Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired hematopoietic stem cell disorder which manifests with hemolysis (classical PNH) due to loss of expression of the CD55 and CD59 proteins, which leads to complement mediated cell lysis.1 Despite being the only curative treatment for PNH,2 allogeneic hematopoietic stem cell transplantation (HSCT) is not recommended as first-line therapy for classical PNH (C-PNH) or for patient with past thrombosis considering the safety and efficacy of Eculizumab.3,4 The European society for Blood and Marrow Transplantation (EBMT) previously reported 30% overall mortality in PNH patients transplanted between 1978 and 2007, with an unacceptable higher risk of mortality in patients with pre-transplant thrombosis history.5 Nowadays, HSCT might thus be considered, in absence of alternative treatment, for 5 to 10% of patients with C-PNH who evolve to myelodysplastic syndromes (MDS) or acute myeloid leukemia4,6 and for 2 to 4% patients with recurrent thrombosis under eculizumab.4,7 HSCT might also be questioned in 1% who may evolve to AA-PNH4 or for 34 to 51% of C-PNH patients who are still transfused under eculizumab.7–11 To date, data on the outcome of HSCT for patients who were previously treated with Eculizumab as well as best management of anti-C5 therapy in the context of HSCT are scarce.12 We report herein the outcome of 21 patients, previously treated with Eculizumab, who underwent HSCT between 2007 and 2017. We show that regardless of the indications for HSCT in PNH patients previously treated with Eculizumab, HSCT is still associated with almost 30% of mortality mainly due to infections and acute graft-versus-host disease (GvHD).
Patients were identified and data were collected through the French PNH registry4 and the registry from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire. An additional questionnaire, which focused on the management of Eculizumab at time of transplantation, was sent to investigators (Online Supplementary Data). Infectious and GvHD complications were graded according to the commonly used scales.13,14 Anti-fungal prophylaxis was conducted according to local policy. Data were described through proportions or median with inter-quartile range (IQR: 25%–75%). Statistical analyses were conducted using SPSS. Fine and Gray’s model of competing-risks regression adjusted according to death, relapse and non-engraftment incidence of acute GvHD (aGvHD) was performed using R Software.15
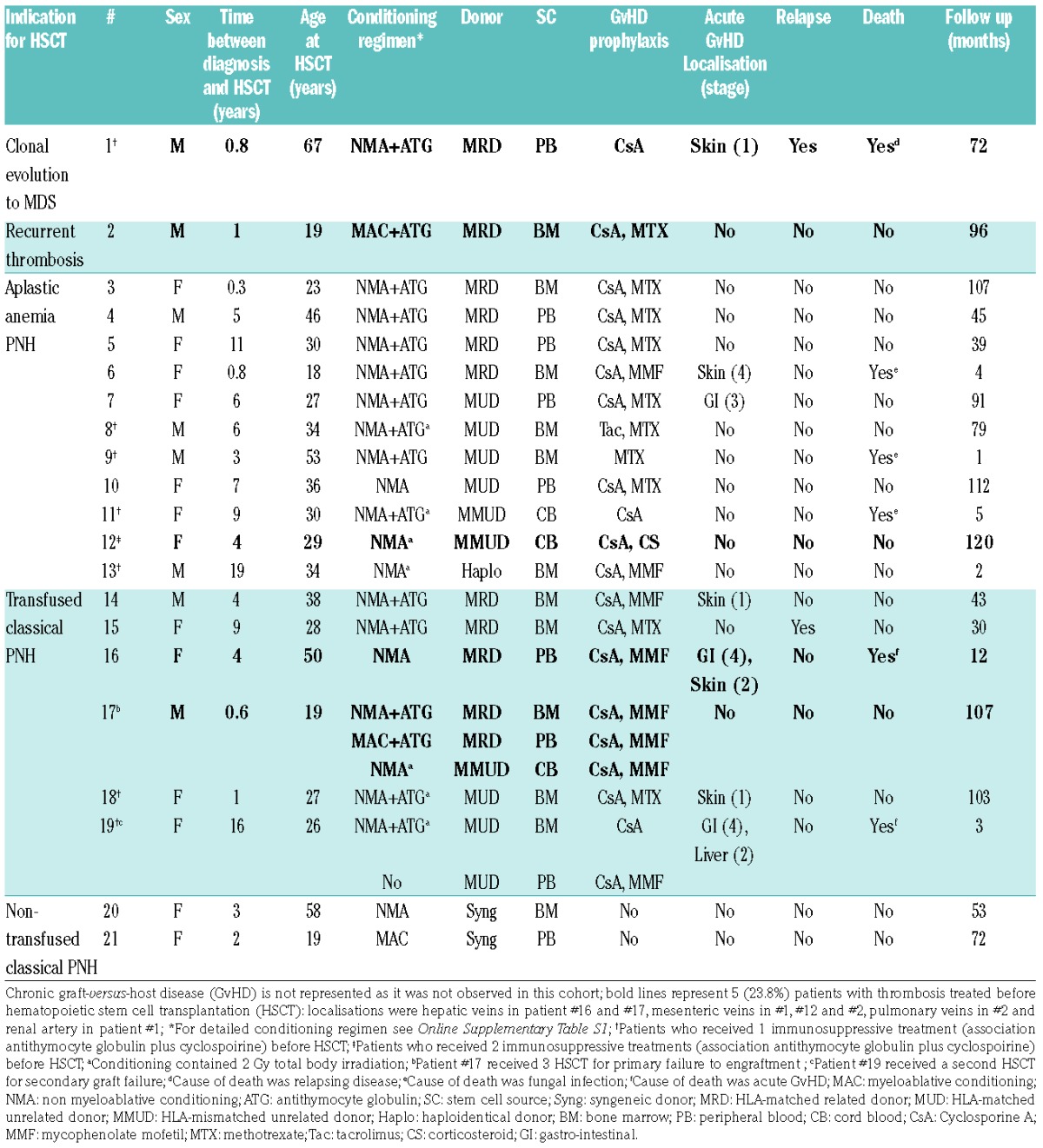
