Abstract
This study aimed to identify motile sperm subpopulations in extended boar semen and to observe the presumptive seasonal variation in their distribution. Data from 4837 boar ejaculates collected over a two-year period were analyzed in terms of kinematic parameters by Computer Assisted Sperm Analysis (CASA). Individual sperm data were used to determine subgroups of motile sperm within the ejaculates using cluster analysis. Four motile sperm subpopulations (SP) were identified, with distinct movement patterns: SP1 sperm with high velocity and high linearity; SP2 sperm with high velocity but low linearity; SP3 sperm with low velocity but high linearity; and SP4 sperm with low velocity and low linearity. SP1 constituted the least overall proportion within the ejaculates (P < 0.05). Season of semen collection significantly influenced the different proportions of sperm subpopulations. Spring was characterized by similar proportions of SP1 and SP4 (NS) and higher proportions of SP3. Summer brought a decrease in both subgroups containing fast sperm (SP1 and SP2) (P < 0.05). During autumn, increases in SP2 and SP4 were recorded. Winter substantially affected the proportions of all sperm subpopulations (P < 0.05) and SP2 became the most represented subgroup, while SP1 (fast and linear) reached its highest proportion compared to other seasons. In conclusion, extended boar semen is structured in distinct motile sperm subpopulations whose proportions vary according to the season of collection. Summer and autumn seem to have a negative impact on the fast and linear subpopulation. Cluster analysis can be useful in revealing differences in semen quality that are not normally detected by classical evaluation based on mean values.
Keywords: Boar, Computer Assisted Sperm Analysis (CASA), Cluster analysis, Season, Sperm subpopulations
Computer Assisted Sperm Analysis (CASA) systems are able to record individual values for a variable number of sperm analyzed during semen examination. Using a multi-step statistical analysis, the individual values obtained allow a further, more detailed evaluation of the semen sample, with classification of the sperm within subpopulations based on their kinematic parameters [1]. This offers the possibility of a detailed profile for each ejaculate.
The starting point for detecting sperm subpopulations originated from the observation made by W.V. Holt that boar ejaculates contain specific kinematic, relatively homogenous subgroups, which can be deduced from the results offered by CASA profiling [2]. Numerous studies on these so-called motile subpopulations have followed in the boar [3,4,5], as well as in bulls [6, 7], stallions [8], donkeys [9], goat bucks [10], deer [11], dogs [12], and even fish [13]. Some authors currently believe that the interpretation of CASA results based only on mean values represents an incomplete approach [14,15,16]. As such, stratifying an ejaculate into sperm subpopulations opens a new perspective on sperm evaluation, which may be helpful in improving seminal dose calculations in assisted reproductive programs [17] and to obtain improved information on ejaculates [18].
Although a consensus on the physiological role of sperm subpopulations has not yet been achieved, the utility of this procedure has been demonstrated by studies that have revealed interesting correlations, such as the link between the distribution of subpopulations and fertility [19], activation of motility in presence of bicarbonate [4], in vitro capacitation and the acrosome reaction [3], or cryoresistance [20]. These findings could be useful in selecting ejaculates with higher cryoresistance and fertility.
Seasonal variation of some seminal parameters in farm animals has previously been demonstrated [21,22,23] and it is thus reasonable to question whether seasonal dynamics may also affect the distribution of motile sperm in specific subpopulations. Although sperm subpopulations analysis based on CASA output has been a well-debated subject during recent years, to date there is no information in the literature regarding seasonal variations in boar. Therefore, the aim of this study was to identify motile sperm subpopulations in extended boar semen using CASA profiling and to describe their seasonal variation.
Materials and Methods
Boars and semen
The study evaluated data from 4837 ejaculates obtained from 702 healthy boars, aged between 9 months and 7 years, belonging to the Pietrain (90%), Landrace (5%), and Duroc (5%) breeds and housed in an artificial insemination (AI) station in southern Germany. All the boars were commonly used for semen collection for commercial purpose and were held in individual pens within the same farm. Boars were fed a standard diet during the year, received water ad libitum, and experienced natural photoperiods through windows facing outdoors. The stables were not furnished with microclimate control systems, so boars were exposed to the natural variations of their environment across seasons. The main climatic data recorded in the region are presented in Table 1.
Table 1. Seasonal mean values for the main climatic factors recorded in the region a and period of study.
| Season b | Temperature (°C) | Relative humidity (%) | Duration of daylight (h) | Atmospheric pressure (hPa) |
| Spring | 9.3 | 73.3 | 13.7 | 1013.7 |
| Summer | 18.4 | 70.0 | 15.4 | 1016.0 |
| Autumn | 10.4 | 87.8 | 10.8 | 1016.5 |
| Winter | 2.6 | 89.1 | 9.1 | 1017.5 |
a Sources of data: https://www.timeanddate.com/sun/germany/nuremberg; http://umweltdaten.nuernberg.de/wetterdaten/messstation-nuernberg-flugfeld/archiv/
b Spring = March–May; Summer = June–August; Autumn = September–November; Winter = December–February.
