Abstract
The marked heterogeneity in glioblastoma (GBM) may be induced through dynamic differentiation and dedifferentiation process of glioma cells. The hypothesis that environmental stimuli induce these phenotypic changes, including dedifferentiation into the stem cell phenotype which contributes to the high invasiveness and resultant poor outcome in GBM patients, is recently being proven. In the process of cancer invasion and metastasis, the phenotypic change has also been described as epithelial-mesenchymal transition (EMT). This biological process is mainly dependent on hypoxic stimuli and also on transforming growth factor-β (TGF-β) released from glioma stem cells, mesenchymal stem cells, and myeloid cells recruited by hypoxia. The tumor microenvironment, especially hypoxia, inducing such dynamic phenotypic changes can be a good therapeutic target in the treatment of GBM.
Keywords: glioma, microenvironment, hypoxia, TGF-β, epithelial-mesenchymal transition, EMT
Introduction
Glioblastoma (GBM) is the most malignant type of gliomas and originates from some precursor cells in the brain intermingled with neurons, glia and some mesenchymal cells.1,2) The most devastating features of GBM are the high invasiveness and apoptosis resistance. In spite of the same morphological characteristics to GBM, patients with this tumor clinically have remarkable heterogeneity in survival periods.3) The known genetic alterations, however, cannot precisely predict patients’ survival.4) The sub-classification of GBM based on The Cancer Genome Atlas shows that a mesenchymal subtype expresses high levels of neural stem cell markers and clinically shows an aggressive phenotype.5) Furthermore, other GBM subtypes frequently acquire the gene expression pattern of the mesenchymal subtype when the tumor recurs after chemotherapy and radiotherapy.5) One of the most important regulators of changing gene expressions is the epigenetic modifications which have been developed to interface environmental alteration with genomic function.6) These clinical and biological data indicate that environmental factors significantly affect phenotypic transition of GBM cells.
A remarkable characteristic of the GBM microenvironment is hypoxia. This is caused by the aberrant structure and distribution of tumor neoangiogenesis and abnormal induction of the intravascular coagulation cascade.7) A hypoxic microenvironment gives rise to a biological process called epithelial-mesenchymal transition (EMT), which induces multiple epigenetic changes to result in dedifferentiation of the cell.8,9) This process is involved in the acquisition of stem cell features.10,11) The most important mediator of EMT among other signaling pathways is transforming growth factor-β (TGF-β).9) TGF-β family is also regarded as an essential player for the induction and maintenance of pluripotent stem cells and cancer stem cells.12) In addition, a hypoxic tumor microenvironment recruits some myeloid cells, such as macrophages or microglia, and mesenchymal stem cells into the tumor tissues.12) These cells secrete high levels of TGF-β to upregulate the transcription factors TWIST or Snail necessary for EMT.9,12) Thus, hypoxia greatly contributes to the devastating and refractory features of GBM through EMT-induced stem cell properties.13,14) The tumor microenvironment inducing dynamic phenotypic change can be a potential therapeutic target in the treatment of GBM.
Plasticity in the stem cell phenotype
Cancer stem cells make asymmetric cell divisions to produce a daughter stem cell for unlimited self-renewal and a daughter progenitor cell for further differentiation to eventually form a tumor mass.15) The most essential question is whether the differentiated glioma cells can be dedifferentiated or reprogrammed into glioma stem cell (GSC). Recently, experimental evidences have been offered regarding the bidirectional plasticity between GSC and non-stem differentiated glioma cells by using patient-derived GSCs.16–18) Berezovsky et al. found Sox2 as a key molecule for maintaining plasticity for bidirectional conversion between cancer stem-like and differentiated glioma cells in a mouse xenograft of human GBM cells.16) Glioma cells can grow in two different phenotypes, as adherent monolayer cells which represent differentiated glioma cells and as free-floating neurospheres which resemble GSC, using specific media supplements for each.17) These phenotypic states are mutually reversible and actually differ in proliferation rate, invasion, migration and chemosensitivity. Natsume et al. reported that an interconversion between glioma stem cells, characterized by Nestin expression, and differentiated glioma cells, characterized by glial fibrillary acidic protein (GFAP) expression, were mutually reversible accompanied with epigenetic modification, depending on the medium condition.17) These evidences strongly suggest that the differentiated glioma cells can go transition to dedifferentiated glioma stem cells according to the external environmental signaling.
