Abstract
Although it is known that B-cell lymphomas occur more frequently in immunocompromised patients, thus far such an association has not been clearly established for T-cell lymphomas. Of the 251 patients who were diagnosed with a T-cell non-Hodgkin lymphoma in our center between 1999 and 2014, at least 25 were identified in immunocompromised patients. Herein, we retrospectively analyzed the clinical and pathological characteristics of these 25 cases. In addition, we searched the literature and present an overview of 605 previously published cases. The actual number of patients with B-cell chronic lymphocytic leukemia and patients on immunosuppressive drugs for inflammatory bowel disease or rheumatoid arthritis in the total cohort of 251 patients diagnosed with T-cell non-Hodgkin lymphoma was much higher than the number of patients expected to have these diseases in this cohort, based on their prevalence in the general population. This, together with the large number of additional cases found in the literature, suggest that the risk of developing T-cell non-Hodgkin lymphoma is increased in immunocompromised patients. Compared to T-cell non-Hodgkin lymphoma in the general population, these lymphomas are more often located extranodally, present at a younger age and appear to have a poor outcome. The observations made in the study herein should raise awareness of the possible development of T-cell non-Hodgkin lymphoma in immunodeficient patients, and challenge the prolonged use of immunosuppressive drugs in patients who are in clinical remission of their autoimmune disease.
Introduction
It has long been recognized that patients with either a primary or acquired immunodeficiency are at increased risk for the development of malignant lymphomas.1,2 Hematopoietic stem cell and solid organ transplant recipients, for example, can develop post-transplant lymphoproliferative disease (PTLD);3 patients infected with the human immunodeficiency virus (HIV), patients with primary immunodeficiencies and patients treated for inflammatory bowel disease (IBD) with immunosuppressive drugs all have an increased risk for developing lymphoma.4–8 Moreover, this complication is seen in patients with autoimmune diseases like rheumatoid arthritis (RA), primary Sjögren syndrome and systemic lupus erythematosus. However, it is not clear whether the lymphomas in these patients are triggered by chronic inflammation caused by the disease itself or by the (immunosuppressive) therapies used.9–13 In all the groups studied, the reported lymphomas are predominantly of B-cell origin.3,5,11
Much less is known about the development of T-cell non-Hodgkin lymphomas (T-NHL) in patients with immunodeficiencies or autoimmune diseases. For HIV patients and solid organ transplant recipients some large case series and reviews on T-NHL have been published.14–20 In IBD patients, the development of a specific and rare subtype of T-NHL, hepatosplenic T-NHL (HSTCL), has been associated with the use of thiopurines, either alone or in combination with tumor necrosis factor (TNF)-α inhibitors.21,22 Even less is known about the development of T-NHL in patients with other immunodeficiencies, as only case reports and some small case series have been published.
Herein, we present a relatively large series of 25 immunodeficient patients in whom T-NHL was diagnosed in a single referral center in the period 1999 to 2014. In this cohort study, we describe the clinical characteristics of these cases and correlate them to the pathological features of T-NHL. Furthermore, we present a review of the literature on T-NHL in immunocompromised patients. To the best of our knowledge, this is the largest series of T-NHL in patients with varying causes of immunodeficiency reported so far.
Methods
Histopathological material and reports from patients treated in or referred to the Academic Medical Center in Amsterdam are stored prospectively in a database. This database was queried for samples on which a T-cell receptor (TCR) gene rearrangement analysis was performed between 1999 and 2014. In our center, analysis of TCR gene rearrangement on tumor tissue is standard practice in the workup if T-NHL is suspected. In cases where the diagnosis of T-NHL was confirmed by histology, molecular testing and clinical features, the corresponding clinical data were searched for the presence of immunodeficiency prior to the diagnosis of T-NHL. For all cases, the biopsies were reviewed and immunohistochemical stains for CD2, CD3, CD4, CD5, CD8, granzyme B, PD1, CD30, ALK1, CD21, CD20, TdT, CD56 and in situ hybridization for Epstein-Barr virus (EBV)-encoded coded ribonucleic acid (RNA; EBER) were analyzed. If these were not performed in the routine diagnostic work-up (especially in older cases), they were additionally performed for this purpose. The lymphomas were (re)classified according to the World Health Organization (WHO) 2008 classification of lymphoid malignancies.23 Clinical information for all patients was collected in an anonymized database. The survival status and the cause of death were determined at the cutoff date of April 1, 2015. For staging, the Ann Arbor system was used for all patients, with the exception of those with cutaneous lymphomas, for whom the Mycosis Fungoides Cooperative Group (MFCG) tumor-node-metastasis (TNM) staging system of cutaneous T-NHL was used.24 Statistical analyses of the data were performed using SPSS (version 23.0 for Windows).
A search in PubMed was performed to find additional cases of T-NHL in patients with varying causes of immunodeficiency using the following search terms: “Immunocompromised”, “immunodeficiency”, “decreased immunity”, “reduced immunity”, “HIV”, “autoimmune disease”, “rheumatoid arthritis”, “IBD”, “inflammatory bowel disease”, “Crohn”, “ulcerative colitis”, “hematologic malignancy”, “Hodgkin”, “Waldenström”, “B-cell lymphoma”, “B-cell lymphoma”, and “leukemia”. These terms were combined using the “AND”-function with the search terms: “T-NHL”, “T-cell lymphoma”, “peripheral T-NHL”, “peripheral T-cell lymphoma” or “PTCL”. When available, Medical Subject Headings (MeSH) terms were used. In order to be included in the review herein, the cases of patients were required to have a pre-existing immunodeficiency due to HIV, immunosuppressive therapy, hematologic malignancies or a primary immunodeficiency before they developed a T-NHL. Reference lists of selected articles were used to identify additional articles. Articles published in a language other than English were excluded. Articles using earlier published cases were checked and duplicate cases were eliminated. Since more comprehensive reviews describing case series and previously published cases are available in the literature for T-NHL occurring in solid organ transplant recipients or in HIV patients, only these papers were included.