Semen was collected over a 24-month period, between March 2013 and February 2015 by manual methods and using an artificial vagina. Ejaculates from the same boar were routinely collected weekly within the AI station. After passing a general exam for quality (65% total sperm motility and ≥ 15 × 109 total sperm count), the ejaculates were diluted using Beltsville Thawing Solution (BTS, Minitube, Tiefenbach, Germany) and submitted for liquid-state preservation at 17°C in doses of 100 ml (90 ml extended semen + 10 ml air).
Analysis of semen
Semen analysis was performed after three days of storage, by means of the CASA system, software SpermVision 3.7 (Minitube of America - MOFA®, Verona, WI, USA), connected to a Zeiss Axio Scope A1 microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany) equipped with a heated stage at 38°C. For the examination, semen samples of 3 µl were placed in four-chamber slides (Leja, Nieuw Vennep, The Netherlands) with a chamber depth of 20 µm. The sperm kinematic parameters were recorded from 7 successive fields per sample. For each examination, the field with sperm motility closest to that calculated as mean value per sample was saved. The following kinematic parameters were calculated: total sperm motility (TMot), progressive sperm motility (PMot), average path velocity (VAP), curvilinear velocity (VCL), straight line velocity (VSL), straightness (STR), linearity (LIN), wobble (WOB), amplitude of lateral head displacement (ALH) and beat cross frequency (BCF).
Individual data of each motile spermatozoon within the field saved by computer were accessed. Thus, calculated values were obtained for a total of 312,444 sperm analyzed over the two-year period. All data were then incorporated in a single dataset.
Cluster analysis
In order to identify the subpopulations, the values were subjected to a series of statistical analyses using IBM SPSS® Statistics version 21 (IBM®, Chicago, IL, USA). The first step was a Principal Component Analysis (PCA) of the eight above-mentioned kinematic parameters. The aim of the PCA is to reduce the number of variables in a database, in order to make data easier to visualize and work with. The analysis is based on identifying correlations among the variables, variation patterns, and subsequent extraction of the elements greatly influencing the variation of the values. We used the Kaiser criterion, retaining only the components with eigenvalue greater than 1. After applying PCA, we identified only two components with eigenvalue greater than 1. We then observed two patterns of variation in the matrix of components; the first characterized VCL, VAP, VSL, BCF, and ALH, while the second was specific to STR, LIN, and WOB. Accordingly, we selected the two variables with the highest values in the matrix of components for further analysis, namely VAP (0.973) and LIN (0.967). The next step was the standardization of values for VAP and LIN such that the mean values would be 0 and the standard deviations 1. The purpose of value standardization is to avoid erroneous calculation of the subgroup centers caused by different value scales of the two parameters. Further, the assignment of sperm into subgroups was performed using the k-means cluster analysis based on Euclidean distances, as described in previous studies [6, 7, 12]. Each spermatozoon was assigned to a cluster (subgroup, subpopulation) such that its movement pattern was similar or close to the other sperm belonging to the same cluster, but significantly different to the movement pattern of the sperm belonging to other clusters. After applying k-means cluster analysis, the cluster membership of each spermatozoon was saved and used to subsequently calculate the proportion of each identified subpopulation. To ascertain if the percentages of motile sperm subpopulations were dependent on a specific season from a statistical standpoint, the χ2 test was applied. Also, to analyze the variance of the share of a certain subpopulation among seasons, the ANOVA test was used. The differences in frequencies between two groups (for example fast sperm vs. slow sperm) were evaluated through the Paired Sample t-test.
Results
Overall kinematic parameters of semen as determined by CASA profiling
All selected kinematic parameters were affected by the season of semen collection, although similarities between some seasons in terms of certain parameters could be observed (Table 2).
Table 2. Overall mean values of selected kinematic parameters of boar semen recorded during the four seasons.
| Parameter | Spring f | Summer | Autumn | Winter |
| TMot (%) | 80.1 ± 10.5 a | 73.4 ± 13.3 b | 73.7 ± 14.4 b | 80.1 ± 10.4 a |
| PMot (%) | 74.1 ± 13.2 a | 64.9 ± 16.5 b | 64.6 ± 17.8 b | 75.2 ± 12.3 a |
| VAP (µm/sec) | 66.4 ± 12.2 a | 59.0 ± 12.7 b | 60.1 ± 14.2 b | 73.5 ± 9.8 c |
| VCL (µm/sec) | 126.8 ± 28.7 a | 112.9 ± 29.1 b | 119.6 ± 32.4 c | 143.4 ± 25.6 d |
| VSL (µm/sec) | 52.7 ± 9.4 a | 47.1 ± 9.9 b | 46.5 ± 10.8 b | 57.2 ± 8.53 c |
| ALH (µm) | 2.98 ± 0.63 a | 2.86 ± 0.65 b | 3.05 ± 0.72 a | 3.33 ± 0.61 c |
| BCF (Hz) | 36.6 ± 3.2 a | 34.5 ± 3.5 b | 33.8 ± 4.5 c | 37.3 ± 2.94 d |
| STR (VSL/VAP) | 0.79 ± 0.08 a | 0.80 ± 0.07 a | 0.77 ± 0.08 b | 0.78 ± 0.08 b |
| WOB (VAP/VCL) | 0.53 ± 0.06 a | 0.53 ± 0.06 a | 0.51 ± 0.06 c | 0.52 ± 0.06 d |
| LIN (VSL/VCL) | 0.42 ± 0.08 a | 0.43 ± 0.08 a | 0.40 ± 0.08 b | 0.41 ± 0.08 c |
Values are reported as means ± SD of 4837 ejaculates from 702 boars. a, b, c, d Within the same row, different superscripts show significant difference at P < 0.05. e Spring = March–May; Summer = June–August; Autumn = September–November; Winter = December–February. TMot = total sperm motility; PMot = progressive sperm motility; VCL = curvilinear velocity; VAP = average path velocity; VSL = straight-line velocity; ALH = amplitude of lateral head displacement; BCF = beat cross frequency; STR = straightness of track; WOB = wobble; LIN = linearity of track.
