Recent approval by the European Medicines Agency has opened doors for the use of lenalidomide (Len; Celgene Corp., Summit, NJ, USA) as a maintenance treatment (LenMT) for multiple myeloma (MM) after autologous hematopoietic stem cell transplantation (HSCT) in Europe. Large studies demonstrate increased progression-free survival,1,2 however, most patients eventually relapse and require further therapy. For second-line treatment, clearly defined algorithms are the subject of ongoing debate. In the setting of gradual asymptomatic serologic progression, the addition of one of the recently approved monoclonal antibodies (mAbs) while continuing LenMT is an intriguing strategy to harness the immune system against myeloma cells in particular.3 Similarly, the combination of Len and bispecific T-cell engaging antibodies (BiTEs) or chimeric antigen receptor (CAR)-modified T cells may increase the efficacy of anti-myeloma therapy. Although conceptually attractive, these approaches have not been validated in clinical studies. Therefore, we and others have argued that more detailed in vivo information about Len-mediated modulation of lymphocytes could shed light on the rationale underlying targeted immunotherapy on a platform of ongoing LenMT.
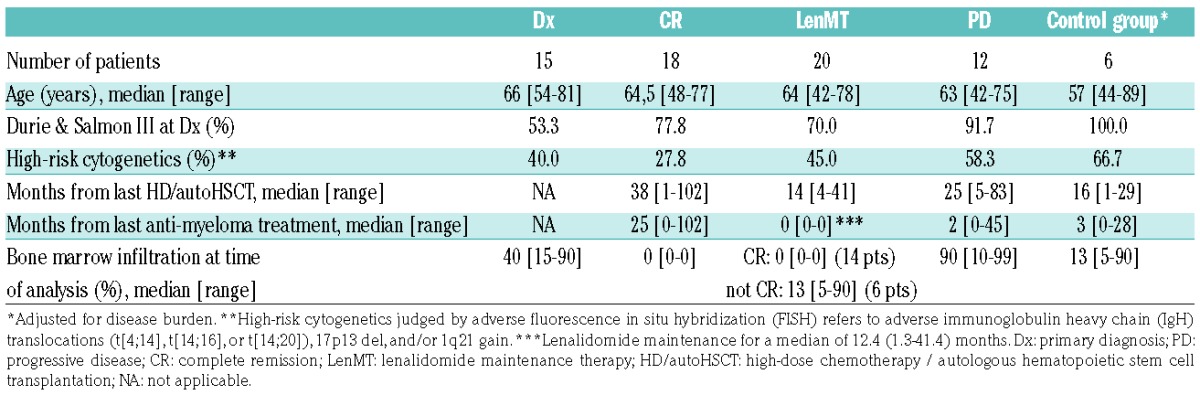
Consequently, we set out to characterize the composition of peripheral blood lymphocyte subsets and their surface expression of programmed death-1 (PD1, CD279) in myeloma patients. We used flow cytometry to evaluate cells from patients during LenMT (n=20; Online Supplementary Figure S1A) and compared them with individuals evaluated at the time of primary diagnosis (Dx; n=15; Online Supplementary Figure S1B), in sustained complete remission (CR; n=18), and at the time of relapse (PD; n=12). A control group, which was matched in terms of disease burden to a subgroup of LenMT patients (n=6), and a reference group of healthy donors (n=7) were also included (Online Supplementary Figure S2). LenMT patients were treated within the framework of our DSMM XII–XIV studies (clinicaltrials.gov identifiers: 00925821, 01090089, and 01685814)4 and received Len (10mg once daily [QD]) continuously; the dose was reduced to 5mg QD in 11 patients. No corticosteroids were administered. The clinical characteristics of all patient cohorts are shown in Table 1.
Table 1.
Clinical characteristics of the study patients.