Another supporting observation regarding GSC phenotypic plasticity is that single stem cell-derived clones of human GBM tumors exhibit functional and morphological heterogeneity, and passage of large or small differentiated subclones produced the heterogeneous cellular populations similar to the original heterogeneity containing GSCs.19) This result does not support the clonal evolution model, but the theory that GSC can exhibit bidirectional plasticity. A theory of malignant transformation of GBM through the dedifferentiation of GBM cells induced by the reversible epigenetic modification of pluripotency-related genes has also been offered by investigating clinical samples.20)
Signal transduction in GSC maintenance
Self-renewal is a crucial function of stem cells, since they should persist throughout the entire lifespan of the organism. The pathways essential for especially preserving self-renewal in addition to the multipotency of GSCs are the Notch, Sonic hedgehog (Shh), and Wnt signaling pathways.21) These factors are all essential for neural stem cell (NSC) survival and self-renewal for the embryonic central nervous system (CNS) development.22–24) In addition, activation of the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of the rapamycin (mTOR) pathway is also essential for the survival of GSC as well as NSC.25) These signaling pathways transduce extracellular signals into the cell, and then the transcription factors inside the cells also have an important role in the maintenance of GSCs (Table 1). Oct4 interacts with Nanog and Sox2 to control the gene expressions related to self-renewal in embryonic stem cells.26) Oct4 and Sox2 are increased in GSCs to inhibit differentiation and to promote tumorigenic activity.27) Nanog is highly expressed in CD133-positive GSCs compared with more differentiated glioma cells with GFAP expressions.28) c-Myc is not only a transcription factor but an important regulator of epigenetic status for maintenance of stem cell phenotype and cellular proliferation.29) Bmi1 is a member of the polycomb repressive complex 1 which composes a suppressive environment in gene expressions of the genome. This structure is required for self-renewal of stem cells and enriched in GSCs.26,30) Glioma patients with higher levels of Bmi1 usually have a shorter survival.30)
Table 1.
Transcription factors enriched in glioma stem cells
| Name | Involvement | Functions |
|---|---|---|
| Oct4 | Octamer-binding transcription factor 4 | Self-renewal of ESC Marker for pluripotency |
| Sox2 | SRY (sex determining region Y)-box 2 | Self-renewal of ESC Marker for pluripotency |
| Nanog | Tir Na Nog (Celtic) | Maintain pluripotency Interact with Oct4 and Sox2 |
| c-Myc | Multifunctional transcription factor | Oncoprotein for many cancers regulating histone acetylation |
| Olig2 | Oligodendrocyte transcription factor | CNS-specific bHLH factor Neuron/glia lineage |
| Gli1 | Glioma-associated oncogene | Zinc finger protein effectors of Hedgehog signaling |
| Bmi1 | B cell-specific moloney murine leukemia virus integration site-1 | Polycomb repressive complex-1 Regulate stem cell functions |
| STAT3 | Signal Transducers and Activator of Transcription 3 | Cytokine signaling of JAK-STAT Cell survival and differentiation |
| Msi1 | RNA-binding protein MUSASHI-1 | Neural stem cell functions Essential for self-renewal |
Notch:
Notch family proteins are transmembrane receptor protein, and its intracellular domain is released from the membrane to the nucleus by enzymatic cleavage by the γ-secretase complex to activate a transcription CSL family (CBF1, Suppressor of Hairless, Lag-1).31) Notch signaling, which mediates various cellular processes, including the regulation of differentiation and apoptosis in NSC, is also highly active in GSC to suppress differentiation and maintain stem cell properties.22) The γ-secretase inhibitors enhance the efficacy of temozolomide treatment in vitro and in vivo for human gliomas.32,33) Endothelial cells express the Notch ligands Delta-like 4 (DLL4) and Jagged-1, and RNAi-mediated knock-down of these ligands in endothelial cells abrogates the tumorigenic potential of co-transplanted GSC.34) HIF-1α-induced activation of the Notch pathway is known to be crucial for hypoxia-induced induction and maintenance of the stem cell phenotype.35)