TMot registered the highest mean value (± SD) during winter (80.1% ± 10.4) and lowest during summer (73.4% ± 13.3). The mean value registered in spring (80.1% ± 10.5) was similar to that obtained in winter (NS), whereas autumn values (73.7% ± 14.4) were not significantly different from those of the summer (NS). The same was observed for PMot, with higher values during winter and spring (75.2% ± 12.3 and 74.1% ± 13.2 respectively), and lower values in autumn and summer (64.6% ± 17.8 and 64.9% ± 16.5, respectively).
Significant variability of sperm velocity was observed among seasons. VCL was the parameter most responsive to the annual changes, showing significant oscillations from one season to another (P < 0.05). The highest values for sperm velocity, reflected by VAP, VCL, and VSL parameters were observed during winter (P < 0.05). No differences between summer and autumn were detected with regards to VAP (NS).
Although characterized by a lower overall velocity the sperm showed a less corrugated trajectory during summer, as described by the parameters STR, LIN, and WOB. Similar values for STR, LIN, and WOB were recorded during summer and spring (NS), and these values were both lower during the autumn and winter months (P < 0.05).
Sperm subpopulations
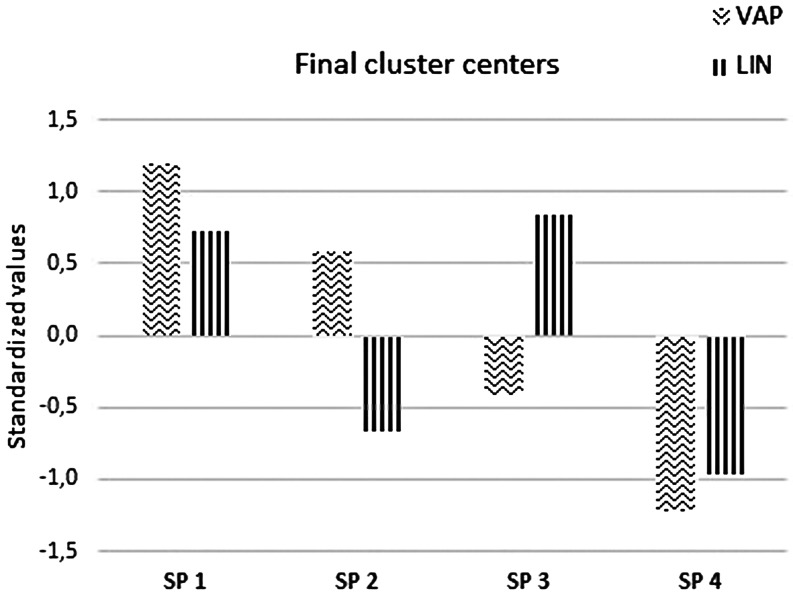
The cluster analysis revealed a clear heterogeneity of sperm populations, with the coexistence of four distinct subgroups of motile sperm characterized by specific values of velocity and linearity. The mean values of kinematic parameters for the four subpopulations are presented in Table 3. Briefly, their main characteristics were as follows (Fig. 1):
Table 3. Kinematic parameters of the four sperm subpopulations (SP1 to SP4).
| Parameter | SP 1 | SP 2 | SP 3 | SP 4 |
| VAP (µm/sec) | 100.1 ± 18.2 a | 81.3 ± 18.9 b | 50.5 ± 16.5 c | 25.4 ± 14.5 d |
| VCL (µm/sec) | 167.1 ± 43.4 a | 183.6 ± 51.4 b | 81.8 ± 30.7 c | 68.5 ± 40.7 d |
| VSL (µm/sec) | 89.0 ± 17.0 a | 51.9 ± 16.3 b | 45.1 ± 15.7 c | 15.9 ± 10.6 d |
| ALH (µm) | 3.57 ± 1.30 a | 4.17 ± 1.48 b | 2.21 ± 1.13 c | 2.38 ± 1.54 d |
| BCF (Hz) | 37.5 ± 9.1 a | 37.1 ± 8.1 b | 36.5 ± 12.6 c | 22.27 ± 13.3 d |
| STR (VSL/VAP) | 0.89 ± 0.08 a | 0.66 ± 0.19 b | 0.89 ± 0.07 c | 0.63 ± 0.21 d |
| WOB (VAP/VCL) | 0.62 ± 0.10 a | 0.45 ± 0.07 b | 0.64 ± 0.12 c | 0.39 ± 0.13 d |
| LIN (VSL/VCL) | 0.55 ± 0.11 a | 0.30 ± 0.10 b | 0.57 ± 0.11 c | 0.24 ± 0.11 d |
Values are means ± SD of 4837 ejaculates from 702 boars. a, b, c, d Within the same row, different superscripts show significant difference at P < 0.05. VCL = curvilinear velocity; VAP = average path velocity; VSL = straight-line velocity; ALH = amplitude of lateral head displacement; BCF = beat cross frequency; STR = straightness of track; WOB = wobble; LIN = linearity of track.
