Ibrutinib (IBR) is an irreversible inhibitor of Bruton’s tyrosine kinase (Btk) which is approved for the treatment of chronic lymphocytic leukemia (CLL). Clinically significant bleedings attributed to platelet inhibition by IBR have been reported and there is great interest toward development of diagnostic and therapeutic strategies to manage these side-effects.1 Btk is involved in platelet activation downstream of the collagen receptor glycoprotein (GP) VI,2 of CLEC-2 receptor3 and of von Willebrand (VWF) receptor, the GPIb-IX-V complex.4 The GPVI-mediated platelet activation has been the most investigated IBR target and several studies have consistently shown that IBR causes inhibition of the collagen-mediated platelet aggregation, which correlates with the occurrence of clinical bleedings.5–7 The CLEC-2-dependent platelet functions are difficult to explore and they have not been investigated in IBR treated patients. The VWF-GPIb mediated pathway has been scarcely investigated and few data are available for these patients. It has recently been reported that ristocetin-induced platelet aggregation (RIPA), which is dependent on vWF binding to GPIb, as measured using whole blood and impedance aggregometry (IA), might be useful for predicting and monitoring bleeding tendency in CLL patients treated with IBR.8 Considering the clinical implications of those results, we measured RIPA in CLL patients treated with IBR using the light transmission aggregometry (LTA), with the aim of confirming previous findings using the gold standard and most widely used method to investigate platelet function. Surprisingly, our results show that no effect of IBR on RIPA could be detected in CLL patients, whereas the aggregation by collagen was severely inhibited in the same patients.
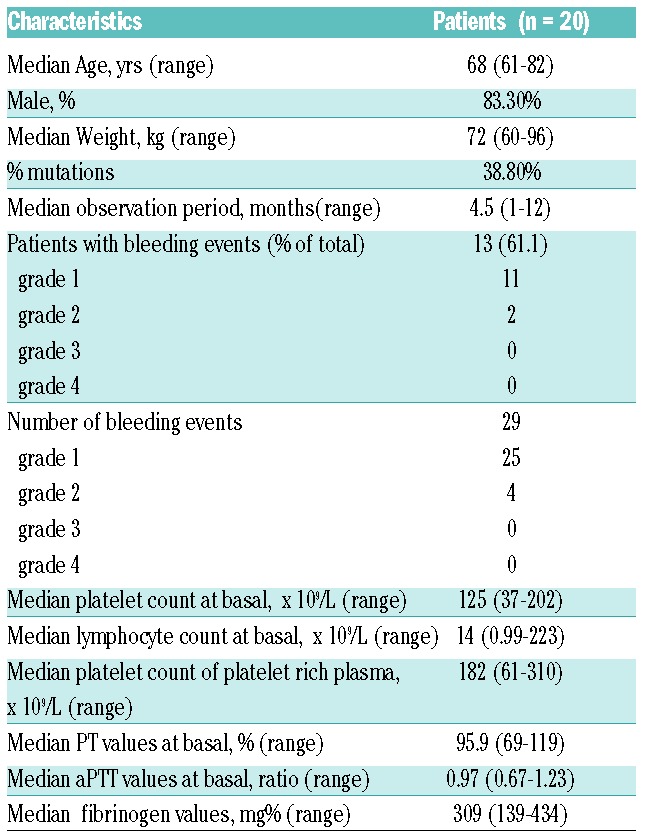
We performed an observational monocentric study including 20 consecutive CLL patients enrolled at the Policlinico Agostino Gemelli in Rome. All 20 patients received 420 mg of IBR once daily according to licensed indication in Europe. Eighteen relapsed/refractory CLL patients received IBR in monotherapy and 2 treatment-naïve CLL patients received IBR in association with anti-CD20 MoAb. Thirteen patients had un-mutated IgVH, and 2 had 17p deletion. Thirteen patients (61.1%) had grade 1–2 bleedings. Patients under anti-thrombotic treatment were excluded by the study. Patient characteristics are reported in Table 1. The study was approved by the ethical committee of Policlinico Agostino Gemelli and informed consent was obtained.
Table 1.
Patient characteristics.
Patients were evaluated at baseline and after 15–30 days, 3,6,9,12 months of IBR treatment. To the date of this report, 16 patients have been evaluated up to 6 months and 4 patients up to 12 months. Four healthy volunteers were recruited for in vitro studies.