Patients’ and transplantations’ characteristics are detailed in Table 1 and Online Supplementary Table S1. Indication for HSCT were clonal evolution to a MDS in 1 (4.7%) patient, recurrent thrombosis under Eculizumab in 1 (4.7%) patient, AA-PNH in 11 (52.4%), transfused C-PNH in 6 (28.6%), and non-transfused C-PNH with a syngeneic donor available in 2 (9.5%) patients. Before HSCT, Eculizumab was infused at the dose of 600 mg every week for 4 weeks followed by infusions of 900 mg every 14 days for every patient. Median duration of Eculizumab was 8 months (IQR: 5–21) and no dose modifications was reported. Among transfused C-PNH, median duration of Eculizumab was only of 6.5 months (IQR: 5–17.75). ABO group incompatibility (4 minor, 3 major, 2 major and minor) and sex-mismatched (female to male in 4 cases) were observed in 9 (42.9%) and 13 (61.9%) patients, respectively.
Table 1.
Characteristics of patients and transplantations.
Among the 21 patients, 20 (95.2%) patients engrafted after HSCT. Median time to engraftment was 20.5 days (IQR: 13.5–23.75). Chimerism was assessed in 17 patients (2 syngeneic transplantations and #16 were not assessed while patient #9 died before evaluation). Full donor and mixed chimerism were observed in 10 (58.8%) and 6 (35.3%) patients, respectively. Patient #17 failed to engraft two times and eventually switched to full donor chimerism at day+27 after a third HSCT. Despite engraftment at day+12, patient #19 received a second transplant for graft failure. Overall, Eculizumab did not seem to interfere with engraftment when compared to 93% engraftment rate of the EBMT cohort.5
During follow up, 7 patients developed aGvHD. Among the 19 patients at risk (2 syngeneic transplantations were excluded), cumulative incidence function (CIF) of aGvHD was 38.1% [±standard error (se) 11.9%]. The median time to onset was 50 days (IQR: 19.50–91.75). Grade III–IV was reached by 4 patients. The overall rate of aGvHD was comparable to the CIF of 40% observed within the EBMT cohort.5 Transfused C-PNH and AA-PNH CIF of aGvHD were 66.6% (±se 24.2%) and 19.3% (±se 13.1%), respectively (P=0.04). In our cohort, transfused C-PNH were exposed to an increased risk for aGvHD. Among the 16 patients at risk, chronic GvHD (cGvHD) was not observed (patients #9 and #19 died before 100 days, patient #13 did not reach day 100 at last follow up and the 2 syngeneic transplantations were excluded for this analysis). Contrariwise, EBMT reported cGvHD CIF of 29% at 5 years. Among those 16 patients, few had risk factors of cGvHD: 6 (37.5%) presented aGvHD, 12 (75%) received ATG in the conditioning regimen, and only 2 (12.5%) patients received HSCT from a HLA-mismatched unrelated donor. It is difficult to draw robust conclusions on the role of Eculizumab in this small series.