Fig. 1.
Characteristics of four motile sperm subpopulations identified in boar semen in terms of average path velocity (VAP) and linearity (LIN). Values are standardized such that the mean values would be 0 and the standard deviations 1.
-
-
Subpopulation 1 (SP1) represented sperm with a high VAP and a high LIN (Fig. 2A). About 15% of the sperm was assigned to this subgroup;
-
-
Subpopulation 2 (SP2) included sperm with a high VAP but a low LIN (Fig. 2B). This subgroup contained 28% of the sperm;
-
-
Subpopulation 3 (SP3) was defined as sperm showing low velocity but high linearity (Fig. 2C). The share of this subgroup was about 35%.
-
-
Subpopulation 4 (SP4) consisted of sperm with a low VAP and a low LIN (Fig. 2D). Approximately 22% of the sperm were part of this cluster.
Fig. 2.
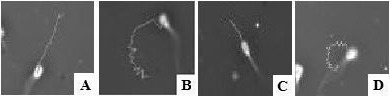
Characteristic motility tracks of boar sperm of the four motile subpopulations (A: subpopulation 1; B: subpopulation 2; C: subpopulation 3; D: subpopulation 4).
Besides the previously mentioned differences in sperm velocity and linearity, there were also differences regarding ALH and BCF (Table 3). The SP2 and SP1 showed higher values for ALH, along with higher values of VAP and BCF. The SP3 contained sperm with the lowest ALH, while the SP4 was not only defined by a low VAP, but also by a low BCF.
The season in which semen was collected influenced the distribution of SPs, as revealed by the χ2 test (P < 0.05) (Fig. 3). Spring was characterized by similar proportions (NS) of SP1 (16.9%) and SP4 (17.9%) and higher proportions of SP3 (37.1%).
Fig. 3.
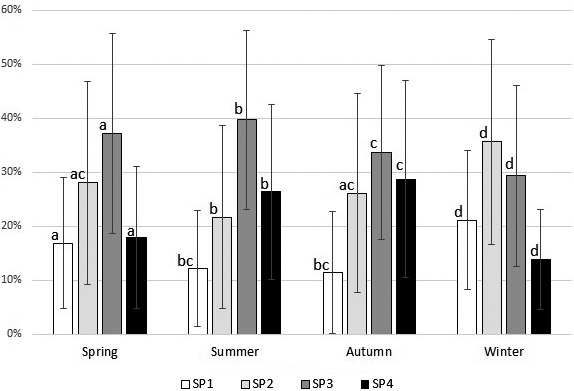
Distribution of sperm subpopulations in boar semen during the four seasons. Values are means ± SD of 4837 ejaculates of 702 boars. Different superscripts indicate significant differences among seasons within the same subpopulation. Spring = March–May; Summer = June–August; Autumn = September–November; Winter = December–February.
During the summer months the proportion of SP1 (12.2%) and SP2 (21.7%) decreased compared to spring, while SP3 (39.7%) and SP4 (26.4%) showed the opposite changes during this season (P < 0.05). Summer had a negative effect on the velocity of sperm and their linearity, as the proportion of both subgroups of rapid sperm (SP1 + SP2) and the subgroup of linear sperm (SP1 + SP3) decreased (P < 0.05). It must be pointed out that the changes in sperm linearity were not detected by the overall analysis of mean values of the LIN parameter (NS) (Table 2). Most sperm during summer were from SP3 and the second most abundant was the SP4 group.
The autumn season also brought some changes in the distribution of motile sperm within subpopulations compared to summer (P < 0.05). SP3 (33.7%) remained the most numerous, but significant increases in SP2 and SP4 were also recorded (P < 0.05). SP1 (sperm with high velocity and high linearity) showed similar values to those of summer (NS). Both in summer and autumn, the SP1 group recorded a much lower share when compared to the other three subpopulations (Fig. 3). Although the overall analysis based on mean values detected no changes (NS) in sperm VAP (Table 2), significantly more sperm were considered to be fast in autumn compared to summer (SP1 + SP2 = 37.6% in autumn versus 33.9% in summer) (P < 0.05).
The cold season significantly changed the proportions of all sperm subpopulations. The SP1 (fast and linear) group increased substantially (21.1%), reaching the highest proportion among the different seasons (P < 0.05). At the same time, the SP4 (slow, non-linear) group decreased considerably (P < 0.05), and was the subgroup with the lowest number of sperm (13.9%). A remarkable rise was also shown by SP2 (35.6%), which became the most frequent subgroup during winter (P < 0.05). The increases in overall sperm velocity (Table 2) during winter were reflected by increases of both subgroups with high velocity (SP1 and SP2). In contrast, the increases in overall linearity (Table 2) were associated only with significant increases in the subgroup with high linearity and high velocity (SP1), while the subgroup with high linearity and low velocity (SP3) showed a lower share compared to autumn (Fig. 3).
