Abstract
Aprior phase I/II trial of bortezomib/tacrolimus/methotrexate prophylaxis after human leukocyte antigen (HLA)-mismatched reduced intensity conditioning allogeneic hematopoietic stem cell transplantation documented low acute graft-versus-host disease incidence, with promising overall and progression-free survival. We performed an open-label three-arm 1:1:1 phase II randomized controlled trial comparing grade II–IV acute graft-versus-host disease between conventional tacrolimus/methotrexate (A) versus bortezomib/tacrolimus/methotrexate (B), and versus bortezomib/sirolimus/tacrolimus (C), in reduced intensity conditioning allogeneic transplantation recipients lacking HLA-matched related donors. The primary endpoint was grade II–IV acute graft-versus-host disease incidence rate by day +180. One hundred and thirty-eight patients (A 46, B 45, C 47) with a median age of 64 years (range: 24–75), varying malignant diagnoses and disease risk (low 14, intermediate 96, high/very high 28) received 7–8/8 HLA-mismatched (40) or matched unrelated donor (98) grafts. Median follow up in survivors was 30 months (range: 14–46). Despite early immune reconstitution differences, day +180 grade II-IV acute graft-versus-host disease rates were similar (A 32.6%, B 31.1%, C 21%; P=0.53 for A vs. B, P=0.16 for A vs. C). The 2-year non-relapse mortality incidence was similar (A 14%, B 16%, C 6.4%; P=0.62), as were relapse (A 32%, B 32%, C 38%; P=0.74), chronic graft-versus-host disease (A 59%, B 60% C 55%; P=0.66), progression-free survival (A 54%, B 52%, C 55%; P=0.95), and overall survival (A 61%, B 62%, C 62%; P=0.98). Overall, the bortezomib-based regimens evaluated did not improve outcomes compared with tacrolimus/methotrexate therapy. clinicaltrials.gov Identifier: 01754389
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is curative in advanced or aggressive hematologic malignancies despite associated toxicities. While a sibling matched at human leukocyte antigen (HLA)-A, -B, -C, and -DRB1 is optimal, only a minority of patients who may benefit from HSCT have such a donor available.1 Utilizing a 7/8 HLA-matched graft increases the likelihood of obtaining an adult donor for all racial and ethnic groups,1 but at the expense of worse outcomes. In reduced-intensity conditioning (RIC) HSCT, retrospective registry studies document increased rates of grade II-IV acute graft-versus-host disease (aGvHD) and non-relapse mortality (NRM), with worse progression-free survival (PFS) and overall survival (OS) for 7/8 vs. 8/8 HLA-matched donors.2
The proteasome-inhibitor bortezomib (bort) can selectively deplete proliferating alloreactive T lymphocytes, reduce Th1 cytokines and Interleukin-6 levels, and block antigen-presenting cell (APC) activation.3–6 It can also spare regulatory T cells (Tregs) that are relevant in GvHD control.7 Administered early after graft infusion, short-course bort can control GvHD in major histocompatibility complex (MHC)-mismatched mouse HSCT and maintain graft-versus-tumor responses,6,8,9 while avoiding the pro-inflammatory colonic toxicity of delayed or prolonged bort.5,8,10
In a phase I/II study of RIC we documented that bort-based GvHD prophylaxis (bort 1.3 mg/m2 IV on d +1, +4, +7, plus conventional tacrolimus [tax] and methotrexate [mtx]) was safe and potentially efficacious in T-replete HLA-mismatched donor (MMD) transplantation, with survival comparable to HLA-matched cohorts.11,12 These data were used to support the inclusion of the bort-based regimen as one novel arm of an ongoing multicenter phase II randomized trial, comparing it to two other novel regimens: post-transplant cyclophosphamide (PTCy), and maraviroc, each compared to a non-randomized ‘conventional care’ cohort (The Blood and Marrow Transplant Clinical Trials Network [BMTCTN] 1203).
We additionally conducted a phase II randomized control trial (RCT) for patients lacking 8/8 HLA-matched sibling donors (DFCI 12–404), directly randomizing conventional tac/mtx vs. two novel bort-based GvHD prophylaxis regimens, whose mature results we report herein. The regimens comprised bort plus tac/mtx (arm B), directly based on our phase I/II data; and bort plus sirolimus (sir)/tac (arm C), to explore the tolerizing effects of enhanced Treg sparing with combined proteasome- and mechanistic target of rapamycin (mTOR)-inhibition during early immune reconstitution.7,13 We chose bort/sir/tac instead of bort-based calcineurin inhibitors (CNI)-free prophylaxis (e.g., bort/sir/mycophenolate mofetil [MMF]) as the sir/MMF doublet has limited clinical efficacy,14 and in our experience, adding bort to sir/MMF did not provide adequate efficacy in GvHD prevention, while the sir/tac doublet has documented efficacy comparable to conventional tac/mtx.15
Methods
This prospective clinical trial was approved by the institutional review board of the Dana-Farber Cancer Institute/Harvard Cancer Center. Written informed consent was obtained prior to patient enrollment.
Study Design
The study was a one-stage randomized phase II trial with the primary objective of comparing the incidence of aGvHD in two bort-based GvHD prophylaxis regimens to conventional tac/mtx. The study was designed for the primary comparisons of the grade II-IV aGvHD rates in Arm A vs. B and Arm A vs. C in parallel, and was powered to test the hypothesis of superiority in Arm B and C (15%), compared to that in Arm A (40%). The accrual goal was 138 patients, randomizing all eligible patients in 1:1:1 ratio to the three regimens. Randomization was stratified by degree of HLA-match (8/8 vs. 7/8).
