A 67-year-old male presented with a one-year history of fatigue, cold intolerance, malaise and an unintentional weight loss of ~80 lbs over the course of the preceding 12 months. Two years prior to presentation, he recalled development of Raynaud’s phenomenon. Five months prior to presentation he developed pruritus, which persisted despite discontinuation of allopurinol. He went on vacation the following month and suddenly developed a foot drop. Upon return, he had an episode of dehydration which required hospitalization. During the hospitalization, his antihypertensives and statin were discontinued. He also developed acute kidney injury and his creatinine increased to 2.9 mg/dl from a baseline of 1.4 mg/dl. As a result, a kidney biopsy was performed which revealed immunoglobulin light chain (AL) amyloidosis with 3+ κ light chain staining with negative λ staining. No other significant immunoglobulin staining was noted. Proteinuria measured by 24-hour collection was 2 g/d. Serum and urine protein electrophoresis and immunofixation were negative. Serum free light chain (FLC) assay showed a κ FLC of 79.3 mg/dl, λ of 12.7 mg/dl, a ratio of 6.24 with a difference of the involved to uninvolved free light chain (dFLC) of 66.6 mg/dl. Bone marrow biopsy showed 5% κ light chain restricted plasma cells. Karyotype was normal but fluorescence in situ hybridization (FISH) was significant for a CCND1/IGH fusion, t(11;14). An echocardiogram showed a left ventricular ejection fraction of 66%, a septal thickness of 14 mm and strain of −14%. Cardiac troponin T (CTnT) was 0.12 ng/ml and N-terminal pro B-type natriuretic peptide (NTproBNP) 5491 pg/ml.
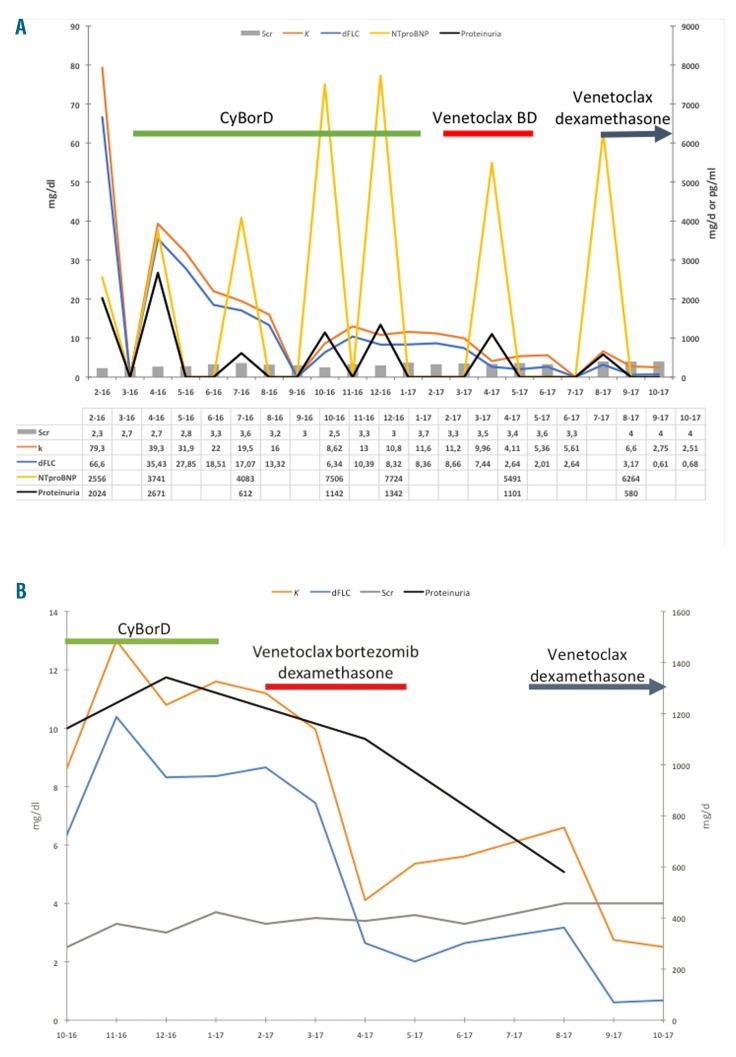
Treatment was started with weekly cyclophosphamide, bortezomib and dexamethasone (CyBorD). The patient had partial response and dFLC dropped to 6.34 mg/dl after six months of therapy. However, his response plateaued and dFLC began to increase up to 8.7 mg/dl (κ FLC = 11.6 mg/dl, Figure 1A). Along with this, NTproBNP also increased to 7724 pg/ml, creatinine increased to 3.5 mg/dl, and proteinuria was at 1.3 g/d. After ten months of CyBorD without any further benefit, the decision was made to substitute cyclophosphamide with venetoclax while continuing bortezomib and dexamethasone. The drug was obtained commercially with the help of a patient assistance grant program from Genentech, which distributes venetoclax in the United States and not as a clinical trial. Venetoclax was given along with weekly bortezomib (1 mg/m2 weekly) and dexamethasone (20 mg weekly). The standard ramp-up dosing for chronic lymphocytic leukemia (CLL) to a maximum dose of 400 mg daily was used and was achieved by the end of cycle 1. The patient developed severe diarrhea which resulted in acute kidney injury with creatinine rising to 4.5 mg/dl, requiring hospitalization and rehydration. The diarrhea eventually resolved and creatinine improved to 3.4 mg/dl. After two months on venetoclax-based therapy, dFLC dropped to 2.7 mg/dl with a κ to λ ratio of 2.5. After three months, the κ to λ ratio was 1.6 and the dFLC was 2.0 mg/dl (Figure 1B). The patient reached an