Discussion
In our study, we observed the coexistence of four subpopulations of sperm with different movement patterns. The presence of four distinct subpopulations in semen has also reported by other authors, both in boar [3, 24] and in other species [8, 25,26,27]. The subpopulation containing sperm with higher velocity and higher linearity was less numerous than the other three subpopulations; this is consistent with previous results [3] and suggests a relatively poor representation of this kind of sperm within boar semen. In comparison, ram semen seems to be mostly constituted of rapid, linear sperm [28].
Seasonal variations of seminal parameters in boar have been studied for years and it is now widely accepted that summer causes a decrease in semen quality and quantity. Previous studies observed the effects of the hot season on a variety of parameters, such as semen volume [29], total number of sperm [30], sperm motility [31], sperm viability [32], sperm morphology [33], sperm agglutination [34], cryoresistance [35], protein content [36], and acrosin activity [37]. All these fluctuations gave rise to discussions of “seasonal infertility”, described in boars, but also in sows, which is defined mostly as reduced fertility occurring during the summer months and in early autumn [38, 39].
Our investigation revealed that the collection season exerts a great influence on the distribution of sperm subpopulations in extended boar semen. The subgroup of fast and linear sperm, which is considered by some authors to be the one with the highest fertilizing potential [12, 28], was best represented during the winter, and was poorly represented during the autumn and summer months. On the other hand, the subgroup of slow and non-linear sperm, which theoretically have lower chances of reaching the oviduct, was more numerous during the autumn and summer and was less numerous during the spring and winter. These data suggest that the previously described decrease in swine fertility during the hot season [39], could also be also related to changes in motile sperm subpopulations.
Seasonal analysis of sperm subpopulations could contribute to a better definition of semen quality throughout the year and could thus have a significant economic impact, as more efficient semen evaluation will lead to an improvement of production of AI doses [40]. Furthermore, seasonal analysis may reveal aspects that may be ignored by usual evaluation methods. For example, in our study the overall percentage of motile sperm was similar in winter and spring. However, when we analyzed individual motile sperm subpopulations we observed a clear difference in the structure of ejaculates, with a significantly higher percentage of rapid sperm present during the winter months. Given the fact that the percentage of motile sperm is still the main criterion for assessing semen quality in production centers [41, 42], one may mistakenly think after a quick look, that the ejaculates collected during spring were similar to those collected during winter, when in fact they were not. Moreover, while the overall analysis based on mean values did not detect significant differences in sperm VAP between summer and autumn, cluster analysis revealed that during the autumn months significantly more sperm could be classified as fast. This means that the succession of seasons does indeed have an effect on the velocity of sperm, but probably not for all subpopulations and not to the same extent.
The fact that seasons modify the distribution of sperm within subpopulations could influence the perception we have on seasonal variations of kinematic parameters in boar semen. Thus, seasonal dynamics in kinematic parameters might be caused by changes in the proportion of sperm assigned to the different subpopulations and not by an overall increase/decrease in values for all the ejaculated sperm. For example, boar sperm velocity seems to be lower during summer [31]. Our study revealed that the subpopulations with fast sperm are less numerous during summer (Fig. 3), which may suggest that the overall decrease of VAP is not necessarily caused by a decrease in velocity of all sperm, but rather by changes in the distribution of sperm within subpopulations, with some sperm “passing” from fast subpopulations to slow subpopulations. This would mean that not all the sperm analyzed suffered during the hot season, but only a certain percentage of sperm whose decreased velocity led to a decline in overall velocity. Furthermore, this might suggest that boar testicles produce “resistant” sperm, which will retain their characteristics even in less favorable environmental conditions and “sensitive” sperm, which will be easily affected by different factors, such as temperature. This hypothesis is also supported by the fact that in our study, the subpopulation containing sperm with high velocity and high linearity remained stable over the summer and autumn, while all the other subpopulations suffered significant changes (Fig. 3).
The main cause for the decrease in the number of fast and linear sperm during the summer months was probably heat stress, as it is well known that the high temperatures specific to the hot season affect spermatogenesis [43,44,45]. There have been a few theories proposed that have attempted to explain the overall seasonal variation in boar semen. Based on previous studies, the main factors implicated in this variation include temperature [22], photoperiod [30], and humidity [36]; some authors have indicated the existence of an ancestral mechanism inherited from the wild boar [37, 46]. A useful step in determining the cause of seasonal variations would be that of finding the basis of the impairment. Sperm gain their mobility during the maturation phase in the epididymis, but it is currently difficult to determine whether the reduction in the number of fast and linear sperm reflects impairment of the epididymal function or that of specific testicular segments involved in spermatogenesis. For example, membrane integrity is strongly correlated with the functional status of sperm mitochondria [47], so disorders in the plasma membrane might result in dysfunctions of the mitochondrial sheath, leading to decreased velocity.