Sonic hedgehog (Shh):
Shh signaling is important in ventral patterning in the embryonal development, proliferation, especially for migration, differentiation, and survival of neural stem cells.24) Shh is also a ligand for GLI1 receptor on the GSC to increase the expressions of the stem genes including CD133, Olig2, Sox2, Oct4, and Nanog.36) This pathway promotes self-renewal and survival of GSCs and supports glioma growth.36,37) Furthermore, Shh is one of the essential soluble factors secreted by the endothelial cells, which may be involved in the formation of a perivascular niche for GSCs.38) GSCs with CD133 expression are mainly found in the areas near Shh-expressing endothelial cells, suggesting a direct interaction between GSC and tumor-associated endothelial cells.39) Reversal of Shh signaling pathway decreases the expression of stem cell-related genes and differentiation.40)
Wnt:
Wnt/β-catenin signaling is a crucial factor for proliferation and differentiation of GSC as well as promoting astroglial lineage differentiation in normal neural development.23,41) Wnt binds to a specific receptor of the Frizzled and the lipoprotein receptor-related protein (LRP) families.41) The signal enables the destruction complex holding β-catenin to be disassembled, and the free β-catenin can translocate into the nucleus to transcribe Wnt-target genes.41) Either gain- and loss-of-function mutations of the Wnt gene is observed in high-grade gliomas and especially in medulloblastoma.42) As another function, FoxM1/β-catenin interaction controls the expressions of Wnt target genes that are required to induce glioma formation.43) Wnt ligands, Wnt1 and Wnt3a, are overexpressed in GSCs and selective knock-down of these ligands decreases their tumorigenic potential.44)
PI3K/Akt/mTOR:
As with many cancer cells, the receptor tyrosine kinase (RTK) induces signal transduction from epidermal growth factor (EGF) or basic fibroblast growth factor (bFGF) in GSC and normal neural stem cells.25) In various cancers, the PI3K/Akt pathway is one of the most important signaling pathways originated from RTKs, which is involved in cell survival and growth.45) epidermal growth factor receptor (EGFR) downstream signaling is constitutively activated in many GBMs and GSC subpopulations through amplification or mutations such as EGFR vIII.46) Transducing astrocytes from p53−/− mice with Akt and c-Myc induces tumorigenicity and increases the expressions of the stem cell markers.47) Furthermore, CD133 is reported to directly activate the PI3K pathway through binding to its p85 regulatory subunit, creating a positive feedback loop to enhance stem cell survival.48)
Microenvironments that maintain the stem cell phenotype
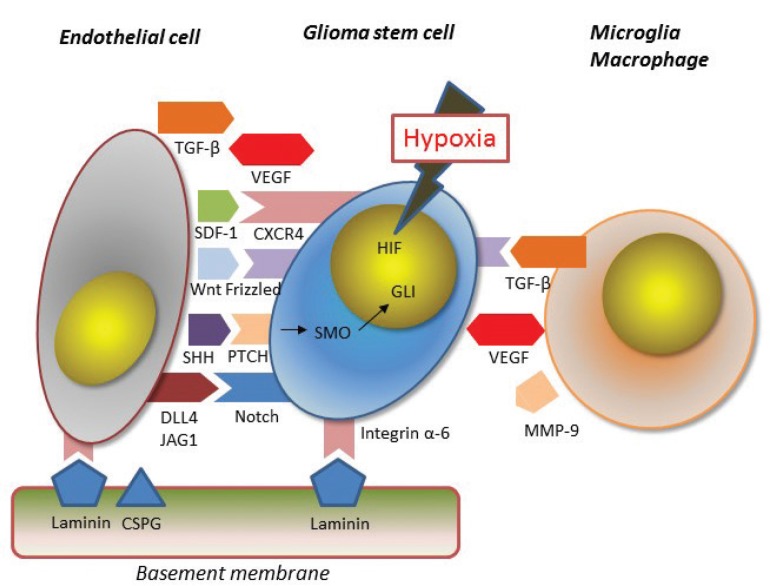
There are three specific anatomical and functional locations which are suitable for maintenance of the stem cell phenotype, namely, the perivascular, perinecrotic, and immune niches.49) Away from such niches, GSCs are considered to go to differentiation processes or apoptosis.50) There is active bidirectional cross talk between GSCs and the cells or extracellular matrix in the niches; the niches enhance the stemness of GSCs and promote the escape of GSCs from apoptosis-inducing stimuli of the immune system or radiotherapy/chemotherapy (Fig. 1).51) On the contrary, GSCs themselves actively promote mesenchymal stem cell migration and angiogenesis around the niches mainly through soluble factors.52,53) This active influence of GSC upon the microenvironments may be one of the causes for establishment of metastatic lesions away from the original sites.
Fig. 1.
There is active bidirectional cross talk between GSCs and endothelial cells or extracellular matrix in the niches through Notch, Sonic Hedgehog, and Wnt/β-catenin signaling.