Infectious complications were the main problem in this population. Fatal fungal infections were observed in 3 (14.3%) patients (#6, # 9, #11). Maximum grade for bacterial infections was 4 in 2 (9.5%) patients (#15, #3). One grade 4 viral infection was documented in one patient (#8) after day 100 (VZV meningitis). Grade 3 fungal (pulmonary aspergillosis), bacterial and viral infections were documented in 1 (4.8%), 8 (38.1%) and 3 (14.3%) patients, respectively. Anti-fungal prophylaxis was not homogeneous between centers and thus cannot be analyzed carefully in such a small number of patients. We observed a significant association between the use of ATG and grade 3 to 5 infections (P=0.01). Statistical association between ATG and infections should be interpreted with caution considering the size of this population. The high rate of infectious complications was expected as i) infectious complications were the main cause of mortality in the EBMT cohort,5 ii) 11 (52.3%) patients received HSCT for AA-PNH, and iii) the known general susceptibility to infection in PNH.4,11 However, there were no fatal infections in C-PNH under Eculizumab from the French PNH registry,4 which highlights the increased risk of fatal infections for transfused C-PNH who may receive HSCT, and the need to closely monitor those patients after transplantation for fungal infections. Finally, patient #9 experienced thrombosis while PNH clone was negative.
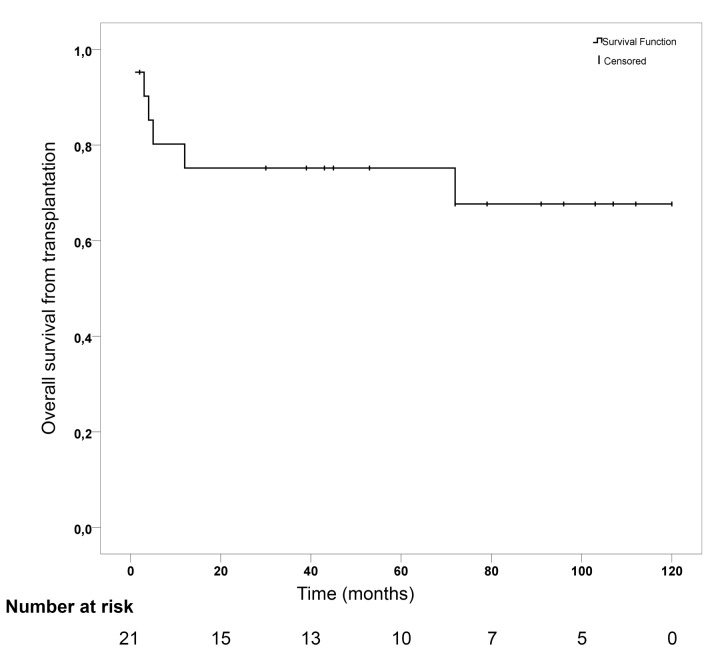
With a median follow-up time of 53 months (IQR: 8.5–99.5), Kaplan-Meier estimated cumulative overall survival (OS) at 6 years was 67.7% (±se 11.2%) (Figure 1). Among the 6 events, causes of death were infections, as mentioned above, in 3 (50.0%) patients, aGvHD in 2 (33.3%) patients, and relapse of MDS in 1 (16.7%) patient. OS at 2 years in AA-PNH and transfused C-PNH were 72.7% (±0.13) and 66.7% (±0.19), respectively. With a median follow-up time of 5 years, the 2 syngeneic HSCT were well tolerated and are alive at the time of this report. Relapse occurred in 2 (9.5%) patients: patient #1 relapsed at 8 months from his MDS and patient #15 relapsed at 2 months with recurrent hemolysis. Thus OS (67.7% versus 68%) and causes of death (50% versus 54.7% of infections and 33.3 versus 28.1% of GvHD) are comparable to the EBMT cohort that focused on non-Eculizumab treated patients transplanted before 2007.5 The 6-years OS of non-transplanted C-PNH patient under Eculizumab was 92% in the French PNH registry.4 Therefore, this may question the role of HSCT in transfused C-PNH under Eculizumab in absence of a syngeneic donor.