The seasonality of reproduction in the swine is once again confirmed by this study. In addition to all the previous studies indicating not only variability in fertility of sows, but also in a large number of seminal parameters in the boar, our study describes the seasonal changes occurring in the distribution of motile sperm in distinct subpopulations based on their movement patterns (Fig. 3). What still remains unclear, however, is the underlying algorithm determining the changes in the proportion of sperm subpopulations. Apparently, some fast and linear sperm pass to another subgroup under stressful conditions. We cannot specify yet in which direction are they migrating. Are they losing velocity by going to the subgroup with slow and linear sperm? Or on the contrary, are they sacrificing the uniformity of their movement to move towards a fast and non-linear subgroup? We cannot exclude that the algorithm of migration differs according to the factors that cause it. For example, in our study the proportion of fast and linear sperm, and also of fast and non-linear sperm, decreased from spring to summer (Fig. 3). At the same time, the proportion of slow and linear, but also of slow and non-linear sperm, increased. This suggests a loss in sperm velocity while maintaining sperm linearity. On the other hand, in the study by Ramió et al. [3], adding progesterone in the in vitro capacitating medium caused an increase in the proportion of rapid, but non-linear sperm indicating that some sperm lose their linearity by passing into the non-linear subgroup and thus follow a different migration algorithm than that observed in our study.
Examiners might consider introducing clustering analysis in their studies on semen. Although the new trend among researchers is to use flow cytometry analysis, CASA systems are still widely used. We believe it would be helpful if the use of CASA for research purposes were extended to the determination of motile sperm subpopulations. This might complicate studies somewhat, but at the same time it would make them more comprehensive. Conceivably the behavior of sperm based on specific parameters should not be generalized to the entire sample, and we should rather study the effects on each subpopulation, identifying which is the most affected. Clustering analysis is no longer a novelty for theriogenologists, and its inclusion as an additional feature of semen examination would only represent a logical step.
In conclusion, extended boar semen seems to be structured in distinct motile sperm subpopulations, defined by specific movement patterns. Sperm could be classified as fast and linear, fast and non-linear, slow and linear, and respectively, slow and non-linear. The proportion of each class varies greatly according to the season, and the hot season seems to have a negative impact on the percentage of fast and linear sperm. These findings might change the way we perceive seasonal variations in overall values of the kinematic parameters in boar semen. Thee variations might be caused by changes in the number assigned to each subpopulation and not by increases or decreases in the values of all ejaculated sperm. We recommend introducing clustering analysis in studies on semen where possible. Sperm subpopulations respond differently to environmental conditions, and the values of kinematic parameters should not be generalized to the entire sample as a whole.
Conflict of interest
There are no conflicts of interest associated with this publication.
Acknowledgments
We would like to thank the Besamungsverein Neustadt/Aisch for their support during the study. Mr Iulian Ibănescu received a scholarship to perform an internship in Neustadt/Aisch under the frame of the European Social Fund, project no. POSDRU/159/1.5/S/132765, through USAMV Iasi.
References
- 1.Martínez-Pastor F, Tizado EJ, Garde JJ, Anel L, de Paz P. Statistical Series: Opportunities and challenges of sperm motility subpopulation analysis. Theriogenology 2011; 75: 783–795. [DOI] [PubMed] [Google Scholar]
- 2.Holt WV. Can we predict fertility rates? Making sense of sperm motility. Reprod Domest Anim 1995; 31: 17–24. [Google Scholar]
- 3.Ramió L, Rivera MM, Ramírez A, Concha II, Peña A, Rigau T, Rodríguez-Gil JE. Dynamics of motile-sperm subpopulation structure in boar ejaculates subjected to in vitro capacitation and further in vitro acrosome reaction. Theriogenology 2008; 69: 501–512. [DOI] [PubMed] [Google Scholar]
- 4.Henning H, Petrunkina AM, Harrison RA, Waberski D. Cluster analysis reveals a binary effect of storage on boar sperm motility function. Reprod Fertil Dev 2014; 26: 623–632. [DOI] [PubMed] [Google Scholar]
- 5.Quintero-Moreno A, Rigau T, Rodríguez-Gil JE. Regression analyses and motile sperm subpopulation structure study as improving tools in boar semen quality analysis. Theriogenology 2004; 61: 673–690. [DOI] [PubMed] [Google Scholar]
- 6.Ferraz MA, Morató R, Yeste M, Arcarons N, Pena AI, Tamargo C, Hidalgo CO, Muiño R, Mogas T. Evaluation of sperm subpopulation structure in relation to in vitro sperm-oocyte interaction of frozen-thawed semen from Holstein bulls. Theriogenology 2014; 81: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 7.Muiño R, Rivera MM, Rigau T, Rodriguez-Gil JE, Peña AI. Effect of different thawing rates on post-thaw sperm viability, kinematic parameters and motile sperm subpopulations structure of bull semen. Anim Reprod Sci 2008; 109: 50–64. [DOI] [PubMed] [Google Scholar]
- 8.Quintero-Moreno A, Miró J, Teresa Rigau A, Rodríguez-Gil JE. Identification of sperm subpopulations with specific motility characteristics in stallion ejaculates. Theriogenology 2003; 59: 1973–1990. [DOI] [PubMed] [Google Scholar]