Perivascular niche:
The perivascular areas harbor numerous myeloid cells including macrophages as well as endothelial cells differentiated from the mesenchymal stem cells.52) The GSCs residing in the perivascular niche are in touch with such cell types and the extracellular matrix, and also under various secreted factors that play a role in maintaining stem cell phenotypes.52) Especially, endothelial cells have the most important role in the maintenance of stem cell features both in direct-contact and secretion of soluble factors.53) Although endothelial cells are considered to be mainly derived from mesenchymal stem cells, which are recruited from peripheral blood to form neo-angiogenesis in the ischemic areas, recent studies have found that some endothelial cells originates from tumor cells.54) A large part of endothelial cells (between 20% and 90%) in GBM contains the same gene alterations as the GBM cells.54) Stromal cell-derived factor-1 (SDF-1) secreted from endothelial cells attracts GCSs through CXCR4 signaling, and some recruited GSCs are finally differentiated into pericytes through endothelial cell-secreted TGF-β.55) The perivascular niche is abundant with the extracellular matrix proteins, such as laminin, collagen, and proteoglycan, while GSCs are enriched for integrin α6 which functions as a receptor for laminin.51) The integrin α6-mediated signaling induces survival and migration of GSC, and silencing of this system makes the self-renewal potential and tumorigenicity of GBM cells reduced.56) Indeed, a major part of the integrin α6-expressing GSCs gather in the perivascular areas of GBM.56)
Perinecrotic niche:
The perinecrotic niche is surrounded by hypoxic areas, which is an essential feature of the glioma microenvironment.57,58) Hypoxia supports GSC survival and induces non-GSCs to acquire stem cell phenotype by some mechanisms such as hypoxia inducible factors (HIF), epigenetics, and metabolic reprogramming described below.16–18,59) Under hypoxic conditions, most importantly, the Notch signaling pathway is activated through HIF-1α in GSCs. This Notch activation, partly by the direct interaction with the ligand expressed on endothelial cells, leads to higher expressions of GSC markers.60) Hypoxia is also a major driving force to recruit the myeloid cells and mesenchymal stem cells through soluble factors produced by GSC such as vascular endothelial growth factor (VEGF).53) The recruited myeloid cells and mesenchymal stem cells are the main producers of pro-inflammatory cytokines, chemokines, proteases, and TGF-β, which can play an essential role for maintaining the stem cell phenotype.61,62)
Immune niche:
Glioma-infiltrating myeloid cells (GIMs) can modulate the tumor microenvironment toward immune-suppression, which allows the GSC to survive against various immunological tumor-eradicating forces.63) GIMs tend to localize in the immediate vicinity of CD133+GSC, which suggests a direct interaction between GSC and GIM or GIM-induced reprogramming of the glioma cells nearby into GSC.64) One of the mechanisms for the conversion of differentiated tumor cells into GSCs is EMT in which TGF-β secreted from GIM usually plays an important role.62,65) In vitro observation also emphasizes the importance of soluble factors including matrix metalloproteinase-9 (MMP-9) produced by microglia to promote migration of glioma cells. GIMs are mainly microglia at the early stage and newly-recruited monocyte-derived macrophages from the systemic circulation at the late stage.66) There are two different phenotypes of macrophages: M1 and M2. The M1 phenotype is associated with active microbial or tumor killing, while the M2 phenotype contributes to the suppression of Th1 adaptive immunity, resolution of inflammation, wound healing, and angiogenesis.67) In other words, the M1 macrophage is not permissive for novel cellular infiltration and the M2 type is permissive and not toxic to the newly infiltrating cells.68) Recent evidence suggests that GIMs are mainly the M2 phenotype, contribute to the formation of immunosuppressive microenvironments, and produce tumor progression.67,68)
Hypoxia as a master regulator for stemness-maintenance
Rapid tumor expansion makes neo-vascularization insufficient and disorganized for being in the hypoxic condition in and around the tumor masses.57,69) For the patients with GBM, hypoxia is a poor prognosis marker and is associated with a rapid tumor progression.70) Hypoxia induces the stem cell phenotype to glioma cells through at least four different mechanisms.
