Abstract
B-cell receptor activation, occurring within lymph nodes, plays a key role in the pathogenesis of chronic lymphocytic leukemia and is linked to prognosis. As well as activation of downstream signaling, receptor ligation triggers internalization, transit to acidified endosomes and degradation of ligand-receptor complexes. Herein, we investigated the relationship between these two processes in normal and leukemic B cells. We found that leukemic B cells, particularly anergic cases lacking the capacity to initiate downstream signaling, internalize and accumulate ligand in acidified endosomes more efficiently than normal B cells. Furthermore, ligation of either surface CD79B, a B-cell receptor component required for downstream signaling, or surface Immunoglobulin M (IgM) by cognate agonistic antibody, showed that the two molecules internalize independently of each other in leukemic but not normal B cells. Since association with surface CD79B is required for surface retention of IgM, this suggests that uncoupling of B-cell receptor internalization from signaling may be due to the dissociation of these two molecules in leukemic cells. A comparison of lymph node with peripheral blood cells from chronic lymphocytic leukemia patients showed that, despite recent B-cell receptor activation, lymph node B cells expressed higher levels of surface IgM. This surprising finding suggests that the B-cell receptors of lymph node- and peripheral blood-derived leukemic cells might be functionally distinct. Finally, long-term therapy with the Bruton’s tyrosine kinase inhibitors ibrutinib or acalabrutinib resulted in a switch to an anergic pattern of B-cell receptor function with reduced signaling capacity, surface IgM expression and more efficient internalization.
Introduction
It is now clear that signaling through the B-cell receptor (BCR) plays a key role in the pathogenesis of chronic lymphocytic leukemia (CLL) and other lymphomas. Several components of this pathway, including Syk,1 Erk,2 Akt,3 NFAT4 and NFκB5 can be constitutively activated and drugs that target BCR signaling, such as the Bruton’s tyrosine kinase inhibitors (BTKi), ibrutinib and acalabrutinib, are proving extremely effective in the clinic.6,7 BCR responsiveness varies markedly between patients with CLL and is linked to prognosis.8 Some cases show features of anergy,4,9 a pattern that is associated with lack of ability to transduce a downstream signal in response to BCR ligation and the presence of markers of good prognosis, including low levels of CD38 and mutated immunoglobulin heavy-chain variable (IGHV) genes. In contrast, cases with responsive or signaling competent BCRs usually express high levels of CD38, have unmutated IGHV genes and a more unfavorable clinical course;10 interestingly, these patients tend to respond more rapidly to BCR antagonists than those with anergic BCRs. Although BTKi therapy is very successful in controlling CLL, it is not curative and many patients are left with low level residual disease, which regrows on discontinuation of drug or when resistance mutations develop.11,12 This persistent disease also suggests that, within individual patients, the tumor may not behave in a homogeneous manner.13
Despite the central importance of BCR signaling in CLL and the efficacy of drugs that block this pathway, there is relatively little known about BCR dynamics in leukemic B cells. Surface levels of IgM and other BCR components are generally lower in CLL compared to normal B cells, and it has been suggested that this might be due to a failure to properly assemble the sIg α/β subunits CD79A and CD79B.14 Recent studies have shown that total IgM and CD79A levels are near normal in CLL but that CD79B expression, which is required for the transport of BCR to the cell surface,15 is reduced, thus trapping IgM within the cell.16 Exposure to interleukin 4 (IL4) increases CD79B expression and allows sIgM levels to increase and BCR signaling capacity to improve.16,17 CLL cell surface BCRs have an immature pattern of glycosylation that matures following ex vivo incubation18 or exposure to IL4,17 in keeping with accelerated BCR turnover induced by chronic activation. It has also been reported that, within the peripheral blood (PB) of individual patients with CLL, leukemic cells with the lowest sIgM expression show biochemical features of recent activation and proliferation, presumably because they have recently been released from lymphoid tissues where BCR stimulation and activation are thought to occur.19,20 Taken together, these previous data suggest that the reduced sIgM levels observed in CLL are due to a combination of increased turnover consequent to chronic activation coupled with defective transport to the cell surface resulting from a deficiency of CD79B. The ability of CLL BCRs to become internalized also has implications for how the tumor interacts with other cells, such as T cells. We, and others, have previously shown that, as in normal lymph nodes (LNs), activated CD4+ T cells colocalize with proliferating tumor cells and, in vitro, can supply signals that cause tumor proliferation.21–24 This process normally involves endocytosis and the processing of antigen bound to BCR, however it is not known whether CLL B cells are capable of providing this function. Herein we investigated whether, like normal B cells, CLL cells can internalize their BCR and to what extent this is linked to downstream signaling in both untreated patients and those receiving therapy with the BTKis ibrutinib and acalabrutinib. Our results shed new light on BCR function in CLL and have implications for understanding the mode of action of this important new class of drugs.
Methods
Patients and samples
PB samples were obtained from 19 healthy volunteers, 40 untreated patients with confirmed CLL (Online Supplementary Table S1), and an additional 15 patients receiving BTKi therapy (Online Supplementary Table S2). LN fine needle aspirate (FNA) and paired matched PB samples were derived from seven untreated CLL patients (Online Supplementary Table S3). Ethical approval was obtained from the National Research Ethics Service (08/H0906/94); all patients provided written informed consent. Patients were classified as having unmutated IGHV genes if homology with germline was >98%25 and as CD38+ if expression levels were 7% or higher (Online Supplementary Tables S1–S3).26
Flow cytometry
Cells were stained according to the manufacturer’s recommendations using fluorochrome-coupled antibodies (Online Supplementary Table S4). Viable CD19+CD5+ CLL and CD19+ normal B cells were acquired on a FACS Canto II flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar).