Results
Patients’ characteristics
A total of 251 T-NHL cases were found in our histopathological database. Forty-two cases, for which no clinical data were available, were excluded (see flowchart, Figure 1). Twenty-five of the remaining 209 cases (12%) were identified in patients with an immunodeficiency. Table 1 and Table 2 give an overview of the clinical characteristics, ordered by the underlying disorder in the latter. The majority of cases of T-NHL were found in patients with an auto-immune disease (56%). Other underlying disorders were hematologic malignancies (24%), solid organ transplantation (16%) and HIV infection (4%). Previously published case reports and case series are summarized in Online Supplementary Table S1, also ordered by the underlying disorder. For comparison, in our PubMed search 605 cases of immunodeficiency-related T-NHL were identified, of which 201 occurred in patients with HIV infection (33%), 197 in transplant recipients (33%), 143 in patients with underlying autoimmune diseases (24%), 55 following previously treated hematologic malignancies (9%) and nine in patients with a primary immunodeficiency (1%).
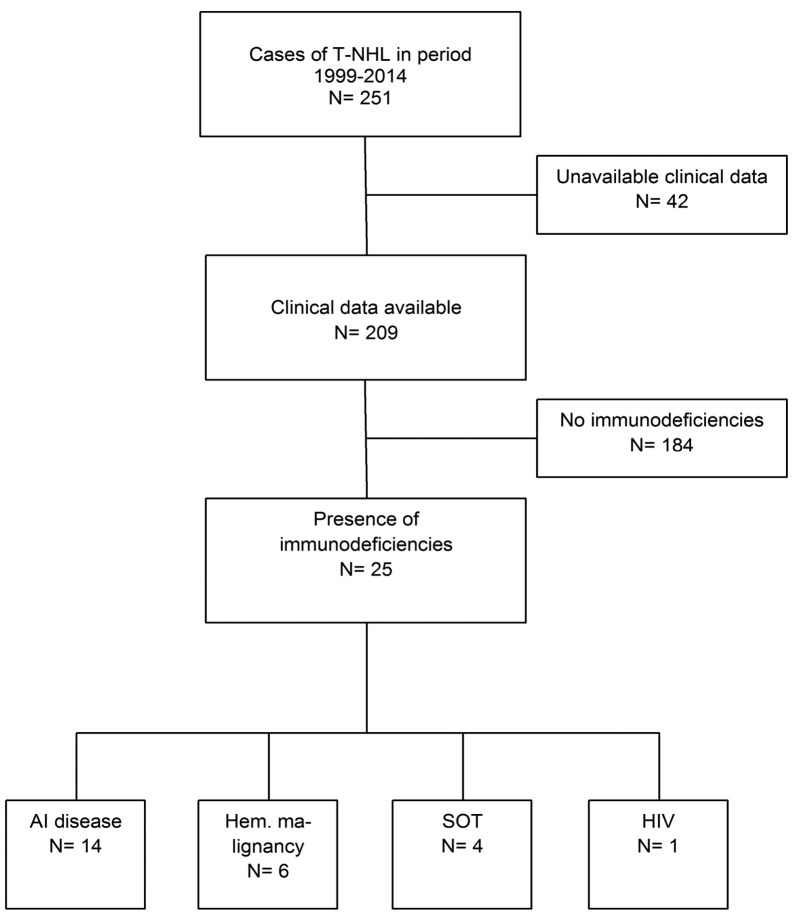
Figure 1.
Cases included. Flowchart of inclusion of cases of T-NHL in patients with immunodeficiencies due to an underlying disorder or immunosuppressive drugs in the period 1999–2014. AI: autoimmune; Hem. malignancy: hematologic malignancy; HIV: human immunodeficiency virus; SOT: solid organ transplantation; T-NHL: T-cell non-Hodgkin lymphoma.
Table 1.
Patient characteristics.
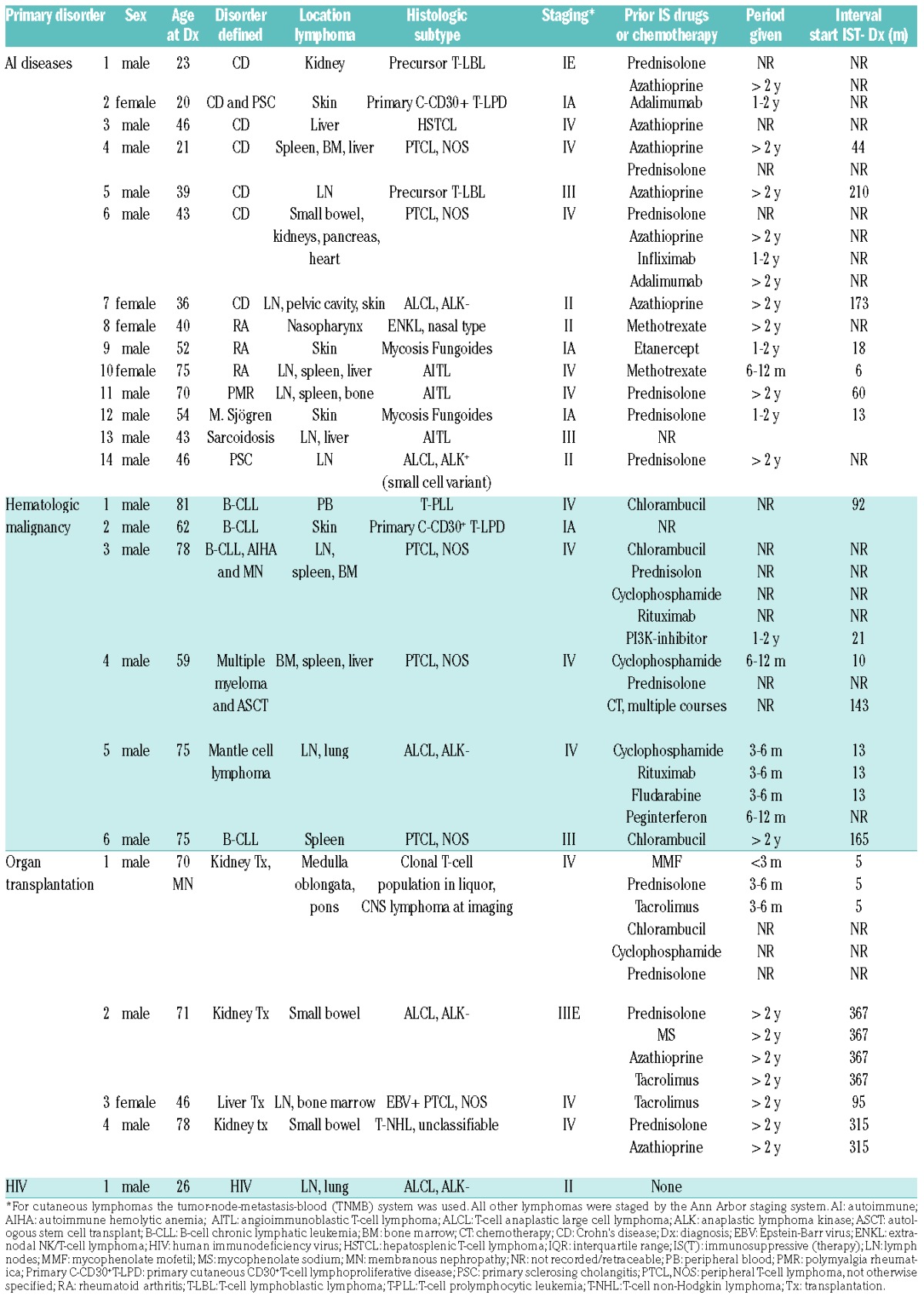
Table 2.