Patients
Adult hematologic malignancy patients lacking an available HLA-matched sibling with an available 8/8 (HLA-A, -B, -C, -DRB1) matched unrelated donor (MUD) or 1-antigen/allele mismatched related donor (MMRD) or mismatched unrelated donor (MMUD) were enrolled on the trial between January 2013 and November 2015. Patients with HIV infection, active hepatitis B or C disease, abnormal renal (serum creatinine > upper limit of normal [ULN] with clearance <40 mL/min/1.73m2 body surface area [BSA]) or liver function (serum total bilirubin >ULN, serum alanine/aspartate aminotransferase >2x ULN), Eastern Cooperative Oncology Group (ECOG) performance status >2, hyperlipidemia (serum cholesterol >300mg/dL; trigylcerides >400mg/dL) despite therapy, and peripheral neuropathy ≥grade 2 within 21 days prior to transplantation were excluded.
Transplantation
Conditioning comprised busulfan (0.8 mg/kg twice daily IV) and fludarabine (30 mg/m2 once daily IV) from days −5 to −2. T-replete donor peripheral blood stem cells (PBSC) dosed at ≥ 2×106 CD34+ cells/kg were infused on day 0. GvHD prophylaxis regimens were: tac/mtx (arm A), bort/tac/mtx (arm B), and bort/sir/tac (arm C). Dosing was: bort (1.3 mg/m2 IV on day +1, +4, +7), mtx (10 mg/m2 IV on day +1, 5 mg/m2 on day +3, +6, +11), sir (target trough level 5–12 ng/ml) and/or tac (target trough level 5–10 ng/ml) from day −3. The tapering of immunosuppression started at around d+100, with the aim of being off immune suppression (IS) by day +180, as applicable per treatment arm.
Participants received levetiracetam for seizure prophylaxis from day −5 to −1 and filgrastim 5 μg/kg daily from day +1 until an absolute neutrophil count (ANC) >1500 cells/μl was attained, and at least 12 months of Pneumocystis jiroveci and herpes simplex virus (HSV)/varicella zoster virus (VZV) prophylaxis. Anti-fungal prophylaxis was not routine.
Immunophenotyping
CD4+ T cells were defined as CD3+CD4+; CD8+ T cells were defined as CD3+CD8+; CD8+ naïve cells were defined as CD8+CD45RO-CD62L+; CD4 Tregs were defined as CD3+CD4+CD25med-highCD127low; CD4 conventional T cells (Tcons) were defined as CD3+CD4+CD25low-negCD127med-high; natural killer (NK) cells as CD56+CD3−; and B cells as CD19+. Aliquots of anti-coagulated whole blood (ethylenediaminetetraacetic acid [EDTA]) were incubated with fluorophore-conjugated monoclonal antibodies: anti-CD3 V450 (clone UCHT1, BD Biosciences), anti-CD4 APC-H7 (clone RPA-T4, BD Biosciences), anti-CD8 Pacific-Orange (clone RPA-T8, Biolegend), anti-CD25 PE-Cy7 (clone M-A251, BD Biosciences), anti-CD127 PE-Cy5 (clone eBioRDR5, eBioscience) for T-cell subsets; anti-CD56 PE (clone B159, BD Biosciences), anti-CD3 V450 (clone UCHT1, BD Biosciences) for NK/NKT cells, and anti-CD19 APC (clone HIB19, BD Biosciences) for B cells. RBC lysis with BD Pharm Lyse was performed either prior to or following incubation with conjugated antibodies. Flow cytometry analysis utilized FACSCanto II (BD Bioscience) or the Fortessa (BD Bioscience) and FACSDiva software (BD Bioscience). There was a change in the use of flow cytometry machines over the course of the study. Both flow cytometers were validated and results were comparable.
Statistical considerations
The primary endpoint was a grade II-IV aGvHD rate by day 180 after stem cell infusion. Secondary endpoints included cumulative incidence of aGvHD, NRM, relapse, chronic (c)GvHD, and PFS, OS and GvHD-free/relapse-free survival (GRFS). The study was designed for the primary comparisons of the grade II-IV aGvHD rates in arm A vs. B and arm A vs. C in parallel. We projected the incidence of grade II-IV aGvHD as 40% in arm A and 15% in arm B and in arm C. With the sample size of 46 per arm, there is an 80% power to detect a 25% difference in grade II-IV aGvHD rate between two arms. This power calculation is based on Fisher’s exact test at one-sided type I error rate of 0.05. The primary analysis was performed per the modified intent-to-treat principle (mITT), i.e., all patients who were randomized and received any amount of the study treatment were included in the primary analysis. Cumulative incidence of aGvHD and cGvHD, relapse and NRM were estimated in the competing risk framework. Relapse with or without death was considered a competing risk for NRM, and similarly NRM for relapse. Death or relapse without developing GvHD was a competing event for GvHD. GvHD incidences occurring after relapse, in the setting of relapse interventions (e.g., immunosuppression [IS] taper, donor lymphocyte infusion [DLI]), were counted in the estimation of cumulative incidence of GvHD in order to obtain comprehensive estimates, and both inclusive and exclusive estimates are presented for aGvHD endpoints. GRFS, PFS and OS were estimated using the Kaplan-Meier method. GRFS was defined as the time from stem cell infusion to incidence of grade III/IV aGvHD, cGvHD requiring systemic immunosuppression agents, relapse or death, whichever occured first. PFS and OS have been defined elsewhere. Cumulative incidences in the presence of competing events were compared using the Gray test, and Kaplan-Meier estimates were compared using the log-rank test.16 For time-to-event endpoints, P-values reflect comparing entire distributions along with point estimates for ease of presentation. For the primary endpoint, incidence rates of grade II-IV aGvHD by day +180 were compared at one-sided significance level of 0.05 using Fisher’s exact test; P-values for secondary endpoints are two-sided at the significance level of 0.05, without adjusting for multiple comparisons. Multivariable analysis adjusting for variables, as listed in Table 1, were performed for OS and PFS using a Cox model, and a Fine and Gray model was used for grade II-IV aGvHD.17 Immunophenotype data were compared using the Wilcoxon rank-sum test at each time point without adjusting for multiple comparisons. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) and R version 3.1.3 (the CRAN project).