Surface (s)IgM and IgD expression was assessed using Quantum™ fluorescein isothiocyanate molecules of equivalent soluble fluorochrome (FITC MESF) microsphere kits and the QuickCal v. 2.3 program, according to the manufacturer’s recommendations. Surface CD79B was quantified using R-phycoerythrin (R-PE) MESF microsphere kits.
BCR signaling competence was determined using the ratiometric Ca2+ detector Indo1-AM (Life Technologies) to measure intracellular calcium (Ca2+) levels and Ca2+ influx following αIgM stimulation, as previously described.4,9
ERK1/2 phosphorylation activation was analyzed in both treatment-naïve and BTKi-treated (collected before and during BTKi therapy) CLL patient B cells. Cells were incubated with or without αIgM, or with phorbol 12-myristate 13-acetate (PMA; served as positive control) for ten minutes at 37°C prior to intracellular and surface staining.
B-cell receptor internalization in normal and CLL B cells
BCR internalization was assessed in two ways. The uptake and retention of ligand/receptor complexes in acidified endosomes was measured using the pH sensitive fluorescent sensor, pHrodo™ Red avidin (Life Technologies) linked to agonistic αIgM or IgD (pHrodo-αIgM or D). Target cells were incubated with either pHrodo-αIgM or D for 30 minutes at 4°C and then 37°C for 1h. Results are expressed as the pHrodo mean fluorescent intensity (MFI) after subtraction of the background signal (MFI of unlabeled anti-IgM). BCR internalization in normal and CLL B cells was also directly assessed by measuring the rate of disappearance of sIgM following ligation by agonistic αIgM. Full methodology is provided in the Online Supplementary Information.
Immunofluorescence Staining
CLL B cells were isolated using a human B-cell negative selection kit without CD43 depletion (StemCell Technologies) according to the manufacturer’s instructions, and purity was confirmed by flow cytometry. Cells were labeled with pHrodo-avidin or pHrodo-αIgM, deposited onto poly-l-lysine coated glass slides by cytospin and stained with CytoPainter Phalloidin-iFluor 488 reagent. Images were acquired on a Nikon Eclipse Ti-E inverted microscope equipped with the Nikon A1R Si confocal imaging system. Image analysis was with Nikon Elements v4.2 software (see Online Supplementary Information).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software version 5 (GraphPad Software, La Jolla, CA, USA). The Shapiro–Wilk test, t-test, Mann–Whitney and/or Wilcoxon’s test were used where indicated. P-values of <0.05 were considered significant.
Results
Internalization of normal and CLL BCRs
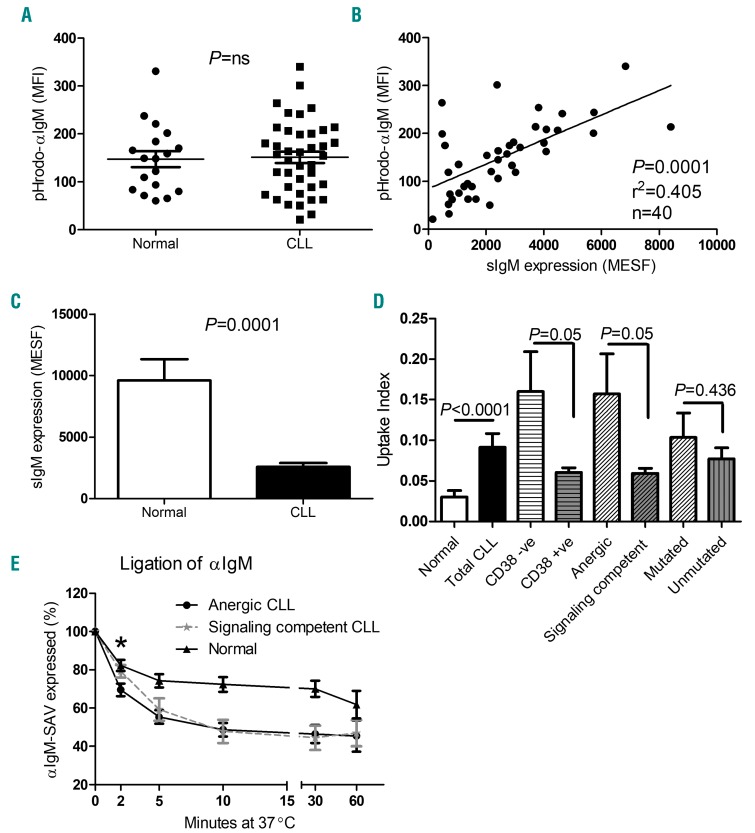
The uptake and retention of pHrodo-αIgM labeled BCRs in acidified endosomes was similar in normal and CLL B cells (MFI ± standard deviation: normal control: 147.5±16.8, n=19, P=0.825, CLL: 151.3±12.0, n=40; Figure 1A) and correlated with sIgM expression (r2=0.405, P=0.0001, n=40; Figure 1B). Confocal immunofluorescence microscopy confirmed that the labeled BCRs accumulate in an intracellular compartment (Online Supplementary Figure S1A) and pre-incubation with excess unlabeled anti-IgM, sodium azide and cytochalasin D showed the process to be specific and dependent on energy and the cytoskeleton (Online Supplementary Figures S1B–S1D). Repeated measurements confirmed the reproducibility of the assay within individual CLL cases (r2=0.874, P<0.0001, n=30; Online Supplementary Figure S1E). It has previously been reported that sIgM expression is lower in CLL compared to normal B cells, and this was also the case in our patients (Figure 1C). Since BCR uptake by normal and CLL B cells is similar and dependent on sIgM expression, this suggested that the process might be more efficient in CLL compared to normal B cells. Correction of the pHrodo-αIgM uptake value for the number of surface IgM molecules (uptake index) confirmed this to be the case (Figure 1D). After correcting for the level of surface IgM expression, the pHrodo-αIgM signal, which reflects accumulation in acidified endosomes, was 3.04 times more efficient in CLL compared to normal B cells (Figure 1D, P<0.0001). The uptake index varied considerably between patients, but was significantly higher in those whose BCRs lacked the ability to mobilize calcium in response to BCR ligation and cases with low CD38 expression (see Online Supplementary Figure S2 and Online Supplementary Table S1).