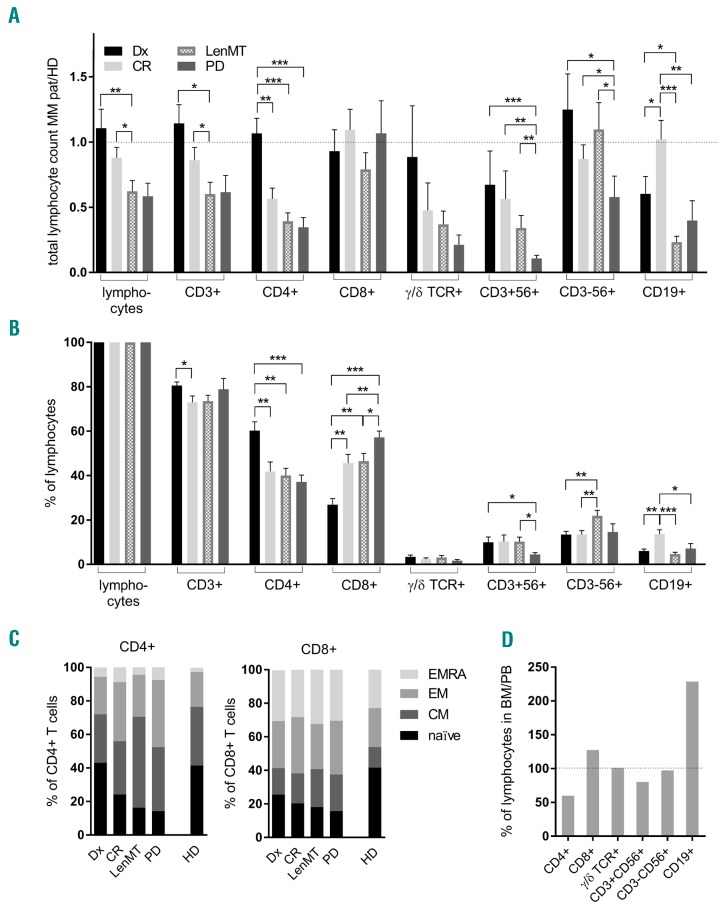
A major anti-myeloma effect of Len in vitro results from propagation and stimulation of natural killer (NK)5 and T cells,6 alongside T-helper 1 (Th1) polarization.7 However, LenMT is also associated with lymphopenia,1 impaired thymic reconstitution,8 and immunosuppressive changes.8 Herein, we found that LenMT-related lymphopenia was due, in large part, to reduced CD4+ T-cell counts (Figure 1A,B), whereby all patients with a previous history of anti-myeloma treatment showed loss of CD4+ T cells with a naïve phenotype (CD45RA+CD62L+; P<0.003, Figure 1C). In addition to age-related changes, this might reflect delayed T-cell output from the thymus following lymphodepletion.9 Moreover, patients undergoing LenMT had lower numbers of terminally differentiated CD4+ effector memory (EMRA; CD45RA+CD62L−) T cells (P<0.049) than patients at Dx or in CR, possibly due to high consumption of mature, and insufficient reproduction of immature T cells. Importantly, CD8+ T-cell counts were not altered significantly during LenMT (Figure 1A). Although LenMT was associated with low absolute CD8+CD45RA+ cell numbers (Figure 1C), the differences between this group and the other patient cohorts was not statistically significant for naïve or EMRA CD8+ T cells (P>0.05). Thus, LenMT-associated lymphopenia coincided primarily with impaired reconstitution and/or increased consumption of the CD45RA+CD4+ T-cell subset.
Figure 1.
Lenalidomide maintenance therapy is associated with numerically intact killer lymphocyte subsets in the peripheral blood (A–C) and bone marrow (D) of myeloma patients. (A–C) Peripheral blood (PB) lymphocyte subsets were evaluated by flow cytometry. (A) Absolute counts in myeloma patients standardized to the mean absolute counts in healthy donors (HD). (B) Proportional counts are shown. Mean values and standard errors of the mean are shown for patients at the time of primary diagnosis (Dx; n=15; black), patients in complete remission (CR; n=18; light gray), patients during lenalidomide maintenance therapy (LenMT; n=20; dashed), and at the time of progressive disease (PD; n=12; dark gray). Significant differences (*P<0.05, **P<0.01, ***P<0.001) between clinical subgroups were investigated using the Mann-Whitney U (MWW) test. Reference values from HDs were taken from the literature (Online Supplementary References1,2). (C) Proportion of naïve (CD45RA+CD45RO−CD62L+; black), central memory (CM; CD45RO+CD62L+; dark gray), effector memory (EM; CD45RO+CD62L−; medium gray), and terminally differentiated effector memory (EMRA; CD45RA+CD62L-; light gray) subsets are indicted for PB CD4+ and CD8+ T cells. (D) Lymphocyte subsets in corresponding bone marrow (BM) and PB samples (n=15) were evaluated by flow cytometry. Proportions of BM compared with PB lymphocytes are shown. MM pat: myeloma patient.