HIF:
Under hypoxia, the HIF family of transcriptional factor becomes stabilized by the inhibition of HIF prolyl-hydroxylase, leads to dimerization, and subsequently binds to the hypoxia-responsive elements (HREs) on the promotor regions of target genes affecting cell survival, proliferation, motility, and metabolisms.71) In contrast to HIF-1α which is expressed in both GSC and non-stem tumor cells, HIF-2α is expressed only in the GSC.72) HIF-1α is known to induce the activation of Notch pathway in GSC.60) The stem cell phenotype maintenance through hypoxic condition in glioma can be inhibited either by a knockdown of HIF-1α or an inactivation of the Notch pathway. HIF-2α up-regulates the expressions of pluripotency-related genes, including KLF4, Sox2 and Oct4.73) HIF-2α expression is also induced by acidic stress as well as hypoxia, and may affect the chromatin structure through activation of an epigenetic modifier such as the histone methyltransferases mentioned above.74) The HIF-2α-target genes include VEGF and the immunosuppressive cytokines, which recruit circulating myeloid cells into glioma tissues.74)
Epigenetics:
The epigenetic mechanism includes DNA methylation, methylation and acetylation of the histone proteins, chromatin structure modification, and non-coding RNAs. Hypoxia directly induces global DNA hypomethylation that is an epigenetic background for stem cell properties.20,75,76) Shahrzad et al. showed a direct reduction of 5-methylcytosine (5mC) levels in the tumor cells from colorectal cancer and melanoma under hypoxic conditions.75) Iwadate et al. reported that DNA hypomethylation in stem cell-related genes such as alkaline phosphatase and CD133 are frequently found around the necrotic foci in the clinical GBM samples.20) In the CD133-negative population of GSC, on the contrary, DNA hypermethylation in the CD133 promotor represses CD133 expression.76) S-adenosyl methionine (SAM), which is a main methyl-donor in human metabolism, could not be synthesized under the hypoxic condition because of the inactivation of methionine adenosyltransferase.77) In contrast, the DNA methylation status is considered rather stable to make a prompt shift to adjust the microenvironments, and histone modification may be the first responsive mechanism for hypoxia at least in some situations.78) Pickaerts et al. found in the MCF7 breast epithelial adenocarcinoma model that hypoxia rapidly induces tri-methylation at histone H3 lysine4 (H3K4me3) and lysine27 (H3K27me3) in the whole genome, and most of the histone methylation is reversed by re-oxygenation.78) Hypoxia induces the histone methyltransferase, mixed-lineage leukemia (MLL1), which increases GSC tumorigenic potential through HIF-2α and its target genes.35) As described above, Bmi1 acts as epigenetic silencers to control stem cell function in GSC as well as during embryonic development.30) This molecule is enriched along with other stem cell markers, CD133 and nestin, in response to hypoxia in GSC cultures.30) Non-coding RNAs play an important role in the embryonal neural development and GBM tumorigenesis.79) Among them, micro RNA (miRNA) has the most important roles in regulating the GSC phenotype.79) Some specific miRNAs work as crucial mediators of the hypoxia signaling in GBM as well as breast cancer.79)
Metabolic reprogramming:
GSCs in the hypoxic condition have altered glucose and glutamine metabolism, which directly impact the epigenetic state.80,81) α-ketoglutarate (α-KG) is a key TCA cycle metabolite that can be derived from glucose and/or glutamine, while GSC usually reduces TCA cycle-dependency, switching to anaerobic glycolysis, which reduces the production of α-KG.82) The Jumonji C family of histone and DNA demethylase use α-KG as a cofactor.82) A higher amount of α-KG promotes histone demethylation and DNA demethylation to maintain pluripotency in embryonic stem cells.6,82) In GSC, increased uptake of glutamine through glutamine transporter ASCT2 may compensate or surpass the decrease of α-KG brought about by anaerobic glycolysis dependency.8,83) Sufficient α-KG metabolized from glutamate is necessary to maintain the pluripotency of embryonal stem cells under the hypoxic conditions.84) Metabolic reprogramming can be accelerated by acidification of tumor microenvironments, and the acidic conditions promote the expressions of GSC markers through epigenetic mechanisms.85)
Soluble factors:
Under hypoxic conditions, GSCs produce high levels of TGF-β which induces the transcription factors essential for EMT, including TWIST, Snail, Slug, and ZEB, and to cause dedifferentiation of the cells.65,86) TGF-β is a major factor in recruitment of mesenchymal stem cells into the glioma tissues and promotes further malignant progression.87) GSCs also produce proangiogenic factors such as VEGF and SDF-1 which drive the migration of mesenchymal stem cells to promote neo-angiogenesis. The recruited mesenchymal cells and myeloid cells also release multiple growth factors including interleukin-6, interleukin-10, and MMPs, as well as TGF-β, VEGF, and SDF-1.88) Mahabir et al. reported that TGF-β released from mesenchymal cells in the tumor microenvironment can accelerate radiation-related induction of EMT in malignant gliomas.89) As mentioned in the Signal transduction section, Shh is an important soluble factor secreted from endothelial cells, which may be involved in the function of perivascular niche for glioma stem cells. Hypoxia also accelerates GSC-mediated immunosuppression through the phosphorylated Signal transducer and activator of transcription (pSTAT) activation, which results in the secretion of immunosuppressive cytokines such as colony stimulating factor-1 (CSF1) and chemokine (C-C motif) ligand-2 (CCL2) to inhibit T cell functions and macrophage phagocytosis.90,91)
Breakdown of the stemness-maintaining signals therapeutic implications
Recent advances in the study of GSC-specific cell surface markers, signaling pathways, and transcription factors have opened the window for novel targeted therapies. Some clinical trials employing γ-secretase inhibitors to suppress Notch signaling and an oral hedgehog antagonist are under investigation. The most important obstacle to be cleared for the development of novel targeted therapies is that there should be limited toxicities to the normal brain and other organs. We should consider the time sequential changes of the target molecules and their heterogeneous expressions within a tumor as well as among patients. Notch signals are essential not only in neural developments but in the maintenance of adult neural functions. The upregulation of HIF-1α is frequently observed in all cancer cells as well as transiently in normal neural progenitors, which limit the therapeutic applications. It is well known that not all GSCs express CD133 and a subgroup of CD133-negative GSCs have been identified in GBM patients. An unavoidable complexity in the targeted therapies is the heterogeneities that exist within each tumor as well as between patients. Thus, understanding the interplay between GSCs and their microenvironments is important to move toward establishment of more effective and personalized treatments for GBM patients.