Table 1.
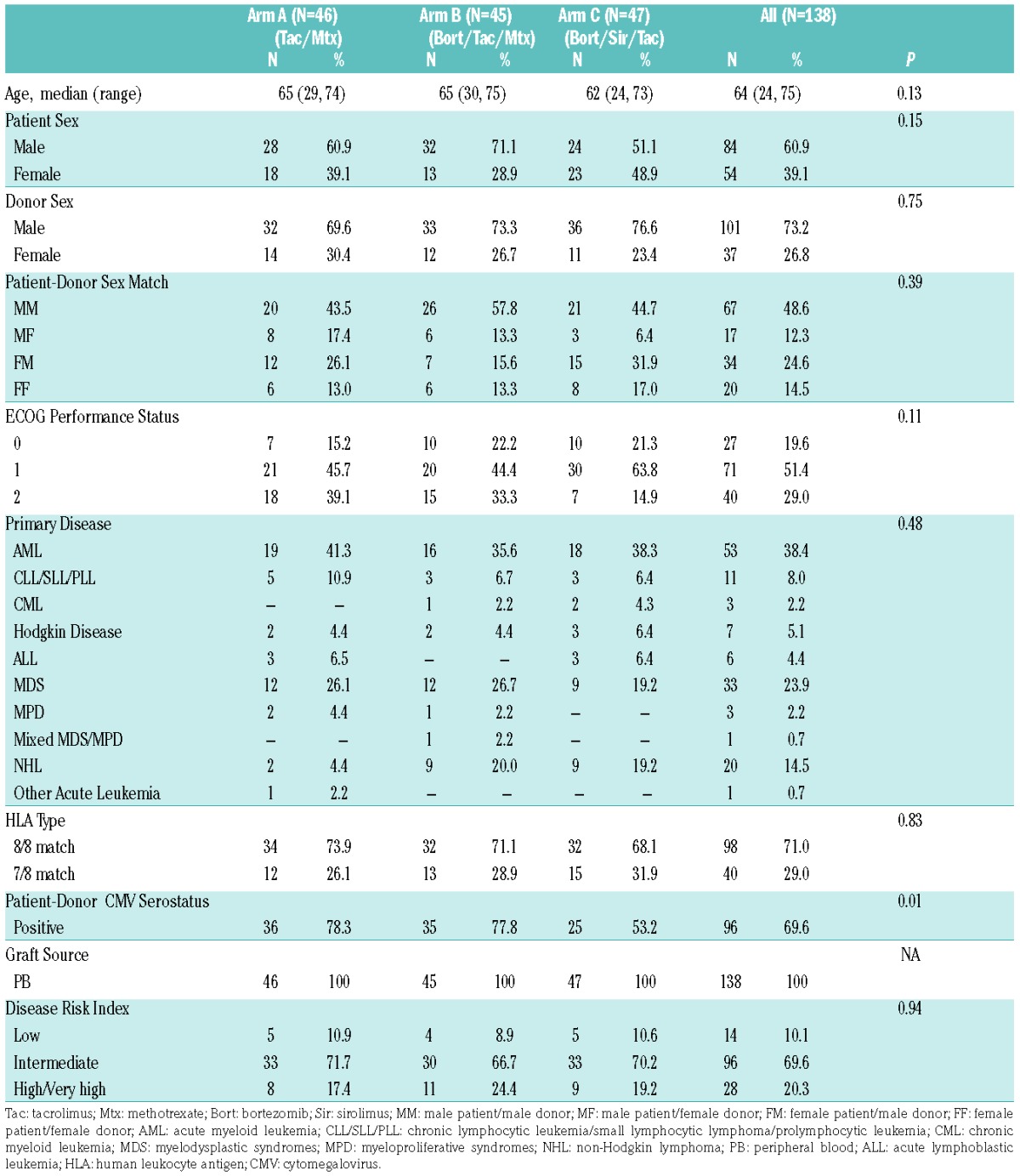
Baseline characteristics of the study cohorts: by treatment arms A, B, C, and overall.
Results
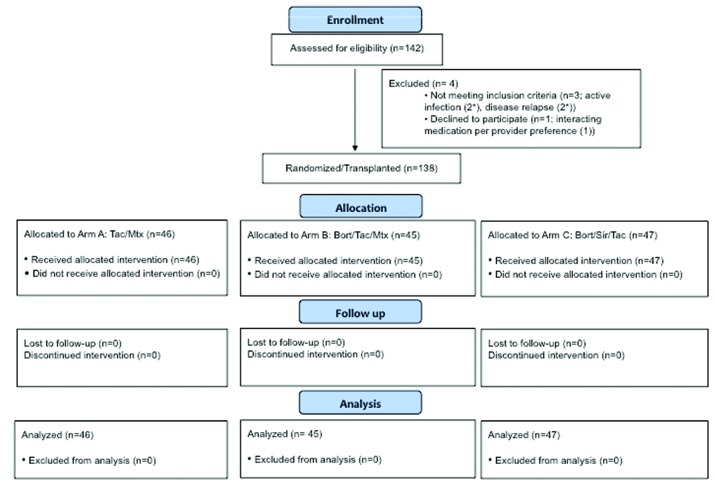
Patient and transplant variables: one hundred and forty-two subjects enrolled and 138 randomized subjects were evaluable per the protocol-specified mITT criteria (four cancelled study participation before receiving transplantation conditioning: two due to acute infection (one with subsequent disease relapse), one due to provider preference to continue protocol-excluded medications, and another due to disease relapse at transplantation admission) (Figure 1). The treatment arms (A 46, B 45, C 47) were balanced for pre-transplant variables (Table 1), except lower cytomegalovirus (CMV) seropositivity in arm C (A, 78.3% vs. B, 77.8% vs. C, 53.2%, P=0.01). Subjects had a median age of 64 years (range: 24–75), varying diagnoses (53 acute myeloid leukemia [AML], 33 myelodysplastic syndrome [MDS], 20 non-Hodgkin lymphoma [NHL], 11 chronic lymphocytic leukemia [CLL], etc.) and disease-risk indices (low 14, intermediate 96, high/very high 28). They received T-replete 8/8 MUD (n=98) or 7/8 MMD (n=40) PBSC grafts. Median follow up in survivors was 30 months (range: 14–46).