Consistent with previous data,5 the percentage of NK cells in the peripheral blood was highest during LenMT (Figure 1B). The lowest absolute NK-cell counts were found in patients in PD (Figure 1A). Antibody-dependent cellular cytotoxicity (ADCC), a central anti-myeloma mechanism mediated by mAbs, relies on the effector function of NK cells. Accordingly, the efficacy of such mAbs in patients with PD and a significant disease load might be compromised; however, LenMT propagates NK cells, which are a prerequisite for the induction of ADCC against myeloma cells.
Furthermore, we found it noteworthy that B-cell counts in patients with active disease (Dx or PD) were below the normal range, and were even lower during LenMT (Figure 1A). However, patients in CR not receiving concurrent immunomodulatory treatment showed completely reconstituted B-cell counts, with an approximate 4-fold increase compared with the LenMT group. This indicates that Len has an inhibitory effect on the recovery of B cells in the peripheral blood after high-dose chemotherapy and autologous HSCT.
To investigate the lymphocyte composition in the bone marrow niche, we next analyzed lymphocyte subsets in 15 myeloma patients for whom corresponding bone marrow and peripheral blood samples were available. Increased numbers of CD38+CD138+ plasma cells were detected in all samples, indicating active disease and high-quality bone marrow aspiration. In conjunction with an increase in CD19+ cells (suggestive of B-cell/plasmablast homing to the bone marrow niche), the proportion of CD4+ T cells in the bone marrow was lower than that in peripheral blood (Figure 1D). The percentages of all other lymphocyte subsets, especially CD8+ T and NK cells, were similar in peripheral blood and bone marrow aspirates.
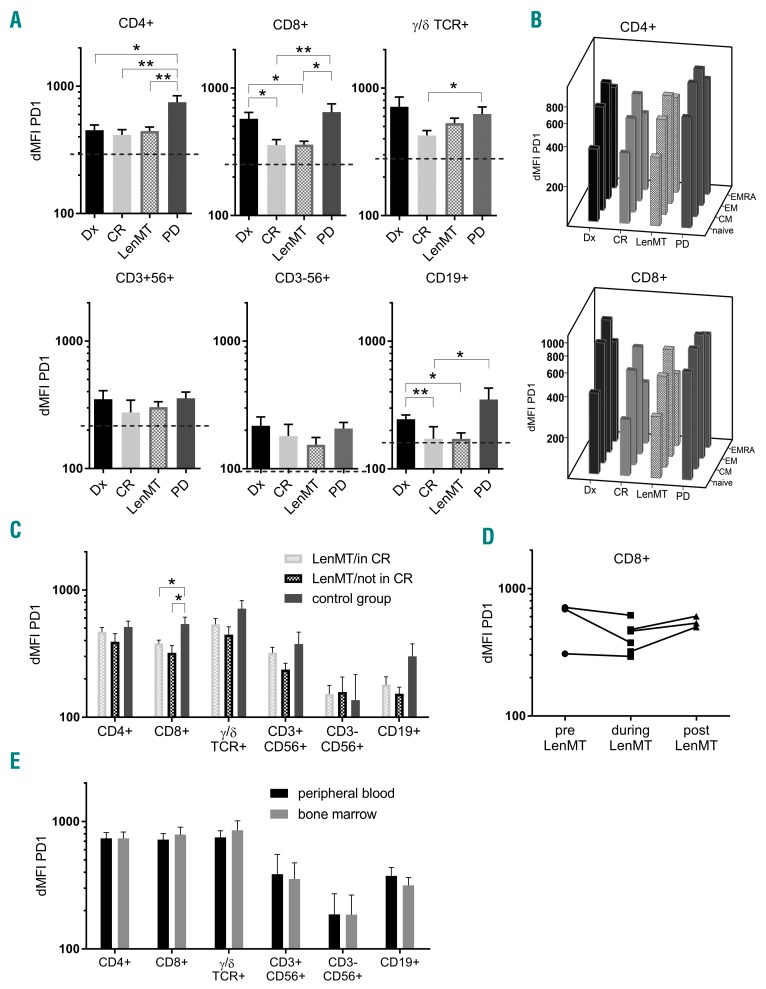
As a correlate of functional status, we next assessed PD1 expression on each of the lymphocyte subsets. The immune checkpoint PD1 is a prototypical marker of T-cell activation, exhaustion or senescence.10 PD1 expression is upregulated on T cells from myeloma patients, but decreases after autologous HSCT.11 As opposed to other inhibitory receptors such as CTLA-4, TIM-3, and LAG-3, increased PD1 levels post-HSCT are associated with early relapse.12 Len reduces PD1 expression on lymphocytes in vitro,13 however, this has not yet been confirmed in vivo.14,15 We found the highest expression of surface PD1 in lymphocytes from patients with active disease (Dx, PD), whereas optimal disease control (CR) almost normalized PD1 levels (Figure 2A). This observation held true for all CD4+ and CD8+ T-cell subsets, with PD1 expression levels being lowest on naïve T cells and highest on effector memory T cells (Figure 2B). Similar to the CR group, but in contrast to the cohorts with active disease, patients undergoing LenMT demonstrated significantly reduced expression of PD1. This effect was most pronounced for CD8+ T cells (Figure 2A).