Patient and lymphoma characteristics, ordered by primary disorder.
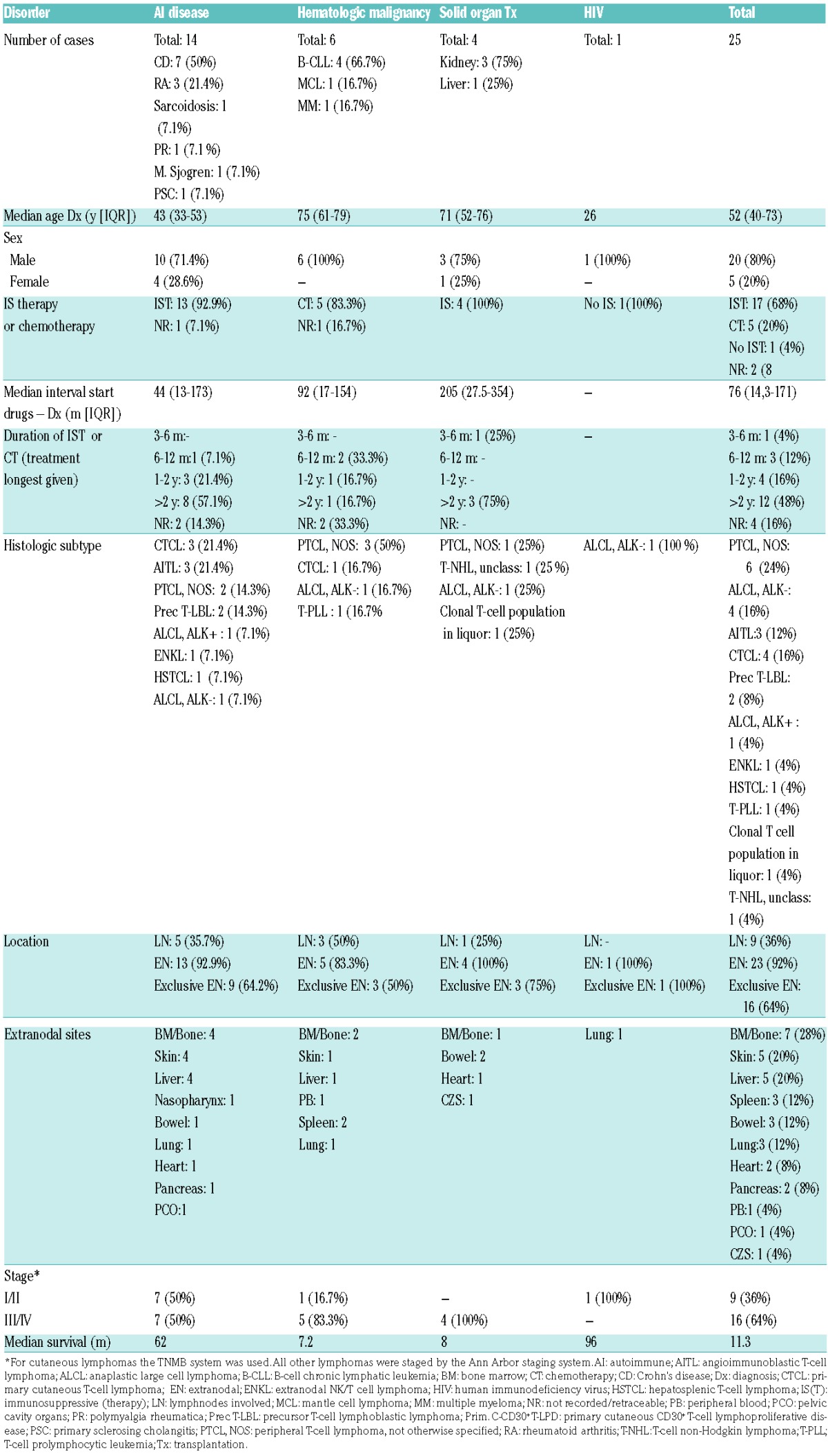
In our series, T-NHL occurred at a median age of 52 years (interquartile range 39.5–73), which is nine to ten years younger than that observed in studies on T-NHL in the general population.25,26 Patients with Crohn’s disease and HIV were younger than patients with hematologic malignancies and solid organ transplantations. This is consistent with the literature on HIV and PTLD, in which the mean/median ages reported were 38–39 and 43.5–57.5 respectively.14,15,17–20
In line with these studies, we found a male predominance (80%).14,15,17–20,26,27 Most patients had been treated with either immunosuppressive therapy or chemotherapy prior to the diagnosis of lymphoma (88%). For patients for whom information was available regarding the exact start date of the therapy with immunosuppressive or chemotherapeutic agents, the median interval between the start of the immunosuppressive treatment and the time of diagnosis was 76 months (interquartile range 14.3–171; N=16). In all patients for whom the duration of the drug use could be deduced from the clinical records, 60% had used one or more immunosuppressive or chemotherapeutic agents for at least two years (N=20). In all 25 patients, most (60%) had used prednisolone for varying periods. Azathioprine was used by 32%, including 86% of the patients with Crohn’s disease; those for whom it was documented (7 out of 8) used this drug for longer than two years. Drugs somewhat less frequently used were chlorambucil and cyclophosphamide (both 16%), mostly by patients with hematologic malignancies, and tacrolimus (12%) by solid organ transplant recipients. A few patients had been treated with adalimumab, infliximab, rituximab, mycophenolate mofetil or sodium, a phosphatidylinositol 3-kinase (PI3K) inhibitor or pegylated interferon (PEG-INF).
