84: 200–207. [DOI] [PubMed] [Google Scholar]
- 30.Yu S, Yan X, Liu H, Cai X, Cao S, Shen L, Zuo Z, Deng J, Ma X, Wang Y, Ren Z. Improved establishment of embryonic stem (ES) cell lines from the Chinese Kunming mice by hybridization with 129 mice. Int J Mol Sci 2014; 15: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin P, Yang Y, Li X, Chen F, Cui C, Hu L, Li Q, Liu W, Jin Y. Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol Reprod Dev 2012; 79: 423–432. [DOI] [PubMed] [Google Scholar]
- 32.Yuan JH, Wang JZ, Lan GC, Sui HS, Yu JN, Tan JH. Expression of steroidogenic enzymes and synthesis of steroid hormones during development of ovarian follicles in prepubertal goats. Domest Anim Endocrinol 2008; 34: 451–460. [DOI] [PubMed] [Google Scholar]
- 33.Mao C, Tai WC, Bai Y, Poizat C, Lee AS. In vivo regulation of Grp78/BiP transcription in the embryonic heart: role of the endoplasmic reticulum stress response element and GATA-4. J Biol Chem 2006; 281: 8877–8887. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida H, Nadanaka S, Sato R, Mori K. XBP1 is critical to protect cells from endoplasmic reticulum stress: evidence from Site-2 protease-deficient Chinese hamster ovary cells. Cell Struct Funct 2006; 31: 117–125. [DOI] [PubMed] [Google Scholar]
- 35.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 2000; 6: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 36.Exley GE, Tang C, McElhinny AS, Warner CM. Expression of caspase and BCL-2 apoptotic family members in mouse preimplantation embryos. Biol Reprod 1999; 61: 231–239. [DOI] [PubMed] [Google Scholar]
- 37.Knijn HM, Gjørret JO, Vos PL, Hendriksen PJ, van der Weijden BC, Maddox-Hyttel P, Dieleman SJ. Consequences of in vivo development and subsequent culture on apoptosis, cell number, and blastocyst formation in bovine embryos. Biol Reprod 2003; 69: 1371–1378. [DOI] [PubMed] [Google Scholar]
- 38.Absalón-Medina VA, Butler WR, Gilbert RO. Preimplantation embryo metabolism and culture systems: experience from domestic animals and clinical implications. J Assist Reprod Genet 2014; 31: 393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 2003; 4: 181–191. [DOI] [PubMed] [Google Scholar]
- 40.Gething MJ, Sambrook J. Protein folding in the cell. Nature 1992; 355: 33–45. [DOI] [PubMed] [Google Scholar]
- 41.Salmen JJ, Skufca F, Matt A, Gushansky G, Mason A, Gardiner CS. Role of glutathione in reproductive tract secretions on mouse preimplantation embryo development. Biol Reprod 2005; 73: 308–314. [DOI] [PubMed] [Google Scholar]
- 42.Gardiner CS, Reed DJ. Status of glutathione during oxidant-induced oxidative stress in the preimplantation mouse embryo. Biol Reprod 1994; 51: 1307–1314. [DOI] [PubMed] [Google Scholar]
- 43.Gardiner CS, Reed DJ. Synthesis of glutathione in the preimplantation mouse embryo. Arch Biochem Biophys 1995; 318: 30–36. [DOI] [PubMed] [Google Scholar]
- 44.Murat B, Idil B, Ozlem GK, Berna S, Isil T, Frederick S, Aydin-Arichi MD, Charles J, Lockwood MD, Umit AK. Unfolded protein response prevents blastocysts formation during preimplantation embryo development in vitro. Fertil Steril 2014; 6: 1777–1784. [DOI] [PubMed] [Google Scholar]
- 45.Zhang JY, Diao YF, Kim HR, Jin DI. Inhibition of endoplasmic reticulum stress improves mouse embryo development. PLoS ONE 2012; 7: e40433. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol 2005; 5: 5–17. [PubMed] [Google Scholar]
- 47.Gupta MK, Uhm SJ, Han DW, Lee HT. Embryo quality and production efficiency of porcine parthenotes is improved by phytohemagglutinin. Mol Reprod Dev 2007; 74: 435–444. [DOI] [PubMed] [Google Scholar]
- 48.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 2002; 36: 592–601. [DOI] [PubMed] [Google Scholar]
- 49.Kim JS, Song BS, Lee KS, Kim DH, Kim SU, Choo YK, Chang KT, Koo DB. Tauroursodeoxycholic acid enhances the pre-implantation embryo development by reducing apoptosis in pigs. Reprod Domest Anim 2012; 47: 791–798. [DOI] [PubMed] [Google Scholar]
- 50.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol 2013; 5: a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi YJ,Gurunathan S, Kim D, Jang HS, Park WJ, Cho SG, Park C, Song H, Seo HG, Kim JH. Rapamycin ameliorates chitosan nanoparticle-induced developmental defects of preimplantation embryos in mice. Oncotarget 2016; 7: 74658–74677. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song BS, Yoon SB, Sim BW, Kim YH, Cha JJ, Choi SA, Jeong KJ, Kim JS, Huh JW, Lee SR, Kim SH, Kim SU, Chang KT. Valproic acid enhances early development of bovine somatic cell nuclear transfer embryos by alleviating endoplasmic reticulum stress. Reprod Fertil Dev 2014; 26: 432–440. [DOI] [PubMed] [Google Scholar]
- 53.Linda LW, Darryl LR, Robert JN, Rebecca LR. Endoplasmic reticulum (ER) stress in cumulus-oocytes complexes impairs pentraxin-3 secretion, mitochondrial membrane potential and embryo development. Mol Endo 2012; 26: 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang P, Sun Q, Zhao C, Ling S, Li Q, Chang YZ, Li Y. HDAC4 protects cells from ER stress induced apoptosis through interaction with ATF4. Cell Signal 2014; 26: 556–563. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev 2003; 66: 143–152. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi T, Igarashi H, Kawagoe J, Amita M, Hara S, Kurachi H. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol Reprod 2009; 80: 493–502. [DOI] [PubMed] [Google Scholar]