Figure 1.
B-cell receptor expression and internalization in CLL patients and healthy controls. (A) PBMCs from CLL patients (n=40) and healthy controls (n=19) were incubated with pHrodo-αIgM and the MFI of cells internalizing pHrodo-labeled avidin was measured within the CD19+5+ (CLL) and CD19+ (normal) B-cell population by flow cytometry. (B) B-cell receptor (BCR) internalization correlates with sIgM expression (Pearson’s correlation); correlation plot comparing the relationship between pHrodo-αIgM uptake and surface IgM (sIgM) expression in CD19+5+ cells derived from 40 CLL patients. (C) Although the level of pHrodo-αIgM uptake was comparable between CLL patients and healthy controls, marked differences in surface expression (sIgM) were observed (P=0.0001). (D) After correcting the level of uptake per molecule of sIgM, the uptake index was measured in both CLL patients and healthy controls. Within the CLL patient subsets, CLL B cells from CD38 negative, anergic and mutated patients were identified as having a greater uptake index than their counterparts, although mutational status was not significant (P=0.05, P=0.05 and P=0.436, respectively; unpaired t-test). (E) To assess the rate of BCR internalization more directly, the disappearance of sIgM following ligation of agonistic αIgM was measured. Accelerated BCR endocytosis was detected (2 min time point) in anergic B cells (n=6) compared to signaling competent (n=6) and normal (n=6) B cells (P=0.02 and P=0.01, respectively*; Mann-Whitney test). MFI: mean fluorescent intensity; MESF: molecules of equivalent soluble fluorochrome; CLL: chronic lymphocytic leukemia; SAV: streptavidin-APC.
Prolonged ex vivo incubation has previously been shown to reverse features of anergy, namely, re-expression of sIgM and restoration of BCR responsiveness.4,9 We therefore examined BCR expression and internalization after 24 hours ex vivo incubation, however, results were heterogeneous with no significant recovery in sIgM expression (Online Supplementary Figure S3A, P=0.83, n=7) or change in BCR internalization efficiency (Online Supplementary Figure S3B, P=0.88, n=7). It was not possible to assess internalization efficiency at later time points as the assay critically depends on metabolic integrity of the cells, which was not consistently maintained after 24 hours.
Since the pHrodo-αIgM signal reflects both uptake into and retention within acidic endosomes, we also assessed the BCR internalization rate more directly by measuring loss from the cell surface following ligation with an agonistic antibody. This showed that all cases of CLL internalize their BCRs more rapidly than normal B cells (Figure 1E; P=0.04, 2 minute time point). More subtle differences were observed between anergic and signal competent cases of CLL, with more rapid initial loss from the surface in the former (Figure 1E; P=0.02 at 2 minute time point).
We also measured sIgD expression and the levels of pHrodo-αIgD uptake using the same assays. All CLL B cells internalized pHrodo-αIgD, and pre-incubation with unlabeled αIgD reduced the level of uptake (Online Supplementary Figure S4A; n=18; P=0.001). No significant correlation between pHrodo-αIgD uptake and surface sIgD expression was observed (Online Supplementary Figure S4B) and there was no difference in the pHrodo-αIgD uptake index between CD38high and low, anergic/non anergic and IGHV-mutated and unmutated patients (Online Supplementary Figure S4C). This suggests that uptake and retention mechanisms differ between IgD and IgM; this was not addressed further herein.
Mechanism of dissociation of BCR signaling and internalization
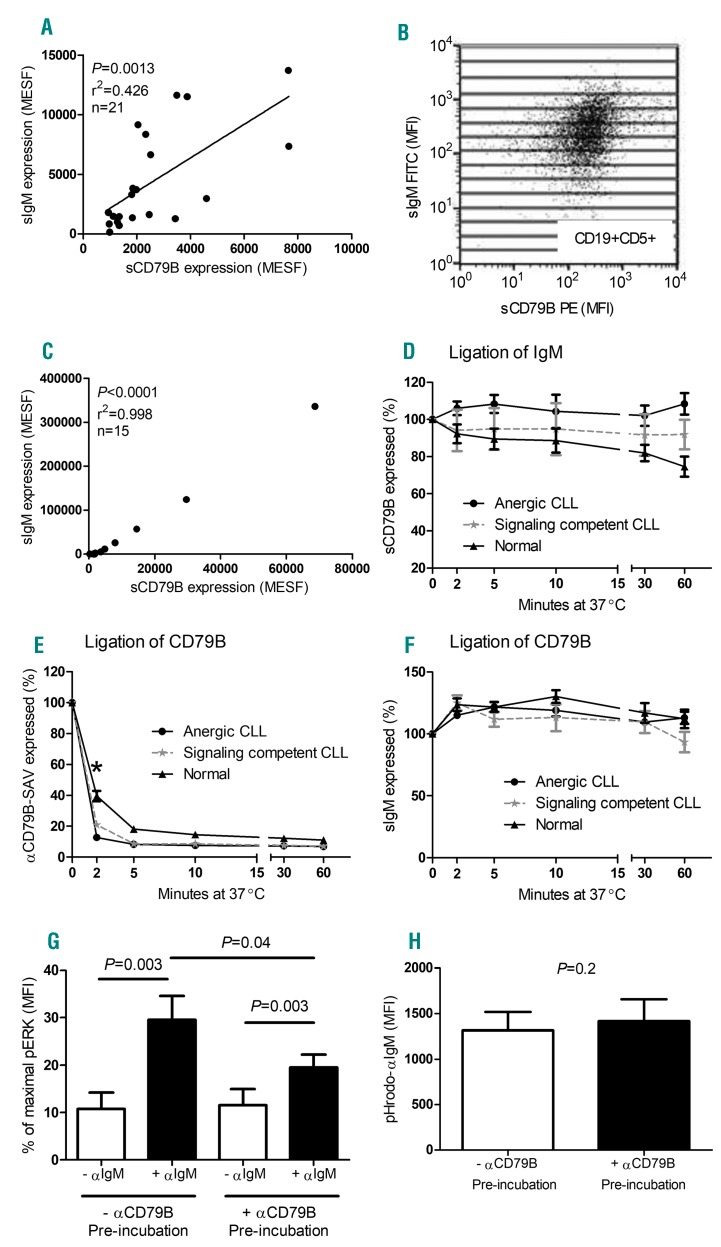