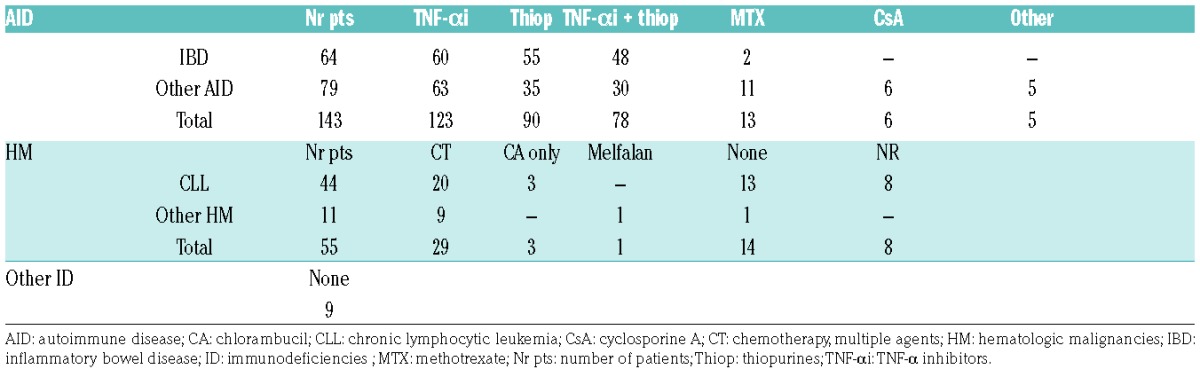
In the cases reported in the literature, the use of thiopurines was also widespread (63% of all patients with autoimmune diseases and 86% of patients with IBD) (Table 3). TNF-α inhibitors like adalimumab, infliximab and etanercept were, in contrast to our series, the most frequently used drugs in patients with autoimmune diseases; 86% was treated a TNF-α inhibitor, often in combination with thiopurines (63% of this group). In the case reports concerning hematologic malignancies most patients were treated with a chemotherapeutic regime containing multiple agents (56%) and a few with chlorambucil only (6%) (Table 3).
Table 3.
Use of drugs in cases reported in the literature.
Lymphoma characteristics
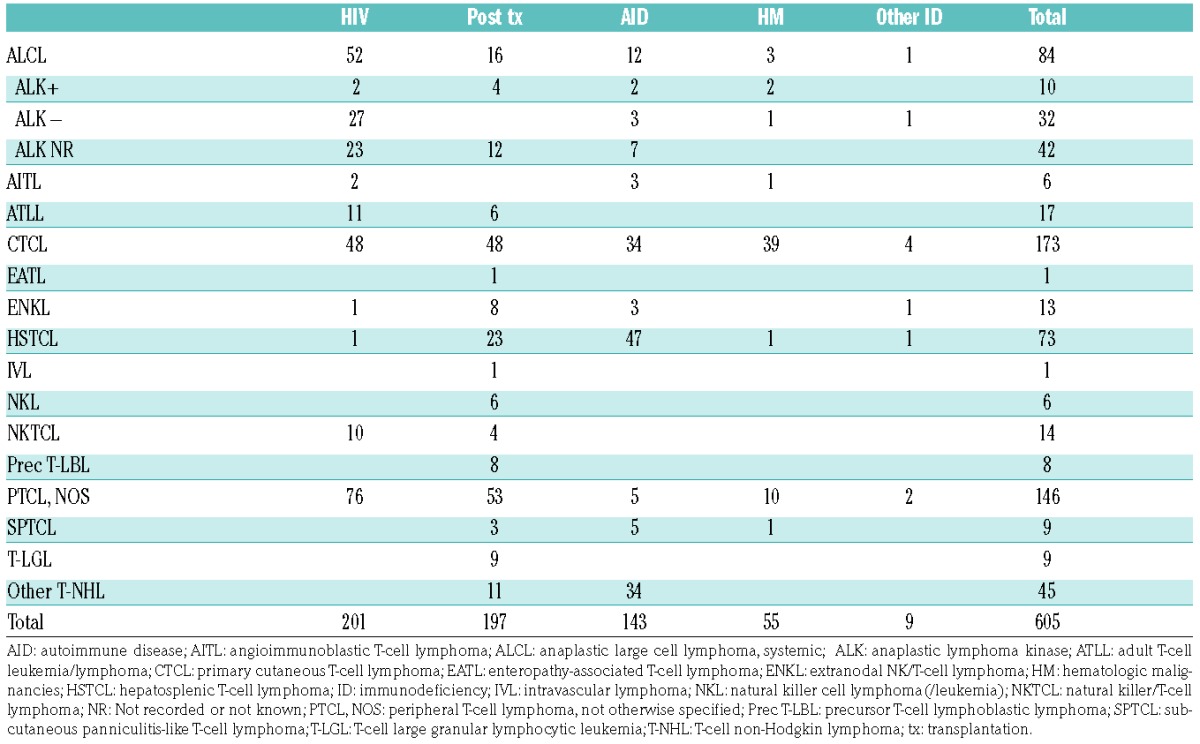
An overview of the histological characteristics of the lymphomas of our patients and those reported in the literature is provided in Table 2 and Table 4, respectively. T-NHL were morphologically and immunophenotypically highly variable. In our series, peripheral T-NHL (PTCL-NOS), was seen most frequently (24%), followed by anaplastic lymphoma kinase (ALK)-negative T-cell anaplastic large cell lymphomas (ALCL) (16%). There were three cases (12%) of angioimmunoblastic T-NHL (AITL), limited to the group of patients with autoimmune diseases. We saw only one case of HSTCL. This distribution was comparable to that in the general European population.26 However, between the subtype distribution in the reported cases and that in the general population there were some differences. Most notable were the high frequency of primary cutaneous T-NHL in the immunocompromised patients (29% vs. 1.7%), a more frequent occurrence of HSTCL (12% vs. 1.4%), and the relative lack of AITL cases (1% vs. 18.5%) in this group.26
Table 4.