As described above, maintenance of the stem cell phenotype depends mainly on hypoxia and recruited myeloid/mesenchymal-stem cells. This fact shows the hypoxic microenvironment could be a good therapeutic target in GBM. To modulate the glioma hypoxic microenvironment, an anti-VEGF therapy including bevacizumab may be a good candidate because vascular structures can be normalized at the early phase of the treatment.7) Vascular normalization improves oxygenation of the tumor tissues which induces differentiation of tumor cells, and enhances the efficacy of radiotherapy and chemotherapy by reforming vascular distribution, decreasing the interstitial pressure and improving drug delivery. In contrast, prolonged use of bevacizumab may result in a diminished vascular system and a resultant hypoxic tumor microenvironment, which leads to increased invasion and more aggressive growth by dedifferentiation to GSC.92) This may explain why bevacizumab is not efficacious for prolonging overall survival (OS) in spite of the improved progression-free survival in the large clinical studies for newly diagnosed patients with GBM. Furthermore, the key player in temozolomide resistance, O6-methylguanine-DNA methyltransferase (MGMT), is highly expressed in the hypoxic regions in GBM.93) The use of bevacizumab would be better limited to the short-term with some cytotoxic drugs administered.92) However, a detailed method of bevacizumab administration, such as when to start, for how long, at what doses, and with what drugs, should be determined in future clinical studies.
Hypoxia is observed not only during the glioma progression but also following radiation and/or chemotherapy. Radiation therapy is toxic to endothelial cells and degenerates the tumor vascular system, leading to further hypoxia in the tumor microenvironments.94,95) The exacerbated hypoxia after radiation therapy brings recurrent tumors more refractory nature. In addition, glioma cells that have survived radiation therapy themselves carry gene expression patterns for EMT-inducing signals that endow tumor cells a more aggressive nature.95) In contrast to the apparent growth suppressive effects against tumor cells, these adverse phenomena could occur in non-lethal tumor cells following radiation therapy. We should take this into account, as well as the neurotoxic effects of radiation therapy to the surrounding normal brain.68) One of the approaches to overcome this may be the targeted inhibition of Notch-1, TGF-β, or HIF-1α, which is suggested to enhance the efficacy of radiation therapy as initial treatment and also be effective as salvage therapy at recurrence.33,39,92,96) The indication of radiotherapy should be limited to really aggressive tumors like GBM, in which its advantages apparently exceed the disadvantages.68)
It is important to design a therapy against GBM not to artificially make a hypoxic microenvironment and to normalize the oxygen distributions in the tumor. A hypoxic microenvironment is a major obstacle for the intrinsic protective mechanisms including the immune system to work against tumor progression in the brain.
Footnotes
Conflicts of Interest Disclosure
The author reports no conflict of interest for this work.