We next investigated the role of sCD79B, a molecule that is closely associated with the BCR and essential for signal transduction, in BCR internalization. As previously reported,27 CLL B cells express lower levels of sCD79B compared to normal B cells, with a particularly reduced expression in anergic compared to signaling competent cases (normal B-cell MFI=2561.2±400.9, anergic CLL MFI=318.2±63.4, signaling competent MFI=397.5±71.1). We also found that sIgM expression correlates with sCD79B both between (Figure 2A) and within a patient with CLL (Figures 2B,C). Despite these findings and the fact that sIgM and sCD79B are known to be associated during BCR signaling, no significant reduction in sCD79B expression occurred following ligation and downregulation of sIgM with agonistic antibody in both anergic and signaling competent cases of CLL (Figure 2D). In normal B cells, a slight but significant reduction in sCD79B expression was observed 30 and 60 minutes after BCR ligation with αIgM. Similarly, downregulation of CD79B with an agonistic anti-CD79B had no effect on the level of sIgM in anergic and signaling competent CLL cells and normal B cells (Figure 2E,F). Thus, although both normal and CLL B cells are capable of internalizing IgM and CD79B following ligation with cognate agonistic antibody, the two molecules do not cointernalize, and thus cannot be closely associated during membrane trafficking.
Figure 2.
Dissociation of BCR signaling and internalization in CLL cells. (A) A correlation was detected between the expression levels of surface (s)IgM and sCD79B on CD19+5+ cells derived from 21 CLL patients (both anergic and signaling competent B cells, Pearson’s correlation). Values are expressed as molecules of equivalent soluble fluorochrome (MESF) and derived from mean fluorescence intensity (MFI) values. (B) To examine the relationship within an individual patient, 15 subsets of CD19+5+ cells were created, as defined by increasing sIgM expression, and mean sCD79B MFI values were recorded within the same gate. (C) A representative patient demonstrating a correlation between sIgM and sCD79B. (D) The percentage of sCD79B receptor expression was measured on CLL and normal B cells following agonistic αIgM ligation; a gradual reduction and internalization of sCD79B on normal CD19+ B cells was detected after 60 min incubation (n=6; P=0.03*; Wilcoxon matched pairs test). In addition, CD79B internalization (E) and sIgM receptor expression (F) was measured upon αCD79B ligation, and compared between anergic, signaling competent and normal B cells. CD79B internalization occurred more rapidly (2 min time point) in anergic B cells compared to signaling competent and normal B cells (P=0.01 and P=0.001, respectively*; Mann-Whitney test), however, the percentage of sIgM receptor expression remained unchanged in all CLL cases. Finally, signaling competent CLL B cells were pre-incubated with agonistic αCD79B for 10mins at 37°C prior to αIgM stimulation to determine the effect of CD79B internalization on αIgM-induced pERK activation (G: n=9; pERK levels were normalized to the positive control), as well as pHrodo-αIgM uptake (H: n=9). Statistical analysis was performed via Wilcoxon matched pairs test. CLL: chronic lymphocytic leukemia; SAV: streptavidin-APC.
Having established that sCD79B is not required for sIgM internalization, we proceeded to assess its role in BCR signaling by measuring the effect of prior sCD79B downregulation on αIgM ERK phosphorylation in CLL cells. As expected, prior sCD79b downregulation reduced, but did not abolish αIgM-induced ERK phosphorylation (Figure 2G). In contrast, depletion of sCD79B was without effect on pHrodo-αIgM uptake (Figure 2H).
In vivo relationship between BCR signaling and internalization
We next investigated the relationship between BCR internalization and signaling in vivo in CLL by studying subsets of PB cells, those from the LN where BCR activation and proliferation is thought to take place and longitudinal samples obtained from patients being treated with BTKis ibrutinib and acalabrutinib.