We measured platelet aggregation using platelet rich plasma (PRP) and LTA. Fresh blood specimens anticoagulated with 3.8 % sodium citrate were centrifuged at 140 g for 15 minutes to obtain PRP and at 2630 g for 15 minutes to obtain platelet poor plasma (PPP). Platelet count was not adjusted with autologous PPP and median platelet counts in whole blood and in PRP at baseline are reported in Table 1. For in vitro experiments, PRP from healthy donors was incubated with 1 μM IBR (PCI-32765, Selleckchem, Houston, USA) or dimethylsulfoxide (DMSO) 2% for 1 h at room temperature. PRP was stirred at 600 rpm at 37 °C in an AggRAM Helena aggregometer, and aggregation of platelets was induced by adding 1.2 mg/ml ristocetin or 3.3 μg/ml collagen. Circulating VWF antigen levels were measured in citrated plasma using immuno-turbidimetric method (HemosIL AcuStar VWF:Ag) on an AcuStar ACL system.
Statistical analysis was performed using a nonparametric Wilcoxon signed rank test to measure differences among groups. P values < 0.05 were considered statistically significant. Results are expressed as median and interquartile range or mean ± SD. Data analysis were performed using SPSS software (Version 17.0, SPSS Inc, Chicago, IL, USA).
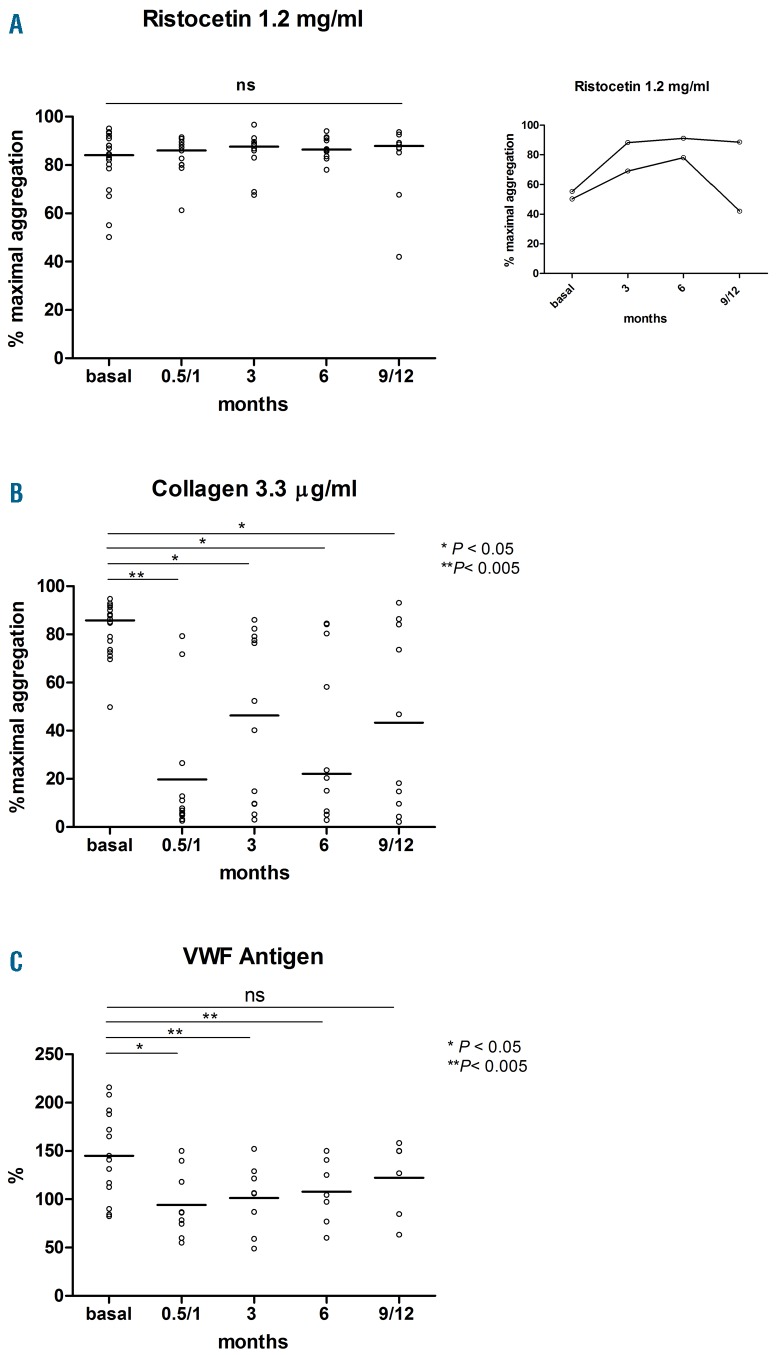
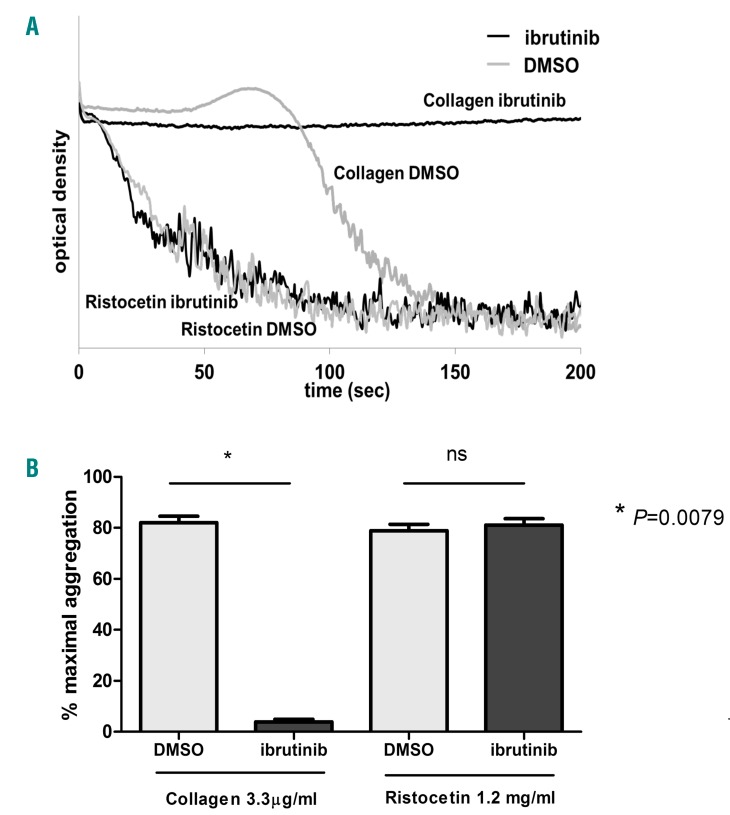
Results reported in Figure 1 clearly show that in our cohort of patients no inhibition of RIPA could be registered at any time during a follow up of 12 months of IBR therapy (Figure 1A). On the contrary, marked inhibition of collagen-induced platelet aggregation was found after 15/30 days of IBR and during a follow up of 12 months, with a trend toward pre-IBR values over time (Figure 1B). Plasma VWF levels were high at baseline and significantly decreased after 15/30 days of IBR, with a trend toward pre-IBR values over time (Figure 1C). In agreement with the findings obtained in patients ex vivo, in vitro added IBR did not affect RIPA of control platelets from 4 healthy subjects, whereas marked inhibition of collagen-induced aggregation was detected in the same conditions (Figure 2 A, B).
Figure 1.
In vivo effect of ibrutinib in CLL patients over a follow-up period of 12 months. (A) Platelet agglutination by 1.2 mg/ml ristocetin. (B) platelet aggregation by 3.3 μg/ml collagen. (C) plasma von Willebrand factor antigen. Horizontal lines indicate median values.
Figure 2.
In vitro effect of ibrutinib on RIPA and on collagen-induced aggregation of control platelets. (A) Agglutination by ristocetin and aggregation by collagen of control platelets in the presence of in vitro added 1 μM ibrutinib or 2% DMSO. Curves are representative of experiments performed on 4 different healthy subjects. (B) LTA responses of control platelets to collagen and ristocetin in the presence of 1 μM ibrutinib or 2% DMSO. Data are reported as mean ± SD (n=4).