Figure 1.
CONSORT Diagram. *1 patient with relapse and infection. Tac: tacrolimus; Mtx: methotrexate; Bort: bortezomib; Sir: sirolimus.
Engraftment, chimerism, safety: the median time to neutrophil (>500/μl) and platelet engraftment (>20,000/μl) among patients who experienced count nadir (i.e., neutrophil count <500/vl and/or platelet count <20,000/μl) was 11 days (range: 2–45) and 19 days (range: 10–57), respectively, with no significant difference between treatment arms (P=0.9 and P=0.55 for neutrophils and platelets, respectively). For the entire cohort, 31% of patients did not experience count nadir (24% in arm A, 27% in arm B, 43% in arm C, P=0.11) and no subject failed neutrophil engraftment. Median total nucleated cell donor chimerism by day 30 was 96% (range: 42–100) and by day 100 was 97% (range: 0–100, with no significant difference between treatment arms (P=0.84 and P=0.83, respectively). The bort-based regimens were well tolerated. No bort doses were delayed or reduced due to toxicity. No serious adverse event (SAE) attributable to bort (e.g., neuropathy) was documented. Common Terminology Criteria for Adverse Events (CTCAE) grade ≥3 organ dysfunction (hepatic, renal, pulmonary) did not differ significantly between treatment arms, including the incidence of acute kidney injury (AKI, 8.7% overall), thrombotic microangiopathy/hemolytic-uremic syndrome (TMA/HUS, 6.5% overall) or hepatic veno-occlusive disease (VOD, 3.3% overall) (P=0.14, P=0.16 and P=0.41, respectively).
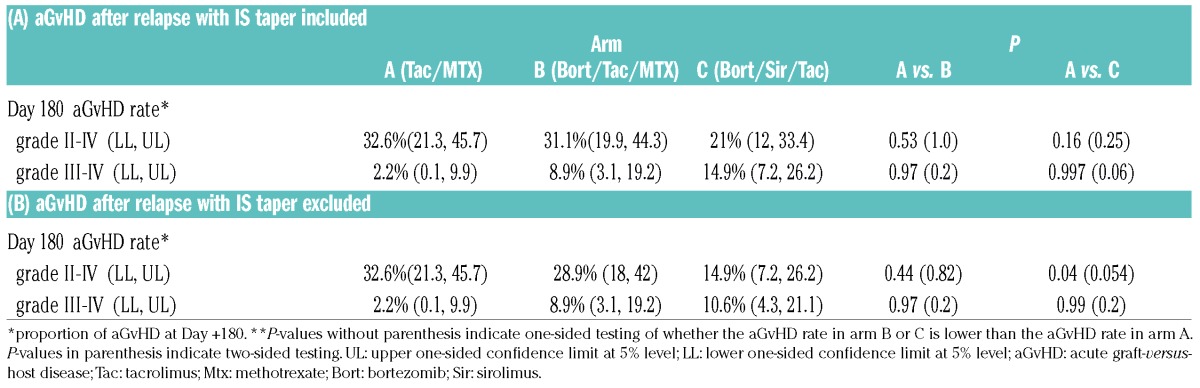
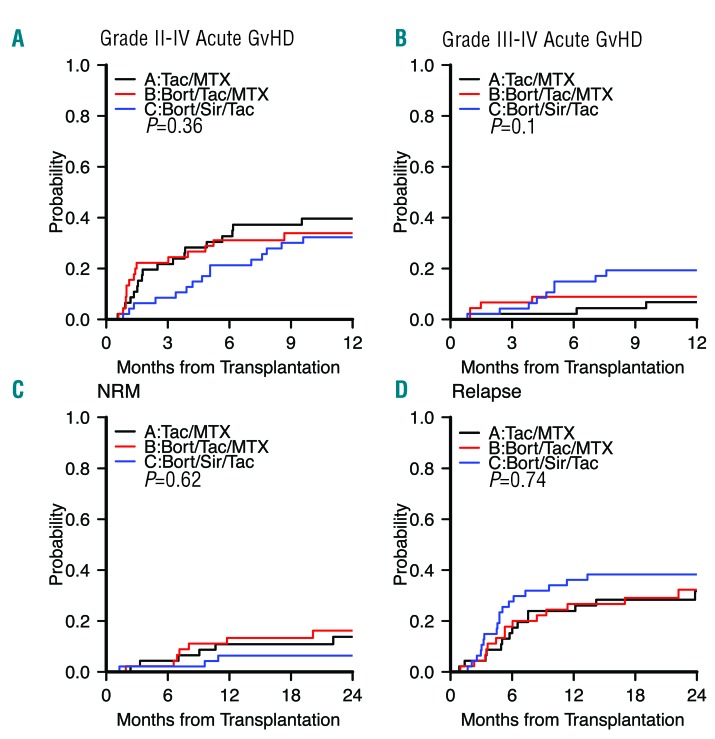
Acute GvHD: The primary endpoint of grade II-IV aGvHD incidence rate by day +180 did not differ significantly between treatment arms, at 32.6% (A) vs. 31.1% (B, one-sided P=0.53 for arm A vs. B) vs. 21% (C, one-sided P=0.16 for arm A vs. C) (Table 2). This result is consistent with the cumulative incidence of aGvHD (P=0.36) (Table 3, Figure 2A). For seven patients, grade II-IV aGvHD occurred after hematologic malignancy relapse (six after early IS taper, and 1 after donor lymphocyte infusions [DLI]). Four of these (B, 1; C, 3) had aGvHD onset prior to day +180, and are included in the primary endpoint. This result was consistent with the result from multivariable analysis: subdistribution hazard ratio (sHR) was 0.87 (95% confidence interval [CI] 0.42–1.78, P=0.7) for arm B vs. arm A, and 0.68 (95% CI 0.33–1.4, P=0.29) for arm C vs. arm A. If patients with aGvHD onset after relapse are excluded from the analysis, the grade II-IV aGvHD incidence rate by day +180 was 32.6% (A) vs. 28.9% (B, one-sided P=0.44 for arm A vs. B) vs. 14.9% (C, one-sided P=0.04 for arm A vs. C) (Table 2). On multivariable analysis, sHR was 0.8 (95% CI 0.39–1.66, P=0.55) for arm B vs. A, and 0.53 (95% CI 0.23–1.18, P=0.12) for arm C vs. A. aGvHD incidence continued to rise after day +180 across arms, and the 1-year cumulative incidence of grade II-IV aGvHD was 40% in arm A, 34% in arm B, and 26% in arm C (P=0.71 for arm A vs. B, P=0.33 for arm A vs. C) (Figure 2A). For grade III-IV severe aGvHD, day +180 cumulative incidence was 2.2% (A) vs. 8.9% (B, P=0.64 for arm A vs. B) vs. 15% (C, P=0.07 for arm A vs. C) (Table 3, Figure 2B).