Recently proliferated and quiescent subsets
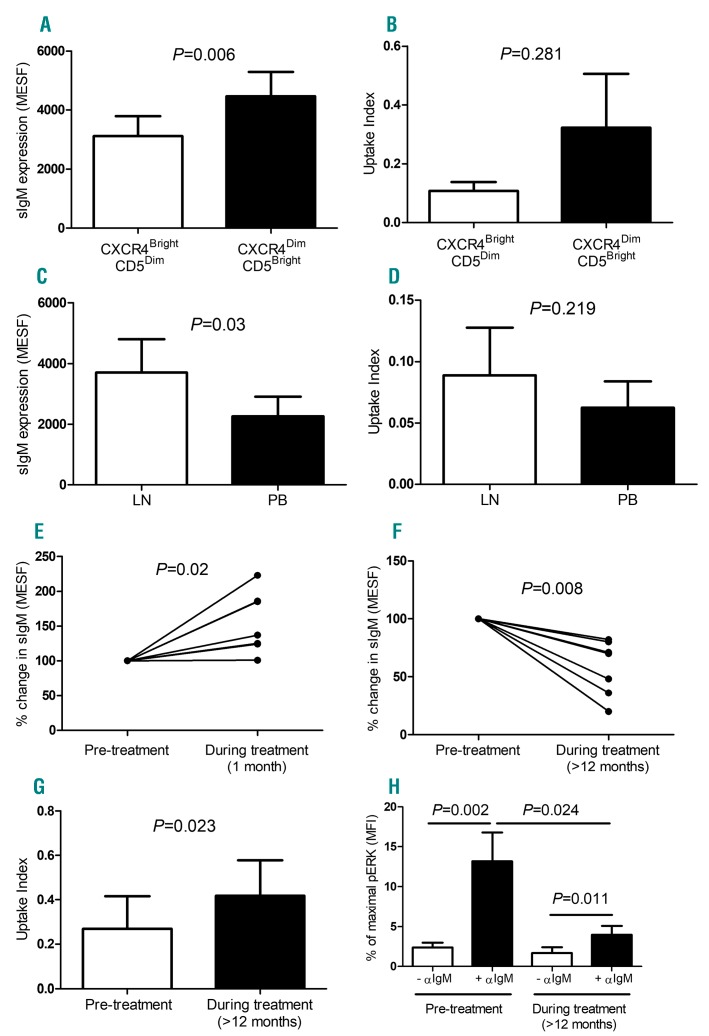
It has previously been shown that recently proliferated LN emigrants express low levels of CXCR4 and high levels of CD5 (CXCR4dimCD5bright).28 Since proliferation within LNs is linked to BCR signaling, we compared BCR expression and internalization efficiency on CXCR4dimCD5bright and CXCR4brightCD5dim subsets. Since the BCR internalization assay involves incubation with agonistic αIgM, we first investigated whether 1-hour incubation with pHrodo-αIgM altered the expression of these markers. As expected, CXCR4 expression was reduced and CD5 increased following ligation of BCR, however, over the 1-hour assay period, the magnitude of change was small (CXCR4 mean fold change = 0.93±0.02 and CD5 mean fold change 1.24±0.04, data not shown). Since the MFI of the top and bottom decade of CXCR4 and CD5 expression differed by 11.8±5.4-fold and 8.7±6.7-fold, respectively, assay-induced changes could not have changed the composition of these subsets. Since the CXCR4dimCD5bright subset are thought to have recently undergone BCR activation within the LN, we expected to find downregulation of sIgM in this fraction, however, this was not the case and levels were higher than in the CXCR4dimCD5bright subset (Figure 3A, P<0.0001). In keeping with post-activation anergy, in the majority of patients (14/21) there was more efficient uptake of pHrodo-αIgM in the CXCR4dimCD5bright subset, however, there was significant variability and, in the whole population, this did not reach statistical significance (Figure 3B; n=21; P=0.281).
Figure 3.
In vivo relationship between BCR signaling and internalization in CLL cells. BCR expression and uptake index were investigated firstly in subsets of peripheral blood (PB) cells (CXCR4brightCD5dim and CXCR4dimCD5bright expressing CLL cells (A-B), and secondly in CD19+CD5+ cells from the matched lymph node (LN) and PB samples from seven unmutated CLL patients (C-D). Thirdly, sIgM expression was measured in CD19+CD5+ PB cells from CLL patients before, one month and at least 12 months after commencing BTKi treatment (ibrutinib, n=6 or acalabrutinib, n=1; presented as a percentage change in surface (s)IgM expression (E-F). Uptake index (G) and the capacity to induce pERK activation following BCR stimulation (H: pERK levels normalized to PMA/positive control) was also examined and compared in CLL B cells, pre- and during treatment. Statistical analysis was performed via Wilcoxon matched pairs test. MFI: mean fluorescent intensity; MESF: molecules of equivalent soluble fluorochrome.
BCR expression and internalization in the PB and LN of CLL patients
Since our results suggested that BCR internalization is influenced by the capacity to initiate downstream signaling, which occur within LNs, we went on to directly compare sIgM levels and uptake of pHrodo-αIgM by LN CLL cells to those derived from simultaneously obtained PB from the same patients. As we have previously shown,29 LN CLL cells expressed higher levels of CD5 than those derived from the PB, in keeping with BCR activation at these sites (data not shown). As was the case for the recently proliferated CXCR4dimCD5bright subset, sIgM levels were significantly higher on cells derived from the LN compared to PB (Figure 3C; P=0.03, n=7; Online Supplementary Figure S5). Again, there was great variability in pHrodo-αIgM uptake efficiency with higher levels in the LN than PB in five out of seven patients, but no significant difference overall (Figure 3D; P=0.218, n=7).
BTKi-treated CLL B-cells display features of anergy
We next investigated the effect of BCR pathway blockade on BCR expression and function. PB samples were collected over time from BTKi-treated patients (ibrutinib and acalabrutinib) with a prolonged lymphocytosis for a minimum of 12 months (Online Supplementary Table S2). On comparison of CD19+CD5+ CLL B-cells before and after one month of treatment, most cases showed an initial increase in sIgM expression (Figure 3E; P=0.02, n=7) followed by a decrease in sIgM levels after at least 12 months of treatment (Figure 3F; P=0.008, n=7). After 12 months or more of BTKi therapy, CLL B cells exhibited more efficient BCR internalization (Figure 3G; P=0.023, n=7) and had reduced ability to activate ERK following BCR stimulation (Figure 3H; P=0.024, n=12).