References
- 1).Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A: Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol 129: 829–848, 2015 [DOI] [PubMed] [Google Scholar]
- 2).Liu C, Sage JC, Miller MR, et al. : Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 146: 209–221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Iwadate Y, Sakaida T, Hiwasa T, et al. : Molecular classification and survival prediction in human gliomas based on proteome analysis. Cancer Res 64: 2496–2501, 2004 [DOI] [PubMed] [Google Scholar]
- 4).Tanaka S, Louis DN, Curry WT, Batchelor TT, Dietrich J: Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat Rev Clin Oncol 10: 14–26, 2013 [DOI] [PubMed] [Google Scholar]
- 5).Phillips HS, Kharbanda S, Chen R, et al. : Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9: 157–173, 2006 [DOI] [PubMed] [Google Scholar]
- 6).Venneti S, Thompson CB: Metabolic reprogramming in brain tumors. Annu Rev Pathol Mech Dis 12: 515–545, 2017 [DOI] [PubMed] [Google Scholar]
- 7).Jain RK: Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307: 58–62, 2005 [DOI] [PubMed] [Google Scholar]
- 8).Cooper LA, Gutman DA, Chisolm C, et al. : The tumor microenvironment strongly impacts master transcriptional regulators and gene expression class of glioblastoma. Am J Pathol 180: 2108–2119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Kalluri R, Weinberg RA: The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Mani SA, Guo W, Liao MJ, et al. : The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN: The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 8: 3274–3284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Ye XZ, Xu SL, Xin YH, et al. : Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol 189: 444–453, 2012 [DOI] [PubMed] [Google Scholar]
- 13).Bao S, Wu Q, McLendon RE, et al. : Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760, 2006 [DOI] [PubMed] [Google Scholar]
- 14).Bhat KPL, Balasubramaniyan V, Vaillant B, et al. : Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 24: 331–346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Schonberg DL, Lubelski D, Miller TE, Rich JN: Brain tumor stem cells: Molecular characteristics and their impact on therapy. Mol Asp Med 39: 82–101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Berezovsky AD, Poisson LM, Cherba D, et al. : Sox2 promotes malignancy in glioblastoma by regulating plasticity and astrocytic differentiation. Neoplasia 16: 193–206, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Natsume A, Ito M, Katsushima K, et al. : Chromatin regulator PRC2 is a key regulator of epigenetic plasticity in glioblastoma. Cancer Res 73: 4559–4570, 2013 [DOI] [PubMed] [Google Scholar]
- 18).Lee G, Auffinger B, Guo D, et al. : Dedifferentiation of glioma cells to glioma stem-like cells by therapeutic stress-induced hif signaling in the recurrent gbm model. Mol Cancer Ther 15: 3064–3076, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Sugimori M, Hayakawa Y, Boman BM, et al. : Discovery of power-law growth in the self-renewal of heterogeneous glioma stem cell populations. PLoS ONE 10: e0135760, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Iwadate Y, Suganami A, Tamura Y, et al. : The Pluripotent Stem-Cell Marker Alkaline Phosphatase is Highly Expressed in Refractory Glioblastoma with DNA Hypomethylation. Neurosurgery 80: 248–256, 2017 [DOI] [PubMed] [Google Scholar]
- 21).Reya T, Morrison SJ, Clarke MF, Weissman IL: Stem cells, cancer, and cancer stem cells. Nature 414: 105–111, 2001 [DOI] [PubMed] [Google Scholar]
- 22).Beatus P, Lendahl U: Notch and neurogenesis. J Neurosci Res 54: 125–136, 1998 [DOI] [PubMed] [Google Scholar]
- 23).Pei Y, Brun SN, Markant SL, et al. : WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development 139: 1724–1733, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Chiang C, Litingtung Y, Lee E, et al. : Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383: 407–413, 1996 [DOI] [PubMed] [Google Scholar]
- 25).Lee J, Kotliarova S, Kotliarov Y, et al. : Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9: 391–403, 2006 [DOI] [PubMed] [Google Scholar]
- 26).Li Z, Wang H, Eyler CE, Hjelmeland AB, Rich JN: Turning cancer stem cells inside out: an exploration of glioma stem cell signaling pathways. J Biol Chem 284: 16705–16709, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Ikushima H, Todo T, Ino Y, et al. : Glioma-initiating cells retain their tumorigenicity through integration of the Sox axis and Oct4 protein. J Biol Chem 286: 41434–41441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Niu CS, Li DX, Liu YH, Fu XM, Tang SF, Li J: Expression of NANOG in human gliomas and its relationship with undifferentiated glioma cells. Oncol Rep 26: 593–601, 2011 [DOI] [PubMed] [Google Scholar]
- 29).Wang J, Wang H, Li Z, et al. : c-Myc is required for maintenance of glioma cancer stem cells. PLoS ONE 3: e3769, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Acquati S, Greco A, Licastro D, et al. : Epigenetic regulation of survivin by Bmi1 is cell type specific during corticogenesis and in gliomas. Stem Cells 31: 190–202, 2013 [DOI] [PubMed] [Google Scholar]