Table 2.
Summary of the primary endpoint
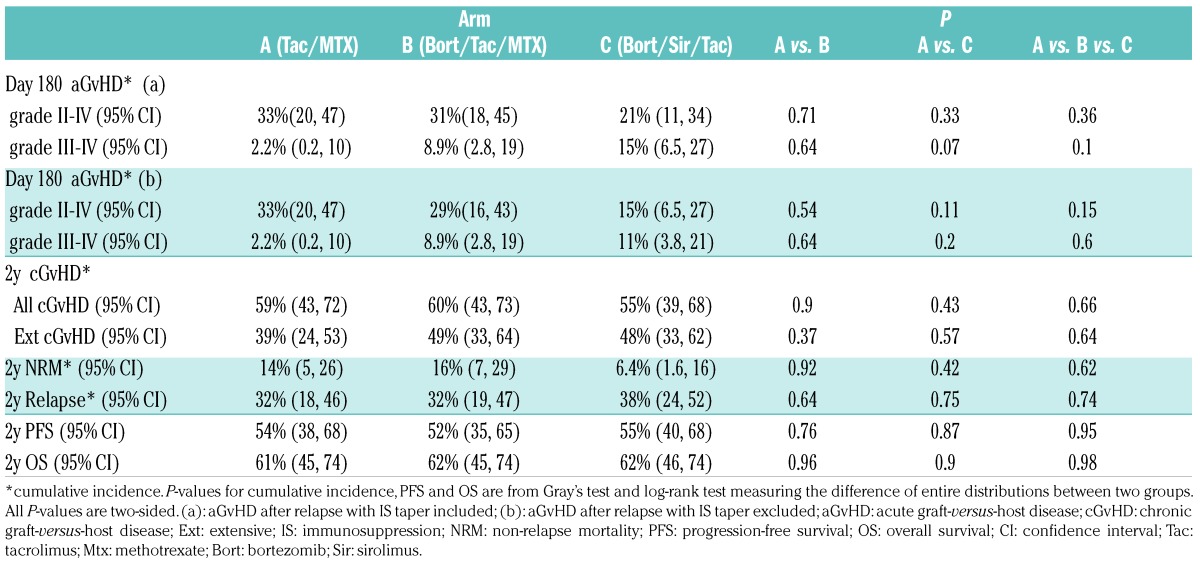
Table 3.
Summary of the secondary endpoints
Figure 2.
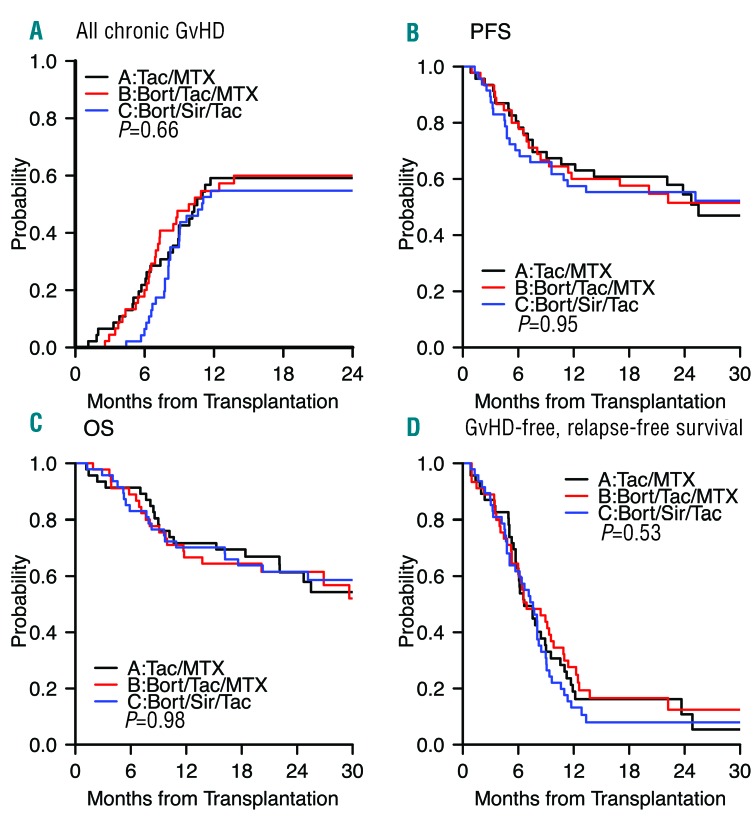
aGvHD: non-relapse mortality and relapse outcomes. Cumulative incidence of (A) grade II-IV aGvHD*, (B) grade III-IV aGvHD*, (C) non-relapse mortality (NRM), and (D) relapse per treatment arm. Black indicates arm A (tac/mtx), red indicates arm B (bort/tac/mtx), and blue indicates arm C (bort/sir/tac). Gray’s test for comparing the entire distributions was used. *: Acute graft-versus-host disease (GvHD) after relapse with IS taper included. Tac: tacrolimus; Mtx: methotrexate; Bort: bortezomib; Sir: sirolimus.