unconfirmed complete response. NTproBNP also declined to 5491 pg/ml and proteinuria was reduced to 1.1 g/d. Around this time, the patient began to experience poor appetite, weakness and weight loss. This was initially attributed to venetoclax and therapy was discontinued after 3 cycles by the treating physician. One month after chemotherapy was discontinued; κ FLC was 5.6 mg/dl with a κ to λ ratio of 1.9 and a dFLC of 2.6 mg/dl. Creatinine increased to 3.6 mg/dl. The patient felt no improvement in his symptoms after stopping chemotherapy. Three months after stopping treatment, κ FLC had risen to 6.66 mg/dl with a dFLC of 3.23 mg/dl and a ratio of 1.94. Creatinine rose to 4.0 mg/dl. Venetoclax 400 mg daily was restarted with dexamethasone without bortezomib in an attempt to avoid the previous side effects. Two months after restarting venetoclax with dexamethasone, κ FLC was 2.51 with a dFLC of 0.68 mg/dl and a ratio of 1.37 (Figure 1B). Creatinine stabilized at 4.0 mg/dl. The patient was able to continue venetoclax without the previous adverse effects.
Figure 1.
Hematologic response and organ function of a patient with AL amyloidosis and t(11;14). (A) This displays the entire clinical course and hematologic response from initiation of treatment to last follow up. Cyclophosphamide, bortezomib and dexamethasone (CyBorD) was used as front-line therapy which achieved a partial response. (B) A close-up of the hematologic response to the chemotherapeutic regimens of only the last 12 months of follow up. A complete response was achieved with the addition of venetoclax to bortezomib dexamethasone (venetoclax BD) and later with venetoclax and dexamethasone. κ serum free light chain and difference between involved and uninvolved free light chain (dFLC) were measured in mg/dl, serum creatinine (Scr) was measured in mg/dl, Proteinuria was measured in mg/d and N-terminal prohormone of brain natriuretic peptide (NTproBNP) was measured in pg/ml.
Venetoclax, formally known as ABT-199, is a highly selective B-cell lymphoma-2 (Bcl-2) inhibitor that targets the Bcl-2 homology 3 (BH3) domain of the Bcl-2 family proteins.1 Bcl-2 gene products are antiapoptotic proteins that are the results of the t(14;18), a hallmark in follicular lymphoma. Venetoclax is the first US Food and Drug Administration (FDA) approved drug for the treatment of patients with CLL with 17p deletion with at least 1 prior therapy.2 Overexpression of Bcl-2 is found commonly in patients with CLL, follicular lymphoma, mantle cell lymphoma, Waldenstrom macroglobulinemia, and diffuse large B-cell lymphoma. Aberrant Bcl-2 expression can also be seen in multiple myeloma with deregulation of cyclin D1 (CCND1).1 Single agent activity was demonstrated by in vitro studies with human multiple myeloma cell lines (HMCLs) and primary myeloma cells.3 In the HMCLs, killing was restricted to cell lines with CCND1 translocation. Similar results were found in the primary myeloma cells. FISH analysis revealed that cell lines with t(11;14) were highly sensitive to venetoclax regardless of the 17p deletion status, suggesting the mechanism of action was independent of P53.