Distribution of histologic subtypes in cases reported in the literature.
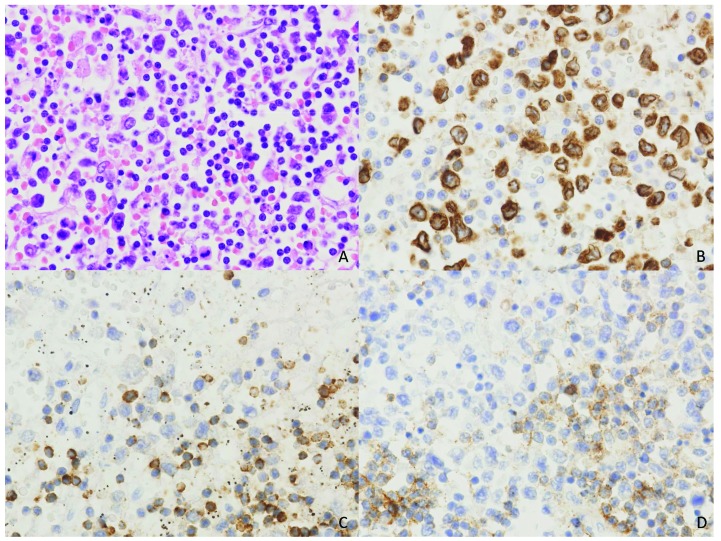
There was no predominance of either CD4+ or CD8+ lymphomas. Of the 19 cases in which CD4/CD8 staining had been carried out, seven (36.8%) were CD8+, six (31.6%) were CD4+, three (15.8%) were CD4+ CD8+, and three cases (15.8%) were CD4−CD8−. EBER was performed in 23 lymphoma cases and the majority was negative (91.3%). EBER was positive in two cases (8.7%), one of which concerned extranodal natural killer (NK)/T-cell lymphoma and the other involving PTCL-NOS with EBV-positive T cells and concurrent EBV-positive B-cell blasts. In two other cases, both AITL, the malignant T cells were negative, but the B-cell compartment was EBV-positive. Figure 2 shows an example of a PTCL-NOS in a patient with B-cell chronic lymphocytic leukemia (B-CLL) as the underlying disorder.
Figure 2.
The hematoxylin and eosin stain shows large atypical cells in a background of small monomorphic lymphocytes. (A) The large atypical cells are positive for CD3 (B) and show loss of expression of CD5. (C) The small lymphocytes in the background are B-cells (CD20) with co-expression of CD5 (D), consistent with residual B-CLL in the background of this T-cell lymphoma.
Extranodal involvement was observed in the vast majority of patients (92%), and 16 patients (64%) showed an exclusively extranodal localization of the lymphoma. The most commonly involved organs were the bone marrow or bone, skin, liver, spleen, small bowel and lung. The heart, pancreas, peripheral blood, pelvic cavity organs and central nervous system were affected in some cases (Table 2). This rate of extranodal involvement is higher than the 65–72% which has been reported in the general, immunocompetent population,25,28 and is consistent with more frequently occurring extranodal localizations of B-cell lymphomas in patients with either primary or acquired immunodeficiencies.3,17,29–31
The majority of patients had Ann Arbor stage III/IV disease at presentation (64%). The four patients staged according to the MFCG TNM staging system of cutaneous T-cell lymphomas had stage I or II disease.
Treatment and outcome
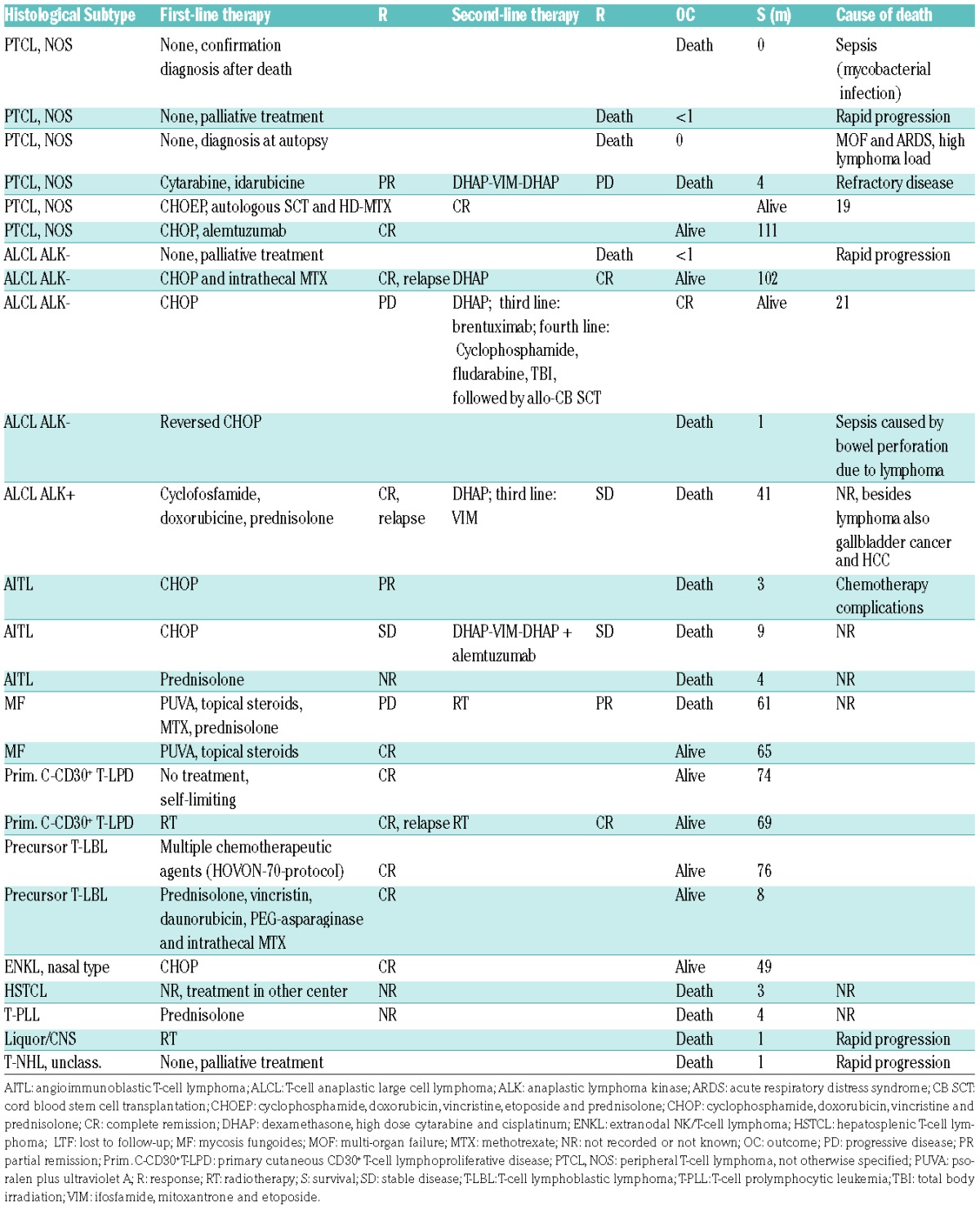
As shown in Table 5, in our series lymphoma treatment was very heterogeneous due to the different histological subtypes and clinical stages of disease.