In an exploratory analysis in line with our protocol stratification, we repeated the analysis by HLA match status (Online Supplementary Table S1A). For the 8/8 HLA-matched recipients, when all grade II-IV aGvHD were counted, the six-month cumulative incidence was 33% (A) vs. 16% (B, P=0.1 for arm A vs. B) vs. 19% (C, P=0.21 for arm A vs. C) (Online Supplementary Figure S1). When arms B and C were combined, the six-month cumulative incidence was 17% (P=0.08 for arm A vs. arm B or C). When we repeated the analysis after excluding aGvHD that occurred after relapse and IS taper, the six-month cumulative incidence was 33% (A) vs. 13% (B, P=0.046 for arm A vs. B) vs. 16% (C, P=0.13 for arm A vs. C). When arms B and C were combined, the cumulative incidence was 15% (P=0.03 for arm A vs. arm B or C). However, for 7/8 MMD recipients such a benefit was not appreciable (albeit with limited sample size) (Online Supplementary Table S1A, Online Supplementary Figure S1).
NRM, relapse, chronic GvHD, and survival: NRM did not differ significantly between treatment arms (Table 3), with a 2-year cumulative incidence of 14% (A, 95% CI, 5–26) vs. 16% (B, 95% CI, 7–29) vs. 6.4% (C, 95% CI, 1.6–16; P=0.62) (Figure 2C). Relapse did not differ significantly between treatment arms, with a 2-year cumulative incidence of 32% (A, 95% CI, 18–46) vs. 32% (B, 95% CI, 19–47) vs. 38% (C, 95% CI, 24–52; P=0.74) (Figure 2D). The 2-year cumulative incidence of cGvHD did not differ significantly between treatment arms, at 59% (A) vs. 60% (B) vs. 55% (C; P=0.66) (Figure 3A). For five patients, cGvHD occurred after documented hematologic malignancy relapse (A, 1; B, 2; C, 2) and these were included in the estimation of cumulative incidence of cGvHD. Two had early IS taper, none received DLI. The 2-year PFS did not differ significantly between treatment arms, at 54% (A) vs. 52% (B) vs. 55% (C; P=0.95) (Figure 3B). The 2-year OS did not differ significantly between treatment arms, at 61% (A) vs. 62% (B) vs. 62% (C; P=0.98) (Figure 3C). The composite 2-year GRFS endpoint did not differ significantly between treatment arms, at 11% (A) vs. 12% (B) vs. 8% (C; P=0.53) (Figure 3D).
Figure 3.
cGvHD: survival and GRFS outcomes. Cumulative incidence of (A) all cGvHD, and Kaplan-Meier survival plots of (B) progression-free survival (PFS), (C) overall survival (OS), and (D) grade III-IV aGvHD/cGvHD requiring systemic IS agents/relapse-free survival (GRFS) per treatment arm. Black indicates arm A (tac/mtx), red indicates arm B (bort/tac/mtx), and blue indicates arm C (bort/sir/tac). Tac: tacrolimus; Mtx: methotrexate; Bort: bortezomib; Sir: sirolimus; GvHD: graft-versus-host disease.
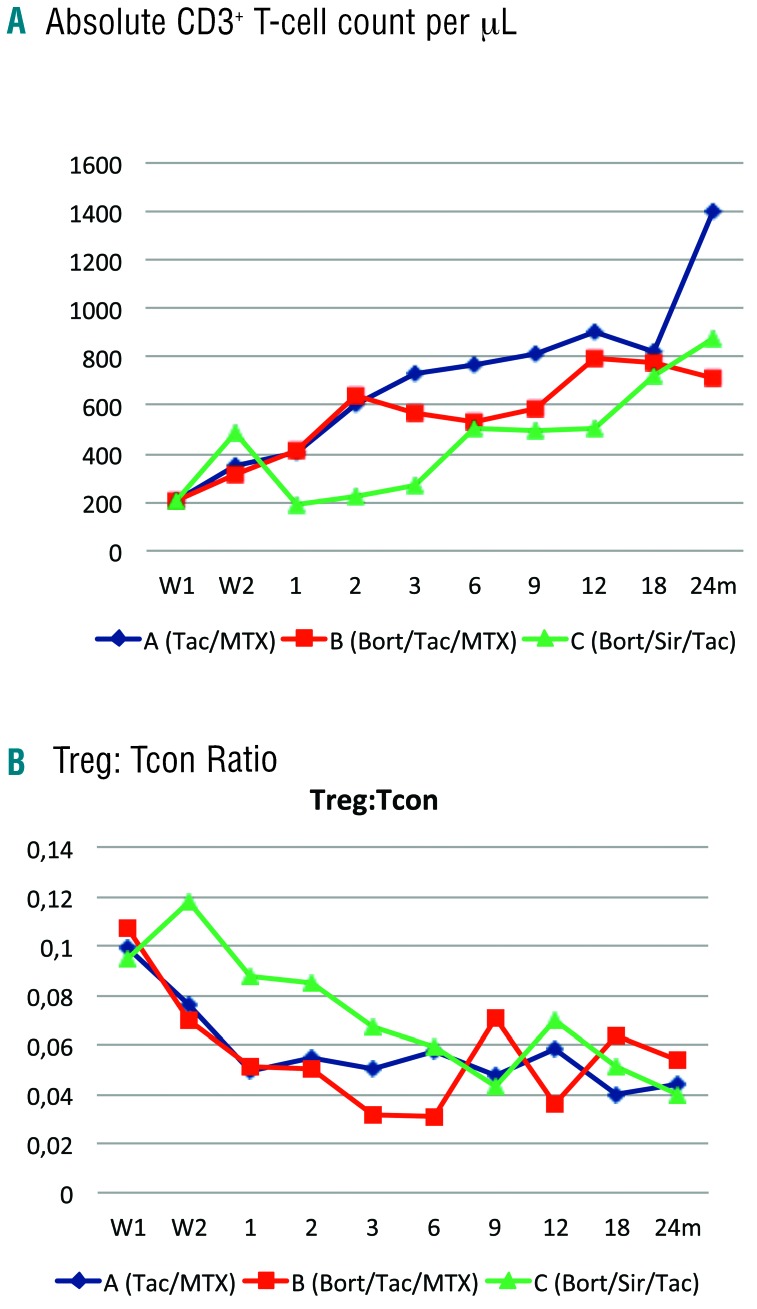
Immune reconstitution: The median total CD3+ T-cell count/μl at one month after transplantation was 401 in arm A (Q1-Q3, 248–681) vs. 414 in arm B (Q1-Q3, 195–898) vs. 190 in arm C (Q1-Q3, 137–340; P<0.0001), and remained lower in arm C through months two and three (A, 602 and 728 vs. B, 639 and 571 vs. C, 221 and 269, respectively; P<0.0001). At six months after transplantation, CD3+ T-cell counts were similar in all three arms at 763 (A, Q1-Q3, 463–1120) vs. 535 (B, Q1-Q3, 333–907) vs. 508 (C, Q1-Q3, 322–728; P=0.10) (Figure 4A). This reflected both a lower median CD8+ and CD4+ T-cell count/μl at one to three months after transplantation in arm C (P<0.0001, data not shown). Similarly, the median CD19+ B-cell and the median CD56+CD3− NK cell count/μl was lower for arm C during the one to three months following transplantation (P<0.05, data not shown).