- 9.Dorado J, Acha D, Ortiz I, Gálvez MJ, Carrasco JJ, Díaz B, Gómez-Arrones V, Calero-Carretero R, Hidalgo M. Relationship between conventional semen characteristics, sperm motility patterns and fertility of Andalusian donkeys (Equus asinus). Anim Reprod Sci 2013; 143: 64–71. [DOI] [PubMed] [Google Scholar]
- 10.Dorado J, Molina I, Muñoz-Serrano A, Hidalgo M. Identification of sperm subpopulations with defined motility characteristics in ejaculates from Florida goats. Theriogenology 2010; 74: 795–804. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Pastor F, Garcia-Macias V, Alvarez M, Herraez P, Anel L, de Paz P. Sperm subpopulations in Iberian red deer epididymal sperm and their changes through the cryopreservation process. Biol Reprod 2005; 72: 316–327. [DOI] [PubMed] [Google Scholar]
- 12.Peña AI, Barrio M, Becerra JJ, Quintela LA, Herradón PG. Motile sperm subpopulations in frozen-thawed dog semen: changes after incubation in capacitating conditions and relationship with sperm survival after osmotic stress. Anim Reprod Sci 2012; 133: 214–223. [DOI] [PubMed] [Google Scholar]
- 13.Gallego V, Cavalcante SS, Fujimoto RY, Carneiro PC, Azevedo HC, Maria AN. Fish sperm subpopulations: Changes after cryopreservation process and relationship with fertilization success in tambaqui (Colossoma macropomum). Theriogenology 2017; 87: 16–24. [DOI] [PubMed] [Google Scholar]
- 14.de Paz P, Mata-Campuzano M, Tizado EJ, Alvarez M, Alvarez-Rodríguez M, Herraez P, Anel L. The relationship between ram sperm head morphometry and fertility depends on the procedures of acquisition and analysis used. Theriogenology 2011; 76: 1313–1325. [DOI] [PubMed] [Google Scholar]
- 15.Amann RP, Waberski D. Computer-assisted sperm analysis (CASA): capabilities and potential developments. Theriogenology 2014; 81: 5–17.e1: 3. [DOI] [PubMed] [Google Scholar]
- 16.Martínez IN, Morán JM, Peña FJ. Two-step cluster procedure after principal component analysis identifies sperm subpopulations in canine ejaculates and its relation to cryoresistance. J Androl 2006; 27: 596–603. [DOI] [PubMed] [Google Scholar]
- 17.Valverde A, Arenán H, Sancho M, Contell J, Yániz J, Fernández A, Soler C. Morphometry and subpopulation structure of Holstein bull spermatozoa: variations in ejaculates and cryopreservation straws. Asian J Androl 2016; 18: 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez-Gil JE. Biological aspects of the mature boar spermatozoon. In: Bonet S, Casas I, Holt WV, Yeste M (eds.), Boar Reproduction - Fundamentals and New Biotechnological Trends. Springer-Verlag Berlin Heidelberg; 2013: 49−64.
- 19.Yániz JL, Palacín I, Vicente-Fiel S, Sánchez-Nadal JA, Santolaria P. Sperm population structure in high and low field fertility rams. Anim Reprod Sci 2015; 156: 128–134. [DOI] [PubMed] [Google Scholar]
- 20.Flores E, Fernández-Novell JM, Peña A, Rodríguez-Gil JE. The degree of resistance to freezing-thawing is related to specific changes in the structures of motile sperm subpopulations and mitochondrial activity in boar spermatozoa. Theriogenology 2009; 72: 784–797. [DOI] [PubMed] [Google Scholar]
- 21.Chemineau P, Guillaume D, Migaud M, Thiéry JC, Pellicer-Rubio MT, Malpaux B. Seasonality of reproduction in mammals: intimate regulatory mechanisms and practical implications. Reprod Domest Anim 2008; 43(Suppl 2): 40–47. [DOI] [PubMed] [Google Scholar]
- 22.Flowers WL. Genetic and phenotypic variation in reproductive traits of AI boars. Theriogenology 2008; 70: 1297–1303. [DOI] [PubMed] [Google Scholar]
- 23.Valeanu S, Johannisson A, Lundeheim N, Morrell JM. Seasonal variation in sperm quality parameters in Swedish red dairy bulls used for artificial insemination. Livest Sci 2015; 173: 111–118. [Google Scholar]
- 24.Rivera MM, Quintero-Moreno A, Barrera X, Palomo MJ, Rigau T, Rodríguez-Gil JE. Natural Mediterranean photoperiod does not affect the main parameters of boar-semen quality analysis. Theriogenology 2005; 64: 934–946. [DOI] [PubMed] [Google Scholar]
- 25.Miró J, Lobo V, Quintero-Moreno A, Medrano A, Peña A, Rigau T. Sperm motility patterns and metabolism in Catalonian donkey semen. Theriogenology 2005; 63: 1706–1716. [DOI] [PubMed] [Google Scholar]
- 26.Dorado J, Alcaráz L, Duarte N, Portero JM, Acha D, Hidalgo M. Changes in the structures of motile sperm subpopulations in dog spermatozoa after both cryopreservation and centrifugation on PureSperm(®) gradient. Anim Reprod Sci 2011; 125: 211–218. [DOI] [PubMed] [Google Scholar]
- 27.Maya-Soriano MJ, Taberner E, Sabés-Alsina M, Ramon J, Rafel O, Tusell L, Piles M, López-Béjar M. Daily exposure to summer temperatures affects the motile subpopulation structure of epididymal sperm cells but not male fertility in an in vivo rabbit model. Theriogenology 2015; 84: 384–389. [DOI] [PubMed] [Google Scholar]
- 28.Bravo JA, Montanero J, Calero R, Roy TJ. Identification of sperm subpopulations with defined motility characteristics in ejaculates from Ile de France rams. Anim Reprod Sci 2011; 129: 22–29. [DOI] [PubMed] [Google Scholar]
- 29.Smital J.Comparison of environmental variations in boar semen characteristics of six breeds and their crossbreds over an eight-year period. Reseach in Pig Breeding 2010; 4: 26–32. [Google Scholar]
- 30.Pinart E, Puigmulé M. Factors affecting boar reproduction, testis function, and sperm quality. In: Bonet S, Casas I, Holt WV, Yeste M (eds.), Boar Reproduction - Fundamentals and New Biotechnological Trends. Springer-Verlag Berlin Heidelberg; 2013: 109−202.