Figure 1.
Kaplan-Meier survival curve for overall survival from transplantation.
Regarding Eculizumab management before HSCT, the last drug administration was in the month preceding transplantation in 18 (85.7%) patients. Among these 18 patients, 16 received Eculizumab during the conditioning regimen. Patients #3 and #7 stopped Eculizumab 2 and 4 months, respectively, before HSCT due to evolving AA-PNH, and patient #13 at 54 months because he was lost to follow up. After HSCT, 18 (85.7%) patients discontinued Eculizumab after engraftment without experiencing signs of hemolysis. Eculizumab was given after HSCT for 3 (14.3%) patients. Patient #11, who presented hemolytic crisis at day+27, was successfully treated with one dose of Eculizumab 900mg. Patient #17 received infusions of 900 mg every two weeks for 2.6 months due to engraftment failure (Eculizumab was discontinued after the third transplantation’s engraftment). For patient #15, Eculizumab was reintroduced due to disease relapse, which is still the case today. Thus, best time for last infusion of Eculizumab seems to be during the conditioning regimen. As expected, administration of the drug is mandatory in case of autologous hematopoiesis (relapse, graft failure or non-engraftment).
To conclude, this study, although retrospective, gives insight into the outcome of transplanted PNH patients in the context of complement blocker. Best timing for last drug infusion seems to be during the conditioning regimen. Allogeneic HSCT for C-PNH was associated with a high rate of aGvHD. Median duration of Eculizumab in transfused patients referred to HSCT was short. Eculizumab seems not to change the risk of HSCT complications in PNH patients, who are still associated with toxicities once referred to HSCT. Therefore, HSCT after complement blocker should only be proposed in the absence of alternative treatment and after careful assessment of the risk-benefit ratio, especially in transfused C-PNH patients.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi Y, McCoy JP, Carvallo C, et al. In vitro and in vivo evidence of PNH cell sensitivity to immune attack after nonmyeloablative allogeneic hematopoietic cell transplantation. Blood. 2004;103(4):1383–1390. [DOI] [PubMed] [Google Scholar]
- 3.Hillmen P, Muus P, Dührsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123–4128. [DOI] [PubMed] [Google Scholar]
- 4.Loschi M, Porcher R, Barraco F, et al. Impact of eculizumab treatment on paroxysmal nocturnal hemoglobinuria: A treatment versus no-treatment study. Am J Hematol. 2016;91(4):366–370. [DOI] [PubMed] [Google Scholar]
- 5.Peffault de Latour R, Schrezenmeier H, Bacigalupo A, et al. Allogeneic stem cell transplantation in paroxysmal nocturnal hemoglobinuria. Haematologica. 2012;97(11):1666–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peffault de Latour R, Mary JY, Salanoubat C, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008;112(8):3099–3106. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840–1847. [DOI] [PubMed] [Google Scholar]
- 8.Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117(25):6786–6792. [DOI] [PubMed] [Google Scholar]
- 9.Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350(6):552–559. [DOI] [PubMed] [Google Scholar]
- 10.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–1243. [DOI] [PubMed] [Google Scholar]
- 11.Schubert J, Hillmen P, Röth A, et al. Eculizumab, a terminal complement inhibitor, improves anaemia in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2008;142(2):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Göker H, Uz Burak, Büyükaşık Y, et al. Eculizumab before and after allogeneic hematopoietic stem cell transplantation in a patient with paroxysmal nocturnal hemoglobinuria. Turk J Haematol. 2011;28(3):233–237. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Common terminology criteria for adverse events v4.0. NCI, NIH, DHHS; May 29, 2009. NIH publication #09-7473. [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GvHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 15.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381–387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.