Discussion
In the study herein, we investigated the capacity of normal and CLL B cells to internalize ligands that bind to the BCR. Using two complementary techniques, we showed that BCR internalization and transit to acidified endosomes occurs in both normal and CLL B cells. When corrected for the level of sIgM expression, we found that BCR internalization and accumulation in endosomes is three times more efficient in CLL than normal B cells, and is highest of all in cases with anergic BCRs. Using agonistic antibodies to sIgM and sCD79B we also showed that internalization of sIgM is not accompanied by internalization of CD79B and vice versa. A comparison of LN with PB CLL cells and PB CXCR4dimCD5bright cells, representing recently proliferated LN emigrants,28 with the CXCR4dimCD5bright subset showed that both the LN and recent emigrants express higher levels of sIgM, however, no consistent difference in BCR internalization efficiency was observed. This was a surprising finding given that BCR activation takes place in the LN and should result in the downregulation of sIgM. Finally, long-term in vivo inhibition of BCR signaling using BTKis resulted in an increase in the number of CLL cells exhibiting features of anergy, including a reduction in sIgM expression and signaling competence as well as an increase in the efficiency of BCR internalization.
These results have a number of implications. First, it is clear that in CLL, BCR internalization is uncoupled from downstream signaling, since anergic CLL B cells internalize their BCRs more efficiently than non-anergic cases and normal B cells. These findings are in keeping with previous murine studies which showed that reduced surface BCR expression and uncoupling of signaling in normal anergic B cells is due to rapid internalization and retention in endosomes.30 Since an enhanced accumulation of BCR in endosomes was observed in every patient studied, the present results show that, at least in this respect, all cases of CLL show some features of anergy.
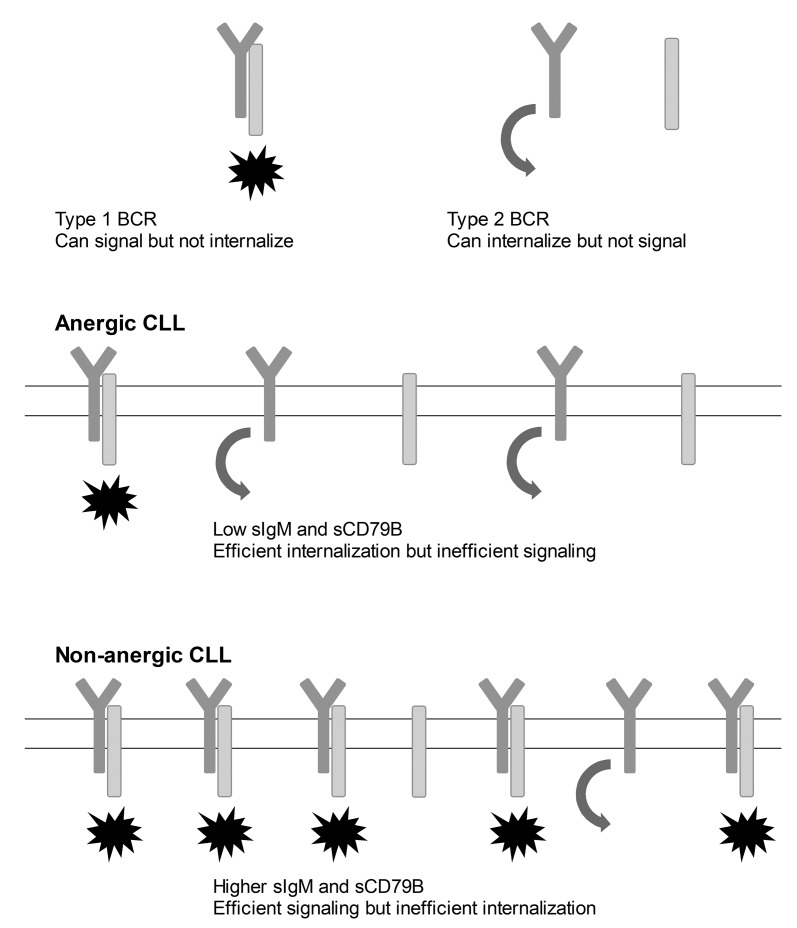
Second, although CD79B is known to associate with sIgM and is essential for the export of IgM to the cell surface and downstream BCR signaling, our data strongly suggest that in CLL B cells, they internalize independently, and thus cannot be associated during endocytosis. In normal B cells a small proportion of sCD79B and sIgM co-internalized, again suggesting minimal association of the two during endocytosis. A plausible explanation for these observations is that BCRs exist in two configurations in CLL (Figure 4); the first in which sIgM and sCD79B are not and do not become associated following ligand binding and a second in which sIgM and sCD79B are already colocalized or are induced to associate following activation. The first configuration could not transduce downstream signals but, because retention of sIgM at the surface requires association with CD79B, would internalize efficiently. Adversely, BCRs in which sIgM and CD79B are associated could initiate signaling, however, the association with CD79B would favor retention at the cell surface, at least until the subunits undergo phosphorylation-induced dissociation.31 Such a model has already been proposed for normal B cells,32 and is thought to favor signaling in response to low-affinity ligands, such as autoantigens of the type recognized by CLL BCRs.33
Figure 4.
Proposed mechanism for the dissociation of BCR signaling and internalization in CLL. Two alternative configurations of the BCR are proposed. Type 1 in which CD79B and surface (s)IgM are already closely associated or become associated following ligand binding. This form of the receptor can transduce downstream signals, but is not internalized until the subunits dissociate. Type 2 BCRs do not contain CD79B either before or after ligand binding. These receptors therefore cannot signal but can become internalized. Possible BCR compositions in anergic and non-anergic CLL are illustrated. In anergic CLL, both sIgM and sCD79B levels are low and there is little potential for association between the two either before or after ligand binding. In this scenario, downstream BCR signaling is minimal but internalization is efficient. In non-anergic cases of CLL, both sIgM and sCD79B levels are higher and there is a greater likelihood of the two becoming associated. Signaling is thus relatively more efficient but the capacity for internalization is less. CLL: chronic lymphocytic leukemia; BCR: B-cell receptor.