Table 5.
Treatment and outcome.
After a median follow-up of six years, 15 patients had died. The median overall survival (OS) was 11.3 months (table 2) and the 1- and 5-year survival rates were 47% (95% confidence interval (CI) 27–67%) and 31% (95% CI 7–55%), respectively. This is somewhat lower than the 5-year survival rate reported in patients with T-NHL in the general population (38–49%),25,28,32 and consistent with a worse survival of HIV and post-transplant patients with T-NHL.14,15,17–19 Since worse outcomes have been reported for certain histological subtypes as compared to others,25,26,28 outcomes were calculated separately for the total number of patients with PTCL-NOS, AITL and HSTCL (n=10). The median OS in this group was 7.5 months with a 5-year survival rate of 20% (95% CI −6–46%), which is indeed lower than the median OS of 60 months and the 5-year survival rate of 41% (95% CI 8–74%) in patients with the remaining histological subtypes.
Twelve of the 15 deceased patients died within four months following diagnosis. The main causes of death, in those cases for which this information was available, were lymphoma progression (N=6) or sepsis (N=2). One patient developed two other malignancies and the exact cause of death remained unknown.
Discussion
In the general population, T-cell neoplasms are uncommon, representing about 5 to 10% of all NHL in Western countries.26 While B-cell lymphomas are known to occur more frequently in transplant recipients and in patients with IBD, HIV or autoimmune diseases,3,5,6,11 for T-NHL this data has not been elucidated as of yet. Since T-NHL are tumors of the immune system, akin to B-cell lymphomas, it is likely that the risk of developing a T-NHL is also increased in patients with an impaired immune system. However, a higher incidence of T-NHL has only been reported for a few specific patient groups, including HIV patients,14,15 solid organ transplant recipients19 and patients with a history of coeliac disease, psoriasis or eczema.33,34 Furthermore, a higher incidence of the rare hepatosplenic T-NHL has been documented in IBD patients on thiopurines.21
The present study of 25 patients is the largest case series of T-NHL in patients with varying causes of acquired immunodeficiencies published thus far. These 25 patients, diagnosed at our center between 1999 and 2014, constitute 12% of the total number of cases of T-NHL in which clinical data were available. Three of these 25 patients developed AITL, which may be accompanied by autoimmune features such as arthritis and synovitis, and can therefore resemble RA.35 The autoimmune diseases in these three patients were RA, polymyalgia rheumatica and sarcoidosis, which had been diagnosed 30 years, five years and three years, respectively, prior to the diagnosis of AITL. These lengthy intervals signify that it is most unlikely that they were manifestations of the AITL itself, since arthritis occurring more than six months in advance of the diagnosis is extremely uncommon.35
To determine whether T-NHL occurs more often in immunocompromised patients than in the general population, the prevalence of patients with an immunodeficiency within the cohort of patients with T-NHL should be compared to the prevalence of patients with an immunodeficiency in the general population. Since no data are available on the overall prevalence of immunodeficiencies in the general population, we compared the frequencies of specific underlying disorders in the Dutch context. We did this for IBD, B-CLL and RA, the three most consistent underlying disorders which we witnessed, apart from solid organ transplantation for which an association with the occurrence of T-NHL is already known.17,19,30 Based on a prevalence of IBD in 432 patients per 100,000 inhabitants in 2010,36 we would expect that 0.90 of the 209 T-NHL would occur in patients with IBD, compared to the seven we actually found. For B-CLL, a prevalence of 5,061 patients (average number in 2013/2014)37 out of a total population of 16,829,28938 leads to an expectation of 0.063 patients with B-CLL in our cohort, within which we identified an actual number of four patients. A similar calculation for RA, which had a prevalence of 116,000 patients in 201139 out of a population of 16,574,98938 leads to an expected number of 1.46 compared to an actual number of 3 patients. The numbers of expected cases for IBD and RA are probably overestimated, since the prevalence of IBD and RA in the general population that we used for these calculations also included all non-treated patients, who were excluded in our case series. Regarding RA, it is known that only about 10% of the patients with this disease who are registered are treated by a rheumatologist or other medical specialist, which generally corresponds with the use of immunosuppressive drugs.39 Using this information, when we recalculated the expected number of patients with RA in our cohort, we found a number of only 0.146 compared to the actual number of three. The probability of observing a certain number of cases, given an expected number of cases, can be calculated via the Poisson distribution. By using this method, we found a probability of 4.34E-05 that at least seven cases of IBD would occur given an expected number of 0.90. The probability of at least four cases of B-CLL compared to an expected number of 0.063 is 6.24E-07. For RA the probability of three cases occurring is 0.00047 when the expected number is 0.146. These calculations, even when bearing in mind the referral bias of our center, suggest that for B-CLL patients and those on immunosuppressive drugs for the treatment of IBD and RA, the risk of developing a T-NHL is higher than in the general population.