Figure 4.
Immune reconstitution outcomes. Reconstitution of (A) median of absolute CD3+ T-cell count/μL, and (B) median values of CD4+ Treg:Tcon cell ratio per treatment arm. Blue indicates arm A (tac/mtx), red indicates arm B (bort/tac/mtx), and green indicates arm C (bort/sir/tac). Treg: regulatory T cells; Tcon: conventional T cells; Tac: tacrolimus; Mtx: methotrexate; Bort: bortezomib; Sir: sirolimus; W1: week 1; W2: week 2; M: month.
While the total CD4+ Tcon cell count/μl at one, two, and three months after transplantation was lower for arm C (P=0.005, P=0.0006, P=0.024 at one, two and three months after transplantation, respectively), the total CD4+ Treg cell count/μl was unimpaired in arm C at those time points, resulting in an improved ratio of Treg:Tcon reconstitution at one and three months after transplantation (A: 0.049 and 0.05 vs. B: 0.051 and 0.032 vs. C: 0.088 and 0.067; P=0.006 and P=0.012, respectively) (Figure 4B).
Discussion
Most adult hematologic malignancy patients who may benefit from allogeneic HSCT lack an available sibling donor and are considered for a MUD or 1-locus MMD, with umbilical cord blood (UCB) and haploidentical (haplo) donors being additional alternative options. Regarding 8/8 MUD, as a result of improvements in DNA-based typing and supportive care, survival outcomes are considered similar to those of MRD HSCT,18,19 although studies indicate that MUD HSCT is associated with increased grade II-IV aGvHD (52% vs. 34%), grade III-IV aGvHD (21% vs.16%), and NRM (RR 2.76; P<0.01).20 The use of 1-locus MMD adds risk. A large registry analysis of 2,588 patients with acute leukemias, MDS or chronic myeloid leukemia (CML) undergoing RIC HSCT compared 7/8 with 8/8 HLA-matched donors, and documented higher rates of grade II-IV aGvHD and NRM, and lower 3-year OS (30% vs. 38%, respectively; P=0.01) with a mismatched graft.2 Novel regimens to improve GvHD outcomes for patients lacking a preferred sibling donor would represent a major advance, and is the focus of our efforts.
Proteasome inhibition with bort has immunomodulatory properties relevant to allogeneic HSCT, as previously highlighted. Based on encouraging phase I/II results in HLA-mismatched T-replete RIC HSCT, we undertook a prospective randomized evaluation of aGvHD prophylaxis with conventional tac/mtx (arm A) vs. two novel regimens of short-course bort for transplantation recipients lacking 8/8 HLA-matched related donors: bort plus tac/mtx (arm B), and bort plus sir/tac (arm C).
In our study, Bort, limited to three doses peritransplantation (day +1, +4 and +7), did not add toxicity. No bort doses were missed or modified. No subjects developed toxicities of prolonged/delayed bort administration (e.g., neuropathy, colonic necrosis). Comparing treatment arms, no increase in hepatic VOD, AKI or TMA/HUS was noted for bort-based regimens vs. tac/mtx.
Engraftment was rapid and sustained, with robust donor chimerism by day 30 that was sustained and comparable across treatment arms. The addition of bort alone did not appear to impair immunologic recovery, with similar counts of T cells (CD4, CD8), B cells and NK cells in the conventional tac/mtx vs. bort/tac/mtx arms. Count recovery for arm C (bort/sir/tac) was lower, as we previously documented for other sir-based cohorts.10 As hypothesized, there was relative sparing of Treg reconstitution with arm C (bort/sir/tac), with no impairment of Treg count recovery and elevated Treg:Tcon ratios.