It is currently believed that BCR activation takes place in LNs and that this, combined with other signals from the microenvironment, leads to tumor proliferation. In other receptor systems, ligand binding is generally accompanied by downregulation of cell-surface receptors,34 and our observation that sIgM levels are actually higher in the LN than PB and in recently proliferated CXCR4dimCD5bright compared to CXCR4dimCD5bright PB CLL cells was therefore unexpected. As recently suggested by others, it is possible that the elevated sIgM levels observed within the LN and CXCR4dimCD5bright subsets are due to IL4-induced upregulation of CD79B within lymphoid tissues.16,17
Our findings also shed further light on the mechanism of action of BTKis. As noted by others,13,35 a persistent low-level lymphocytosis is frequently seen in such patients and complete remissions are rare. In the early period following the commencement of BTKi therapy, we observed an increase in sIgM expression in PB CLL cells. Since we have shown that cells in the LNs express higher levels of sIgM than those in the PB, this most likely reflects the previously documented redistribution of the tumor from the former compartment to the later.35 Over the longer term, however, the opposite is the case, with a significant reduction in sIgM that is accompanied by an increase in BCR internalization efficiency and a reduced capacity to phosphorylate ERK. These findings indicate that long-term BTKi therapy causes the emergence of a population of neoplastic B cells with anergic phenotypic and functional properties. This may occur either through reprograming of the tumor into a more anergic state or because there is subclonal heterogeneity within the tumor and selection of cells with anergic features by BTKi therapy. The latter possibility is supported by in vitro studies of leukemic B-cell migration29 and in vivo observations using heavy water or glucose labeling36 that suggest the existence of subclonal heterogeneity at a functional level in CLL.
Finally, since BCR internalization into endosomes is the first event in antigen processing, our data support the theory that, under some circumstances, CLL B cells might act as antigen presenting cells. We have recently shown that LN derived CLL B cells express higher levels of costimulatory molecules, form immune synapses and stimulate an allogeneic mixed lymphocyte reaction more efficiently than those from the PB.29 In addition, elution of peptides from CLL major histocompatibility complex (MHC) class II reveals presentation of a range of autologous peptides,37 with evidence for expansion of cognate T-cell clones in CLL but not normal PB. Furthermore, a number of groups have documented the presence of T cells in CLL patients that are capable of responding to a range of other molecules, including Rh antigen,38 tumor idiotype23 immunoglobulin framework,39 or CDR3 motifs40 as well as broader responses against tumor lysates41 or intact leukemic cells.42 It is therefore plausible that the abnormal phenotype, repertoire and function of CLL T cells might be a consequence of excessive and aberrant antigen presentation occurring within lymphoid tissues.
In summary, we have shown that, as in normal B-cell anergy, CLL B cells internalize ligands that bind to the BCR more efficiently than normal. This process is uncoupled from downstream signaling and does not involve association with CD79B. We demonstrate that BTKi therapy induces or selects for cells with anergic properties that persist in the long term. Understanding how this occurs will be important in order to optimize the efficacy of this important new class of drugs.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/3/497
Funding
This work was supported by research funding from Bloodwise (grant number: 15012) and the British Society of Haematology (BSH; grant number: 34721/start up). The authors also acknowledge financial support from the UK Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
References
- 1.Gobessi S, Laurenti L, Longo PG, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23(4):686–697. [DOI] [PubMed] [Google Scholar]
- 2.Muzio M, Apollonio B, Scielzo C, et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood. 2008;112(1):188–195. [DOI] [PubMed] [Google Scholar]
- 3.Zhuang J, Hawkins SF, Glenn MA, et al. Akt is activated in chronic lymphocytic leukemia cells and delivers a pro-survival signal: the therapeutic potential of Akt inhibition. Haematologica. 2010;95(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apollonio B, Scielzo C, Bertilaccio MT, et al. Targeting B-cell anergy in chronic lymphocytic leukemia. Blood. 2013;121(19):3879–3888, S1-8. [DOI] [PubMed] [Google Scholar]
- 5.Hewamana S, Alghazal S, Lin TT, et al. The NF-kappaB subunit Rel A is associated with in vitro survival and clinical disease progression in chronic lymphocytic leukemia and represents a promising therapeutic target. Blood. 2008;111(9):4681–4689. [DOI] [PubMed] [Google Scholar]
- 6.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374(4):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesano A, Perbellini O, Evensen E, et al. Association between B-cell receptor responsiveness and disease progression in B-cell chronic lymphocytic leukemia: results from single cell network profiling studies. Haematologica. 2013;98(4):626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mockridge CI, Potter KN, Wheatley I, Neville LA, Packham G, Stevenson FK. Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood. 2007;109(10):4424–4431. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118(16):4313–4320. [DOI] [PubMed] [Google Scholar]
- 11.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guieze R, Robbe P, Clifford R, et al. Presence of multiple recurrent mutations confers poor trial outcome of relapsed/refractory CLL. Blood. 2015;126(18):2110–2117. [DOI] [PubMed] [Google Scholar]