Taking into account the additional 596 cases we found in the literature of T-NHL in patients with impaired immunity due to HIV, hematologic malignancies and immunosuppressive drugs, this observation might be extrapolated to all patients with secondary immunodeficiencies. Whether patients with primary immunodeficiencies are also at risk for developing T-NHL is less clear, since we found only nine such cases in the literature. In this particular group of patients polyclonal T-cell proliferations are seen more often than overt T-cell lymphomas.8
The pathogenesis of T-NHL in immunodeficient patients is unclear. In our series the majority of lymphomas were EBV-negative, suggesting that, in contrast to B-cell lymphoproliferative disorders in immunocompromised patients, EBV does not play a role in this setting. Infection by other viruses, such as human T-lymphotropic virus type 1 (HTLV1) and human herpes virus (HHV)-6, possibly plays a role in development, as is suggested for T-cell PTLD.40–42 Another possible mechanism is that immune dysfunction contributes to lymphomagenesis by diminished immunosurveillance when malignant mutations arise in lymphoid cells due to other causes, for instance chronic antigenic stimulation or environmental factors, including mutagenic effects of chemotherapeutic or immunosuppressive drugs.42,43 Furthermore, a common biological basis of the first and second malignancy in hematologic malignancies has been suggested, ie., due to malignant transformation of a stem cell with the capacity to differentiate in either a B- or a T-NHL or a shared genetic predisposition for the two lymphomas.44,45 An example of this is the ten-eleven translocation 2 (TET2) mutation. TET2 is a dioxygenase that plays an important role in hematopoietic stem cells and progenitor cells by catalyzing multiple steps of 5-methylcytosine oxidation. TET2 mutations are commonly found in myeloid cancers, in about 11.9 % of all T-NHL, particularly in AITL and PTCL, in NOS, and in about 2% of B-NHL. The same TET2 mutations, for example, have been found in patients with AML/MDS secondary to a previous lymphoma, suggesting a shared genetic origin.46,47 Moreover, it has been observed that TET2 loss in mice leads to hypermutagenicity in hematopoietic stem cells and progenitor cells, resulting in an increased risk of various hematologic malignancies.48
Considering that most patients had been treated with immunosuppressive drugs or chemotherapy for intervals of longer than two years, it seems that prolonged treatment with these types of drugs increases the risk of malignant lymphoma development. An association between the use of thiopurines alone or combined with TNF-α inhibitors and the development of HSTCL in IBD patients has been reported previously.15 The majority of patients with IBD in our series and in the reported cases had been using azathioprine. It is possible, therefore, that the use of thiopurines also contributes to the development of other T-NHL.
Obviously, inherent to its retrospective design, our study has some limitations. The clinical data were not complete for all patients, thus making it difficult to assess the temporal relationship between the underlying disorders or drug use and the development of T-NHL for some. In addition, the group was too small to run subgroup analyses. Moreover, since the overall prevalence of immunodeficiencies in the general population is not known, we could only compare the prevalence of specific underlying disorders in our cohort of patients with T-NHL to those in the general population.
Conclusion
The 25 cases presented herein, together with the 596 cases found in the literature of T-NHL in patients with varying causes of immunodeficiencies suggest that patients with a secondary immunodeficiency are at increased risk for the development of T-NHL. Prolonged treatment with immunosuppressive or chemotherapeutic drugs seems to contribute to the risk. T-NHL in immunodeficient patients are histologically very heterogeneous. The distribution of subtypes resembles that present in the general population, with the exception of primary cutaneous T-NHL and HSTCL, both of which have been reported more often in patients with an immunodeficiency, and AITL, which has been reported less frequently in this group. Overall, the prognosis seems worse compared to T-NHL of similar subtypes in the general population. T-NHL occur predominantly in men, and in immunodeficient patients they tend to be more often located extranodally, which is in line with B-cell lymphomas in this group of patients. In our series of immunodeficient patients, the lymphomas occurred on average nine to ten years earlier than T-NHL in the general population.
The observations in the study herein should raise awareness of the possible development of T-NHL in immunodeficient patients and challenge the prolonged use of immunosuppressive drugs in patients who are in clinical remission of their autoimmune disease.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/3/486
Funding
The study was supported by Lymph&Co and by a grant from the Egbers Foundation.
References
- 1.Gatti RA, Good RA. Occurrence of malignancy in immunodeficiency diseases. A literature review. Cancer. 1971;28(1):89–98. [DOI] [PubMed] [Google Scholar]
- 2.Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant Proc. 1969;1(1):106–112. [PMC free article] [PubMed] [Google Scholar]
- 3.Parker A, Bowles K, Bradley JA, et al. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients - BCSH and BTS Guidelines. Br J Haematol. 2010;149(5):675–692. [DOI] [PubMed] [Google Scholar]
- 4.Beral V, Peterman T, Berkelman R, Jaffe H. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991;337(8745):805–809. [DOI] [PubMed] [Google Scholar]
- 5.Levine AM, Seneviratne L, Espina BM, et al. Evolving characteristics of AIDS-related lymphoma. Blood. 2000;96(13):4084–4090. [PubMed] [Google Scholar]
- 6.Bewtra M. Lymphoma in inflammatory bowel disease and treatment decisions. Am J Gastroenterol. 2012;107(7):964–970. [DOI] [PubMed] [Google Scholar]
- 7.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374(9701):1617–1625. [DOI] [PubMed] [Google Scholar]
- 8.Gratzinger D, Jaffe ES, Chadburn A, et al. Primary/congenital immunodeficiency: 2015 SH/EAHP workshop report-part 5. Am J Clin Pathol. 2017;147(2):204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50(6):1740–1751. [DOI] [PubMed] [Google Scholar]