- 31.Yeste M, Sancho S, Briz M, Pinart E, Bussalleu E, Bonet S. A diet supplemented with L-carnitine improves the sperm quality of Piétrain but not of Duroc and Large White boars when photoperiod and temperature increase. Theriogenology 2010; 73: 577–586. [DOI] [PubMed] [Google Scholar]
- 32.Barranco I, Ortega MD, Martinez-Alborcia MJ, Vazquez JM, Martinez EA, Roca J. Season of ejaculate collection influences the freezability of boar spermatozoa. Cryobiology 2013; 67: 299–304. [DOI] [PubMed] [Google Scholar]
- 33.Janett F, Fuschini E, Keo S, Hässig M, Thun R. Seasonal changes of semen quality in the boar. Reprod Domest Anim 2005; 40: 356 (abstract). [Google Scholar]
- 34.Bollwein H, Petschow K, Weber F, Leiding C, Stolla R. The incidence of agglutination and its influence on sperm quality and fertility of boar semen(in German).Berl Munch Tierarztl Wochenschr 2004; 117: 327–333. [PubMed] [Google Scholar]
- 35.Cheon YM, Kim HK, Yang CB, Yi YJ, Park CS. Effect of season influencing semen characteristics, frozen-thawed sperm viability and testosterone concentration in Duroc boars, Asian-Aust. J Anim Sci 2002; 15: 500–503. [Google Scholar]
- 36.Murase T, Imaeda N, Yamada H, Miyazawa K. Seasonal changes in semen characteristics, composition of seminal plasma and frequency of acrosome reaction induced by calcium and calcium ionophore A23187 in Large White boars. J Reprod Dev 2007; 53: 853–865. [DOI] [PubMed] [Google Scholar]
- 37.Ciereszko A, Ottobre JS, Glogowski J. Effects of season and breed on sperm acrosin activity and semen quality of boars. Anim Reprod Sci 2000; 64: 89–96. [DOI] [PubMed] [Google Scholar]
- 38.Peltoniemi OA, Tast A, Love RJ. Factors effecting reproduction in the pig: seasonal effects and restricted feeding of the pregnant gilt and sow. Anim Reprod Sci 2000; 60−61: 173–184. [DOI] [PubMed] [Google Scholar]
- 39.Kraeling RR, Webel SK. Current strategies for reproductive management of gilts and sows in North America. J Anim Sci Biotechnol 2015; 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martí JI, Aparicio IM, Leal CL, García-Herreros M. Seasonal dynamics of sperm morphometric subpopulations and its association with sperm quality parameters in ram ejaculates. Theriogenology 2012; 78: 528–541. [DOI] [PubMed] [Google Scholar]
- 41.Ibănescu I, Leiding C, Ciornei ŞG, Roșca P, Sfartz I, Drugociu D. Differences in CASA output according to the chamber type when analyzing frozen-thawed bull sperm. Anim Reprod Sci 2016; 166: 72–79. [DOI] [PubMed] [Google Scholar]
- 42.Ciornei SG, Rosca P, Drugociu D, Mare M, Nechifor F, Ibanescu I. Reproduction biotechnologies in Mangalita breed boars. Res J Biotech 2013; 8: 51–56. [Google Scholar]
- 43.Sonderman JP, Luebbe JJ. Semen production and fertility issues related to differences in genetic lines of boars. Theriogenology 2008; 70: 1380–1383. [DOI] [PubMed] [Google Scholar]
- 44.Orgal S, Zeron Y, Elior N, Biran D, Friedman E, Druker S, Roth Z. Season-induced changes in bovine sperm motility following a freeze-thaw procedure. J Reprod Dev 2012; 58: 212–218. [DOI] [PubMed] [Google Scholar]
- 45.Malama E, Zeron Y, Janett F, Siuda M, Roth Z, Bollwein H. Use of computer-assisted sperm analysis and flow cytometry to detect seasonal variations of bovine semen quality. Theriogenology 2017; 87: 79–90. [DOI] [PubMed] [Google Scholar]
- 46.Tast A, Hälli O, Ahlström S, Andersson H, Love RJ, Peltoniemi OA. Seasonal alterations in circadian melatonin rhythms of the European wild boar and domestic gilt. J Pineal Res 2001; 30: 43–49. [DOI] [PubMed] [Google Scholar]
- 47.Bollwein H, Fuchs I, Koess C. Interrelationship between plasma membrane integrity, mitochondrial membrane potential and DNA fragmentation in cryopreserved bovine spermatozoa. Reprod Domest Anim 2008; 43: 189–195. [DOI] [PubMed] [Google Scholar]