Figure 2.
High expression of programmed death-1 on lymphocytes occurs in active myeloma and can be reversed by lenalidomide maintenance therapy. (A–D) Differential mean fluorescence intensity (dMFI) of programmed death-1 (PD1) expression on peripheral blood (PB) lymphocyte subsets was evaluated by flow cytometry. (A) Mean values and standard errors of the mean are shown for patients at the time of diagnosis (Dx; black), complete remission (CR; light gray), during lenalidomide maintenance therapy (LenMT; dashed), and at the time of progressive disease (PD; dark gray). Significant differences (*P<0.05, **P<0.01) between clinical subgroups were investigated using the Mann-Whitney U (MWW) test. Mean values for healthy donors (n=7) are indicated by the dashed line. (B) Mean values for naïve, central memory (CM), effector memory (EM), and terminally differentiated effector memory (EMRA) CD4+ and CD8+ T cells are depicted for the indicated clinical cohorts. (C) Mean values and standard errors of the mean are shown for patients in CR undergoing LenMT (LenMT/in CR; n=14; dashed, light gray), patients with a measurable disease load during LenMT (LenMT/not in CR; n=6; dashed, black), and patients with matched measurable disease burden in the absence of Len (control group; n=6; dark gray). Disease burden (according to bone marrow (BM) infiltration) was quantified by immunohistochemistry (median 13% [range: 5–90]) and remission status (according to the the International Myeloma Working Group criteria [very good partial response: n=3; partial response: n=2; progressive disease [PD]: n=1]). Significant differences (*P<0.05) between subgroups were investigated using the MWW test. (D) dMFI of PD1 expression on CD8+ T cells from six myeloma patients at two different time points before (pre) and during, or during and after (post), exposure to Len are illustrated. (E) dMFI of PD1 expression was evaluated on lymphocyte subsets in corresponding PB and BM samples by flow cytometry. Median values and standard errors of the mean are shown. Statistical differences in dMFI between PB or BM lymphocyte subsets were assessed using a paired samples Student’s t-test (P>0.05).
We next performed subgroup analyses of the LenMT cohort and compared patients in CR (LenMT/in CR; n=14) to patients with measurable disease (LenMT/not in CR; n=6). We found no significant differences in PD1 expression (Figure 2C). Thus, we hypothesized that Len counteracts the increase in PD1 expression by lymphocytes that occur in the presence of myeloma cells. Therefore, we analyzed a control group of myeloma patients not exposed to Len or other immunomodulatory drugs (n=6). For this control group, patients were drawn from our database and matched to those patients who were not in CR during LenMT. The matching criteria included serological remission status (according to the International Myeloma Working Group consensus) and degree of plasma cell infiltration of the bone marrow (evaluable by immunohistochemistry) (Table 1; Online Supplementary Figure S1B). We observed a trend towards reduced PD1 expression on T and B cells from the patient cohort during LenMT, with significant reduction for CD8+ T cells (P<0.05, Figure 2C). Hence, to the best of our knowledge, this is the first study to demonstrate in vivo that continuous administration of Len inhibits myeloma-associated increases in PD1 expression by CD8+ T cells, thereby counteracting the development of an exhausted/senescent T-cell phenotype.
Next, we analyzed replicate patient samples obtained prior to, during, and after LenMT. Albeit individual checkpoint expression levels on CD8+ T cells were highly variable, the lowest PD1 levels for all (n=6) patients were observed during LenMT (Figure 2D). Of note, we found a mild reduction in PD1 expression in one patient who had already achieved CR following high-dose chemotherapy and autologous HSCT before starting LenMT. Moreover, we observed a marked increase in PD1 expression in another patient after cessation of LenMT (at the patient’s request), notwithstanding an ongoing remission. Therefore, sequential sampling confirmed the capacity of Len to thwart myeloma-induced PD1 elevation on CD8+ T cells.