The discrepancy between our results and those obtained by Kazianka et al. could be, at least partially, explained by the different method used to assess the VWF-GPIb dependent platelet activation.
Firstly, we used PRP instead of whole blood, hence the effects of leukemic cells on the test could be ruled out in our conditions. Whether the presence of CLL cells in whole blood samples might affect the vWF-GPIb-dependent platelet function assays is a possible hypothesis. To this regard, in the paper by Kazianka et al., 11 patients had a baseline median RIPA value of 17 U (range 3–70) which decreased to a median value of 9U (range 0–19) after IBR. Indeed, the reported in-house normal range of the test was 44–176 U, but only 4 out of the 11 patients had RIPA values greater than 44 U before IBR, while 7 patients had values below the normal range, suggesting that most patients included in the analysis had platelet dysfunction not dependent on IBR. We can speculate that the presence of leukemic cells in the sample or the effect of antithrombotic drugs could explain the results. In another paper, the effect of IBR on the vWF-GPIb-dependent platelet activation was investigated by measuring the adhesion of platelets to vWF spread on a coverslip.5 Significant reduction of platelet adhesion was found in 3 bleeding patients compared to 3 non-bleeding patients after 15/30 days of treatment. Reduction of adhesion of normal platelets to vWF was also found when IBR was added in vitro. However, whole blood was used in this assay and, although the authors specified that hyperleukocytosis was not correlated to ex vivo platelet dysfunction, adhesion to vWF of patient platelets before IBR was reduced compared to that of control platelets. Whether the disease or the drug or the association of both factors can be responsible for the platelet dysfunction in this clinical setting is still a matter of debate.7,9
To this regard, we highlight that all our patients were evaluated before and after initiation of IBR in order to discriminate the effect of the disease and that of IBR on platelet function.7 In our cohort of patients, neither the disease nor IBR had any short- and long-term effect on RIPA, which resulted at any time similar to that of control subjects (median 72 %, range 65–90 %)(Figure 1A). Two patients had basal low RIPA values (<60%), which improved during IBR therapy. In one patient, RIPA returned low while developing relapse of CLL (Figure 1A inset).