Human studies with venetoclax in multiple myeloma have begun. Venetoclax has been reported to produce a very good partial response (VGPR) in a patient who became refractory to multiple regimens, including two immunomodulatory-proteasome inhibitor combinations, transplant, alkylator-proteasome inhibitor combination, and daratumumab.4 Single agent activity was demonstrated in a phase I trial in patients with relapsed refractory multiple myeloma (RRMM). In this cohort of heavily treated (median regimen = 6) population, responses were observed in 15% of patients with t(11;14) both of whom achieved a complete response. No responses were seen in patients without t(11;14). Venetoclax was combined with bortezomib and dexamethasone in a phase Ib trial of patients with RRMM. Response was noted in 50% of the evaluable patients, all of whom were Velcade responsive or naïve. In both trials, doses of up to 1200 mg a day were used. Additional trials are currently ongoing.
AL amyloidosis is a protein folding disorder that is most often the result of a plasma cell dyscrasia.5 The majority of patients do not meet the criteria for multiple myeloma.6 However, treatment with anti-myeloma therapy has been very successful, especially if a VGPR (dFLC < 40 mg/l or 4 mg/dl) could be achieved.7 The most common cytogenetic abnormality identified in patients with AL amyloidosis is t(11;14), which can occur in nearly half of the patients.8,9 A recent study found that patients with t(11;14) have inferior outcomes when treated with bortezomib than patients with other cytogenetic abnormalities. The poorer survival was attributed to the inability to achieve a deep response in patients with the t(11;14) abnormality. Since bortezomib-based therapies are the current standard treatment for AL amyloidosis, this could represent a major problem.10
Herein, we presented a patient with AL amyloidosis with t(11;14) who plateaued at a partial response with CyBorD therapy. The addition of venetoclax to bortezomib and dexamethasone made it possible to achieve a complete response. The duration of response was short, and the κ FLC began increasing within three months of stopping treatment along with serum creatinine. Fortunately, the patient quickly responded to venetoclax again upon reintroduction, this time without bortezomib. To the best of our knowledge, this is the first patient with AL amyloidosis who has been successfully treated with venetoclax. Since t(11;14) is the most common cytogenetic abnormality in Al amyloidosis, this could represent a major advance in the treatment if this could be confirmed. Our results, although very preliminary, support larger studies via a clinical trial.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Zhu H, Almasan A. Development of venetoclax for therapy of lymphoid malignancies. Drug Des Devel Ther. 2017;11:685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;28;374(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touzeau C, Dousset C, Le Gouill S, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia. 2014;28(1):210–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touzeau C, Le Gouill S, Mahe B, et al. Deep and sustained response after venetoclax therapy in a patient with very advanced refractory myeloma with translocation t(11;14). Haematologica. 2017;102(3):e112–e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349(6):583–596. [DOI] [PubMed] [Google Scholar]
- 6.Leung N, Bridoux F, Hutchison CA, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120(22):4292–4295. [DOI] [PubMed] [Google Scholar]
- 7.Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541–4549. [DOI] [PubMed] [Google Scholar]
- 8.Bochtler T, Hegenbart U, Cremer FW, et al. Evaluation of the cytogenetic aberration pattern in amyloid light chain amyloidosis as compared with monoclonal gammopathy of undetermined significance reveals common pathways of karyotypic instability. Blood. 2008;111(9):4700–4705. [DOI] [PubMed] [Google Scholar]
- 9.Bryce AH, Ketterling RP, Gertz MA, et al. Translocation t(11;14) and survival of patients with light chain (AL) amyloidosis. Haematologica. 2009;94(3):380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palladini G, Sachchithanantham S, Milani P, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126(5):612–615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.