In order to relate our observations to conditions in the myeloma bone marrow niche, we compared PD1 levels on lymphocyte subsets in bone marrow and peripheral blood samples obtained concurrently (when available) (Figure 2E, n=15). Given that no significant differences were apparent, our data are analogous to those obtained for peripheral blood lymphocytes, making the counteraction of PD1 upregulation on lymphocytes a potential further piece in the jigsaw representing the mode of action of Len. Nevertheless, PD1 levels remained above the normal range in all patients with measurable disease, making combination treatment with checkpoint inhibitors conceptually attractive.16
In summary, in-depth profiling of the lymphocyte compartment in myeloma patients underlines the potential utility of Len as a combination partner for targeted immunotherapy beyond its direct anti-myeloma efficacy. First, our parallel comparison suggests that low NK cell counts at the advanced stage of disease can be increased by Len, providing elevated numbers of effector cells for mAbs like elotuzumab and daratumumab to induce ADCC. Second, we are the first to present in vivo data showing that Len preserves CD8+ T-cell numbers and reduces expression of the exhaustion/senescence marker PD1, enabling synergistic effects alongside checkpoint inhibitors. Most importantly, these data provide a strong rationale for the combined use of Len and novel therapies, which rely on a functional T-cell compartment, such as BiTEs and CAR-modified T cells.
Supplementary Material
Acknowledgments
The authors would like to thank all collaborators within the Deutsche Studiengruppe Multiples Myelom (DSMM), especially Florian Bassermann, Monika Engelhardt, Martin Gramatzki, Christian Langer, and Christian Straka, as well as all patients and their families for their support.
Footnotes
Funding: SD was supported by the IZKF Würzburg (Interdisziplinäres Zentrum für Klinische Forschung) and the Else-Kröner-Forschungskolleg Würzburg. MH was supported by the Junges Kolleg der Bayerischen Akademie der Wissenschaften.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782–1791. [DOI] [PubMed] [Google Scholar]
- 3.Hofmeister CC, Lonial S. How to integrate elotuzumab and daratumumab into therapy for multiple myeloma. J Clin Oncol. 2016;34(36):4421–4430. [DOI] [PubMed] [Google Scholar]
- 4.Knop S, Langer C, Engelhardt M, et al. Lenalidomide, adriamycin, dexamethasone for induction followed by stem-cell transplant in newly diagnosed myeloma. Leukemia. 2017;31(8):1816–1819. [DOI] [PubMed] [Google Scholar]
- 5.Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210–216. [DOI] [PubMed] [Google Scholar]
- 6.Haslett PA, Hanekom WA, Muller G, Kaplan G. Thalidomide and a thalidomide analogue drug costimulate virus-specific CD8+ T cells in vitro. J Infect Dis. 2003;187(6):946–955. [DOI] [PubMed] [Google Scholar]
- 7.Luptakova K, Rosenblatt J, Glotzbecker B, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 2013;62(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clave E, Douay C, Coman T, et al. Lenalidomide consolidation and maintenance therapy after autologous stem cell transplant for multiple myeloma induces persistent changes in T-cell homeostasis. Leuk Lymphoma. 2014;55(8):1788–1795. [DOI] [PubMed] [Google Scholar]
- 9.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19(5):318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–772. [DOI] [PubMed] [Google Scholar]
- 11.Rosenblatt J, Glotzbecker B, Mills H, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34(5):409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung DJ, Pronschinske KB, Shyer JA, et al. T-cell exhaustion in multiple myeloma relapse after autotransplant: optimal timing of immunotherapy. Cancer Immunol Res. 2016;4(1):61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorgun G, Samur MK, Cowens KB, et al. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res. 2015;21(20):4607–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer I, Engelhardt M, Fichtner S, et al. Lenalidomide enhances myeloma-specific T-cell responses in vivo and in vitro. Oncoimmunology. 2016;5(5):e1139662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busch A, Zeh D, Janzen V, et al. Treatment with lenalidomide induces immunoactivating and counter-regulatory immunosuppressive changes in myeloma patients. Clin Exp Immunol. 2014;177(2):439–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badros A, Hyjek E, Ma N, et al. Pembrolizumab, pomalidomide and low dose dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;130(10):1189–1197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.