Secondly, we used LTA instead of IA. Impedance aggregometry has the advantage of being faster and more user-friendly to study the platelet response to aggregating agents, but it has not been widely adopted and, at variance with LTA, fails to provide additional diagnostic information that can identify a defect of platelet function.10 We were quite surprised that using LTA we could not confirm the results obtained by IA. It is known that IA is greatly influenced by the platelet count within the physiologic range, whereas LTA is not.11,12 Hence, lower platelet counts in CLL patients might have affected the results obtained using IA.
Thirdly, we measured VWF levels both to rule out an acquired VWF disease, which is known to occur in LLC patients and could have been potentially responsible for the bleedings,13 and to investigate whether RIPA could have been influenced by abnormal VWF plasma levels. No acquired VW disease was present in our cohort of patients (Figure 1C). On the contrary, VWF levels were high before IBR and significantly decreased during IBR, thus ruling out their effects on RIPA. These results confirm previously reported data.7
Finally, it has to be emphasized that, unlike the study by Kazianka et al., patients under antithrombotic treatments, either antiplatelet and/or anticoagulant, were excluded in the present study to avoid confounding factors, since these drugs might impact on platelet function assays. Two patients who were under aspirin treatment were not included in the study and they both showed grade 1–2 bleedings during IBR.
In conclusion, we show that RIPA measured by LTA standard method is not affected in IBR-treated CLL patients and, therefore, cannot be used to identify patients at higher risk of bleeding, as previously suggested. The exploration of the bleeding tendency in these patients needs to be studied using other tests. These results may be relevant for clinicians treating CLL patients with ibrutinib, whose bleeding side effects may severely impact on a population of elderly patients who are often under chronic anti-thrombotic treatments.
Supplementary Material
Footnotes
Funding: this work was supported by Università Cattolica del Sacro Cuore – Facoltà di Medicina e Chirurgia, Roma, Italia
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Shatzel JJ, Olson SR, Tao DL, McCarty OJT, Danilov AV, DeLoughery TG. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J Thromb Haemost. 2017;15(5):835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quek LS, Bolen J, Watson SP. A role for Bruton’s tyrosine kinase (Btk) in platelet activation by collagen. Curr Biol. 1998;8(20):1137–1140. [DOI] [PubMed] [Google Scholar]
- 3.Manne BK, Badolia R, Dangelmaier C, et al. Distinct pathways regulate Syk protein activation downstream of immune tyrosine activation motif (ITAM) and hemITAM receptors in platelets. J Biol Chem. 2015;290(18):11557–11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Fitzgerald ME, Berndt MC, Jackson CW, Gartner TK. Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood. 2006;108(8):2596–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levade M, David E, Garcia C, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124(26):3991–3995. [DOI] [PubMed] [Google Scholar]
- 6.Kamel S, Horton L, Ysebaert L, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2014;29(4):783–787. [DOI] [PubMed] [Google Scholar]
- 7.Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazianka L, Drucker C, Skrabs C, et al. Ristocetin-induced platelet aggregation for monitoring of bleeding tendency in CLL treated with ibrutinib. Leukemia. 2016;31(5):1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam CS, Kamel S, Comment on Lipsky et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2016;101(3):e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo M, Cerletti C, Harrison P, et al. Recommendations for the standardization of light transmission aggregometry: a consensus of the working party from the Platelet Physiology Subcommittee of SSC/ISTH. J Thromb Haemost. 2013;11(6):1183–1189. [DOI] [PubMed] [Google Scholar]
- 11.Seyfert UT, Haubelt H, Vogt A, Hellstern P. Variables influencing Multiplate(TM) whole blood impedance platelet aggregometry and turbidimetric platelet aggregation in healthy individuals. Platelets. 2007;18(3):199–206. [DOI] [PubMed] [Google Scholar]
- 12.Femia EA, Scavone M, Lecchi A, Cattaneo M. Effect of platelet count on platelet aggregation measured with impedance aggregometry (Multiplate analyzer) and with light transmission aggregometry. J Thromb Haemost. 2013;11(12):2193–2196. [DOI] [PubMed] [Google Scholar]
- 13.Alattar ML, Ciccone M, Gaballa MR, et al. Bleeding diathesis associated with acquired von Willebrand Syndrome in three patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56(12):3452–3454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.