- 11.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165(20):2337–2344. [DOI] [PubMed] [Google Scholar]
- 12.Askling J, Fored CM, Baecklund E, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64(10):1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54(3):692–701. [DOI] [PubMed] [Google Scholar]
- 14.Castillo JJ, Beltran BE, Bibas M, et al. Prognostic factors in patients with HIV-associated peripheral T-cell lymphoma: a multicenter study. Am J Hematol. 2011;86(3):256–261. [DOI] [PubMed] [Google Scholar]
- 15.Gilardin L, Copie-Bergman C, Galicier L, et al. Peripheral T-cell lymphoma in HIV-infected patients: a study of 17 cases in the combination antiretroviral therapy era. Br J Haematol. 2013;161(6):843–851. [DOI] [PubMed] [Google Scholar]
- 16.Biggar RJ, Engels EA, Frisch M, Goedert JJ. Risk of T-cell lymphomas in persons with AIDS. J Acquir Immune Defic Syndr. 2001;26(4):371–376. [DOI] [PubMed] [Google Scholar]
- 17.Herreman A, Dierickx D, Morscio J, et al. Clinicopathological characteristics of post-transplant lymphoproliferative disorders of T-cell origin: single-center series of nine cases and meta-analysis of 147 reported cases. Leuk Lymphoma. 2013;54(10):2190–2199. [DOI] [PubMed] [Google Scholar]
- 18.Perez K, Castillo J, Dezube BJ, Pantanowitz L. Human immunodeficiency virus-associated anaplastic large cell lymphoma. Leuk Lymphoma. 2010;51(3):430–438. [DOI] [PubMed] [Google Scholar]
- 19.Swerdlow SH. T-cell and NK-cell post-transplantation lymphoproliferative disorders. Am J Clin Pathol. 2007;127(6):887–895. [DOI] [PubMed] [Google Scholar]
- 20.Seckin D, Barete S, Euvrard S, et al. Primary cutaneous posttransplant lymphoproliferative disorders in solid organ transplant recipients: a multicenter European case series. Am J Transplant. 2013;13(8):2146–2153. [DOI] [PubMed] [Google Scholar]
- 21.Deepak P, Sifuentes H, Sherid M, Stobaugh D, Sadozai Y, Ehrenpreis ED. T-cell non-Hodgkin’s lymphomas reported to the FDA AERS with tumor necrosis factor-alpha (TNF-alpha) inhibitors: results of the REFURBISH study. Am J Gastroenterol. 2013;108(1):99–105. [DOI] [PubMed] [Google Scholar]
- 22.Kotlyar DS, Osterman MT, Diamond RH, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9(1):36–41 e1. [DOI] [PubMed] [Google Scholar]
- 23.Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues (4th ed). Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 24.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(6):1713–1722. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Guillermo A, Cid J, Salar A, et al. Peripheral T-cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification. Ann Oncol. 1998;9(8):849–855. [DOI] [PubMed] [Google Scholar]
- 26.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. [DOI] [PubMed] [Google Scholar]
- 27.Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112(12):4384–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arrowsmith ER, Macon WR, Kinney MC, et al. Peripheral T-cell lymphomas: clinical features and prognostic factors of 92 cases defined by the revised European American lymphoma classification. Leuk Lymphoma. 2003;44(2):241–249. [DOI] [PubMed] [Google Scholar]
- 29.Ferreri AJ. Risk of CNS dissemination in extranodal lymphomas. Lancet Oncol. 2014;15(4):e159–169. [DOI] [PubMed] [Google Scholar]
- 30.Zucca E, Roggero E, Bertoni F, Cavalli F. Primary extranodal non-Hodgkin’s lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol. 1997;8(8):727–737. [DOI] [PubMed] [Google Scholar]
- 31.Tran H, Nourse J, Hall S, Green M, Griffiths L, Gandhi MK. Immunodeficiency-associated lymphomas. Blood Rev. 2008;22(5):261–281. [DOI] [PubMed] [Google Scholar]
- 32.Gisselbrecht C, Gaulard P, Lepage E, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood. 1998;92(1):76–82. [PubMed] [Google Scholar]
- 33.Ekstrom Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang SS, Flowers CR, Kadin ME, et al. Medical history, lifestyle, family history, and occupational risk factors for peripheral T-cell lymphomas: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. 2014;2014(48):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsochatzis E, Vassilopoulos D, Deutsch M, Filiotou A, Tasidou A, Archimandritis AJ. Angioimmunoblastic T-cell lymphoma-associated arthritis: case report and literature review. J Clin Rheumatol. 2005;11(6):326–328. [DOI] [PubMed] [Google Scholar]
- 36.De Groof J, Rossen N, van Rhijn B, et al. P644 Epidemiology and characteristics of inflammatory bowel disease in a large population-based cohort in the Netherlands. Poster presentations European Crohn’s and Colitis Organisation, 2015. [Google Scholar]
- 37.The Netherlands Cancer Registry.
- 38.Statistics Netherlands (CBS).
- 39.National Institute of Public Health and the Environment (RIVM).
- 40.Hoshida Y, Li T, Dong Z, et al. Lymphoproliferative disorders in renal transplant patients in Japan. Int J Cancer. 2001;91(6):869–875. [DOI] [PubMed] [Google Scholar]
- 41.Lin WC, Moore JO, Mann KP, Traweek ST, Smith C. Post transplant CD8+ gammadelta T-cell lymphoma associated with human herpes virus-6 infection. Leuk Lymphoma. 1999;33(3–4):377–384. [DOI] [PubMed] [Google Scholar]
- 42.Fisher SG, Fisher RI. The epidemiology of non-Hodgkin’s lymphoma. Oncogene. 2004;23(38):6524–6534. [DOI] [PubMed] [Google Scholar]
- 43.Barzilai A, Trau H, David M, et al. Mycosis fungoides associated with B-cell malignancies. Br J Dermatol. 2006;155(2):379–386. [DOI] [PubMed] [Google Scholar]
- 44.Campidelli C, Sabattini E, Piccioli M, et al. Simultaneous occurrence of peripheral T-cell lymphoma unspecified and B-cell small lymphocytic lymphoma. Report of 2 cases. Hum Pathol. 2007;38(5):787–792. [DOI] [PubMed] [Google Scholar]
- 45.Herro E, Dicaudo DJ, Davis MD, Weaver AL, Swanson DL. Review of contemporaneous mycosis fungoides and B-cell malignancy at Mayo Clinic. J Am Acad Dermatol. 2009;61(2):271–275. [DOI] [PubMed] [Google Scholar]
- 46.Ko M, An J, Pastor WA, Koralov SB, Rajewsky K, Rao A. TET proteins and 5-methylcytosine oxidation in hematological cancers. Immunol Rev. 2015;263(1):6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solary E, Bernard OA, Tefferi A, Fuks F, Vainchenker W. The Ten-Eleven Translocation-2 (TET2) gene in hematopoiesis and hematopoietic diseases. Leukemia. 2014;28(3):485–496. [DOI] [PubMed] [Google Scholar]
- 48.Pan F, Wingo TS, Zhao Z, et al. Tet2 loss leads to hypermutagenicity in haematopoietic stem/progenitor cells. Nat Commun. 2017;8:15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.