The clinical impact of these systematic immunologic differences, however, remains unclear. Importantly, the 2-year NRM incidence was low (ranging from 6.4% to 16%) and did not differ significantly between treatment arms. Relapse was in the expected range after RIC HSCT (ranging from 24% to 36%), and did not differ significantly between treatment arms. In this study, survival, while not differing significantly between treatment arms, with 2-year OS ranging from 61% to 62% (P=0.98), appeared better than anticipated for 7/8 and 8/8 HLA-matched recipients, plateauing at 50% and 66%, respectively, in contrast to 1- and 3-year registry benchmark survivals recently reported for 7/8 and 8/8 donor recipients, of 48% → 30% and 55%→38%, respectively2. We highlight a lack of significant difference between treatment arms with regard to the primary endpoint of grade II-IV aGvHD rate by day +180.
In the context of delayed T-cell reconstitution noted for arm C, this suggests that the combination of a CNI (tac) plus mTOR inhibitor (sir) provides IS without long term tolerizing effects. While it is possible that longer duration of tac/sir immunosuppression in arm C beyond day +180 may have better prevented aGvHD, ultimately, aGvHD deferral rather than long-term amelioration remains a possibility. In subgroup analysis for the 8/8 HLA-matched recipients, bort-based regimens had a borderline trend towards grade II-IV aGvHD benefit vs. tac/mtx, with a cumulative incidence of 17% vs. 33%, respectively, (P=0.08). However, grade III-IV severe aGvHD rates were not improved, and for 7/8 MMD grafts a similar trend for aGvHD benefit of bort-based regimens was not appreciable, though sample size was limited.
Our trial has strengths due to its appropriate size in the phase II context, and its direct prospective randomization to conventional tac/mtx vs. two novel bort-based GvHD prophylaxis regimens. Concerns regarding open-label treatment assignment are ameliorated by the minimal dropout rate and the mITT analysis to further avoid bias, while the ‘hard’ endpoints of GvHD, NRM, relapse and survival additionally obviate assessment bias concerns.
Our primary finding is that, as tested, bort-based regimens did not appear to provide additional benefit for grade II-IV aGvHD prevention in T-replete PBSC RIC HSCT, failing to meet the protocol-specified 25% reduction in aGvHD incidence for success. Our phase II RCT requirements for success were stringent, which limits our ability to detect lower, albeit potentially clinically meaningful benefit with bort. However, it is also notable that conventional tac/mtx outcomes were better than anticipated, a finding similar to that recently reported in other contemporary randomized HSCT trials.21
These data highlight the inadequacy of non-randomized comparators for evaluating early phase single arm interventional studies, and document, as an updated standard, the improved MMD and MUD RIC HSCT outcomes achievable with conventional tac/mtx in this study (2-year OS of 58% and 62%, respectively). In the future, prospective trials of alternative donors (e.g., UCB, haplo) and novel GvHD prophylaxis regimens (e.g., maraviroc, PTCy) may need to benchmark these outcomes. In contrast, the bar for the novel GRFS endpoint appears far lower, ranging from 8% to 12% in our study, with no significant difference between treatment arms (P=0.53).
In summary, mature data from this open-label 1:1:1 three-arm phase II RCT indicates that the bort-based regimens evaluated appear to provide lower than anticipated grade II-IV aGvHD benefit compared to conventional tac/mtx in T-replete PBSC RIC HSCT. While we note the potential benefit of bort for 8/8 HLA-matched transplants, direct phase III prospective randomization is required in order to confirm such a benefit. Overall, however, the lack of benefit for other transplantation outcomes (NRM, relapse, chronic GvHD, and survival) suggests limited utility for bort prophylaxis in the doses and combinations assessed. Alternative proteasome inhibitor combination regimens (e.g., with PTCy) should also be considered.
Supplementary Material
Acknowledgments
We thank clinical research nurses Susan Stephenson RN and Mildred Pasek RN. JK is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/3/522
References
- 1.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verneris MR, Lee SJ, Ahn KW, et al. HLA mismatch is associated with worse outcomes after unrelated donor reduced-intensity conditioning hematopoietic cell transplantation: an analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(10):1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nencioni A, Schwarzenberg K, Brauer KM, et al. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood. 2006;108(2):551–558. [DOI] [PubMed] [Google Scholar]
- 4.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107(9):3575–3583. [DOI] [PubMed] [Google Scholar]
- 5.Pai CC, Hsiao HH, Sun K, et al. Therapeutic benefit of bortezomib on acute graft-versus-host disease is tissue specific and is associated with interleukin-6 levels. Biol Blood Marrow Transplant. 2014;20(12):1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun K, Welniak LA, Panoskaltsis-Mortari A, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci USA. 2004;101(21):8120–8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JS, Lee JI, Shin JY, et al. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88(12):1349–1359. [DOI] [PubMed] [Google Scholar]
- 8.Sun K, Wilkins DE, Anver MR, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106(9):3293–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107(2):827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang Y, Ma S, Zhang Y, et al. IL-1beta and TLR4 signaling are involved in the aggravated murine acute graft-versus-host disease caused by delayed bortezomib administration. J Immunol. 2014;192(3):1277–1285. [DOI] [PubMed] [Google Scholar]
- 11.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114(18):3956–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30(26):3202–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston L, Florek M, Armstrong R, et al. Sirolimus and mycophenolate mofetil as GVHD prophylaxis in myeloablative, matched-related donor hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47(4):581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray R. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16(3):1141–1154. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Kiehl MG, Kraut L, Schwerdtfeger R, et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol. 2004;22(14):2816–2825. [DOI] [PubMed] [Google Scholar]
- 19.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24(36):5695–5702. [DOI] [PubMed] [Google Scholar]
- 20.Ringden O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113(13):3110–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soiffer RJ, Kim HT, McGuirk J, et al. A prospective randomized double blind phase 3 clinical trial of anti-T lymphocyte globulin (ATLG) to assess impact on chronic graft-versus-host disease (cGVHD) free survival in patients undergoing HLA matched unrelated myeloablative hematopoietic cell transplantation (HCT). Blood. 2016;128(22):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.