- 13.Woyach JA, Smucker K, Smith LL, Lozanski A, Zhong Y, Ruppert AS, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014. March 20;123(12):1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vuillier F, Dumas G, Magnac C, et al. Lower levels of surface B-cell-receptor expression in chronic lymphocytic leukemia are associated with glycosylation and folding defects of the mu and CD79a chains. Blood. 2005;105(7):2933–2940. [DOI] [PubMed] [Google Scholar]
- 15.Williams GT, Venkitaraman AR, Gilmore DJ, Neuberger MS. The sequence of the mu transmembrane segment determines the tissue specificity of the transport of immunoglobulin M to the cell surface. J Exp Med. 1990;171(3):947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo B, Zhang L, Chiorazzi N, Rothstein TL. IL-4 rescues surface IgM expression in chronic lymphocytic leukemia. Blood. 2016;128(4):553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguilar-Hernandez MM, Blunt MD, Dobson R, et al. IL-4 enhances expression and function of surface IgM in CLL cells. Blood. 2016;127(24):3015–3025. [DOI] [PubMed] [Google Scholar]
- 18.Krysov S, Potter KN, Mockridge CI, et al. Surface IgM of CLL cells displays unusual glycans indicative of engagement of antigen in vivo. Blood. 2010;115(21):4198–4205. [DOI] [PubMed] [Google Scholar]
- 19.Coelho V, Krysov S, Steele A, et al. Identification in CLL of circulating intraclonal subgroups with varying B-cell receptor expression and function. Blood. 2013;122(15):2664–2672. [DOI] [PubMed] [Google Scholar]
- 20.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghia P, Strola G, Granziero L, et al. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. Eur J Immunol. 2002;32(5):1403–1413. [DOI] [PubMed] [Google Scholar]
- 22.Patten PE, Buggins AG, Richards J, et al. CD38 expression in chronic lymphocytic leukemia is regulated by the tumor microenvironment. Blood. 2008;111(10):5173–5181. [DOI] [PubMed] [Google Scholar]
- 23.Os A, Burgler S, Ribes AP, et al. Chronic lymphocytic leukemia cells are activated and proliferate in response to specific T helper cells. Cell Rep. 2013;4(3):566–577. [DOI] [PubMed] [Google Scholar]
- 24.Pascutti MF, Jak M, Tromp JM, et al. IL-21 and CD40L signals from autologous T cells can induce antigen-independent proliferation of CLL cells. Blood. 2013;122(17):3010–3019. [DOI] [PubMed] [Google Scholar]
- 25.Ghia P, Stamatopoulos K, Belessi C, et al. ERIC recommendations on IGHV gene mutational status analysis in chronic lymphocytic leukemia. Leukemia. 2007;21(1):1–3. [DOI] [PubMed] [Google Scholar]
- 26.Krober A, Seiler T, Benner A, et al. V(H) mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood. 2002;100(4):1410–1416. [PubMed] [Google Scholar]
- 27.Cabezudo E, Carrara P, Morilla R, Matutes E. Quantitative analysis of CD79b, CD5 and CD19 in mature B-cell lymphoproliferative disorders. Haematologica. 1999;84(5):413–418. [PubMed] [Google Scholar]
- 28.Calissano C, Damle RN, Hayes G, et al. In vivo intraclonal and interclonal kinetic heterogeneity in B-cell chronic lymphocytic leukemia. Blood. 2009;114(23):4832–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasikowska M, Walsby E, Apollonio B, et al. Phenotype and immune function of lymph node and peripheral blood CLL cells are linked to transendothelial migration. Blood. 2016;128(4):563–573. [DOI] [PubMed] [Google Scholar]
- 30.Blery M, Tze L, Miosge LA, Jun JE, Goodnow CC. Essential role of membrane cholesterol in accelerated BCR internalization and uncoupling from NF-kappa B in B cell clonal anergy. J Exp Med. 2006;203(7):1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilen BJ, Nakamura T, Cambier JC. Antigen-stimulated dissociation of BCR mIg from Ig-alpha/Ig-beta: implications for receptor desensitization. Immunity. 1999;10(2):239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou P, Araujo E, Zhao T, et al. B cell antigen receptor signaling and internalization are mutually exclusive events. PLoS Biol. 2006;4(7):e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanemo Myhrinder A, Hellqvist E, Sidorova E, et al. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood. 2008;111(7):3838–3848. [DOI] [PubMed] [Google Scholar]
- 34.Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb Perspect Biol. 2013;5(5):a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herman SE, Niemann CU, Farooqui M, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia. 2014;28(11):2188–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herndon TM, Chen SS, Saba NS, et al. Direct in vivo evidence for increased proliferation of CLL cells in lymph nodes compared to bone marrow and peripheral blood. Leukemia. 2017;31(6):1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kowalewski DJ, Schuster H, Backert L, et al. HLA ligandome analysis identifies the underlying specificities of spontaneous antileukemia immune responses in chronic lymphocytic leukemia (CLL). Proc Natl Acad Sci U S A. 2015;112(2):E166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall AM, Vickers MA, McLeod E, Barker RN. Rh autoantigen presentation to helper T cells in chronic lymphocytic leukemia by malignant B cells. Blood. 2005;105(5):2007–2015. [DOI] [PubMed] [Google Scholar]
- 39.Trojan A, Schultze JL, Witzens M, et al. Immunoglobulin framework-derived peptides function as cytotoxic T-cell epitopes commonly expressed in B-cell malignancies. Nat Med. 2000;6(6):667–672. [DOI] [PubMed] [Google Scholar]
- 40.Rezvany MR, Jeddi-Tehrani M, Rabbani H, et al. Autologous T lymphocytes may specifically recognize leukaemic B cells in patients with chronic lymphocytic leukaemia. Br J Haematol. 2000;111(2):608–617. [DOI] [PubMed] [Google Scholar]
- 41.Goddard RV, Prentice AG, Copplestone JA, Kaminski ER. Generation in vitro of B-cell chronic lymphocytic leukaemia-proliferative and specific HLA class-II-restricted cytotoxic T-cell responses using autologous dendritic cells pulsed with tumour cell lysate. Clin Exp Immunol. 2001;126(1):16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krackhardt AM, Harig S, Witzens M, Broderick R, Barrett P, Gribben JG. T-cell responses against chronic lymphocytic leukemia cells: implications for immunotherapy. Blood. 2002;100(1):167–173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.