- 31).Kopan R: Notch: a membrane-bound transcription factor. J Cell Sci 115: 1095–1097, 2002 [DOI] [PubMed] [Google Scholar]
- 32).Gilbert CA, Daou MC, Moser RP, Ross AH: Gamma-secretase inhibitors enhance temozolomide treatment of human gliomas by inhibiting neurosphere repopulation and xenograft recurrence. Cancer Res 70: 6870–6879, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Saito N, Aoki K, Hirai N, et al. : Effect of Notch expression in glioma stem cells on therapeutic response to chemo-radiotherapy in recurrent glioblastoma. Brain Tumor Pathol 32: 176–183, 2015 [DOI] [PubMed] [Google Scholar]
- 34).Zhu TS, Costello MA, Talsma CE, et al. : Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res 71: 6061–6072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Heddleston JM, Wu Q, Rivera M, et al. : Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell tumorigenic potential. Cell Death Differ 19: 428–439, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG: Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol 177: 1491–1502, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A: HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 17: 165–172, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Yan GN, Yang L, Lv YF, et al. : Endothelial cells promote stem-like phenotype of glioma cells through activating the hedgehog pathway. J Pathol 234: 11–22, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Ulasov IV, Nandi S, Dey M, Sonabend AM, Lesniak MS: Inhibition of sonic hedgehog and notch pathways enhances sensitivity of CD133+ glioma stem cells to temozolomide therapy. Mol Med 17: 103–112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Zhu T, Li X, Luo L, et al. : Reversion of malignant phenotypes of human glioblastoma cells by β-elemene through β-catenin-mediated regulation of stemness-, differentiation- and epithelial-to-mesenchymal transition-related molecules. J Transl Med 13: 356, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Clevers H: Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480, 2006 [DOI] [PubMed] [Google Scholar]
- 42).Rossi M, Magnoni L, Miracco C, et al. : β-catenin and Gli1 are prognostic markers in glioblastoma. Cancer Biol Ther 11: 753–761, 2011 [DOI] [PubMed] [Google Scholar]
- 43).Zhang N, Wei P, Gong A, et al. : FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell 20: 427–442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Kaur N, Chettiar S, Rathod S, et al. : Wnt3a mediated activation of Wnt/β-catenin signaling promotes tumor progression in glioblastoma. Mol Cell Neurosci 54: 44–57, 2013 [DOI] [PubMed] [Google Scholar]
- 45).Hambardzumyan D, Squatrito M, Carbajal E, Holland EC: Glioma formation, cancer stem cells, and akt signaling. Stem Cell Rev 4: 203–210, 2008 [DOI] [PubMed] [Google Scholar]
- 46).Moscatello DK, Holgado-Madruga M, Emlet DR, Montgomery RB, Wong AJ: Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J Bil Chem 273: 200–206, 1998 [DOI] [PubMed] [Google Scholar]
- 47).Radke J, Bortolussi G, Pagenstecher A: Akt and c-Myc induce stem-cell markers in mature primary p53−/− astrocytes and render these cells gliomagenic in the brain of immunocompetent mice. PLoS ONE 8: e56691, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Wei Y, Jiang Y, Zou F, et al. : Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proc Natl Acad Sci USA 110: 6829–6834, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Codrici E, Enciu A-M, Popescu I-D, Mihai S, Tanase C: Glioma stem cells and their microenvironments: Providers of challenging therapeutic targets. Stem Cell Int 2016: Article ID 5728438, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Sharma A, Shiras A: Cancer stem cell-vascular endothelial cell interactions in glioblastoma. Biochem Biophys Res Commun 473: 688–692, 2016 [DOI] [PubMed] [Google Scholar]
- 51).Christensen K, Schrøder HD, Kristensen BW: CD133+ niches and single cells in glioblastoma have different phenotypes. J Neurooncol 104: 129–143, 2011 [DOI] [PubMed] [Google Scholar]
- 52).Calabrese C, Poppleton H, Kocak M, et al. : A perivascular niche for brain tumor stem cells. Cancer Cell 11: 69–82, 2007 [DOI] [PubMed] [Google Scholar]
- 53).Folkins C, Shaked Y, Man S, et al. : Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res 69: 7243–7251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Ricci-Vitiani L, Pallini R, Biffoni M, et al. : Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468: 824–828, 2010 [DOI] [PubMed] [Google Scholar]
- 55).Cheng L, Huang Z, Zhou W, et al. : Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153: 139–152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Lathia JD, Gallagher J, Heddleston JM, et al. : Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 6: 421–432, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Carmeliet P, Jain RK: Angiogenesis in cancer and other diseases. Nature 407: 249–257, 2000 [DOI] [PubMed] [Google Scholar]
- 58).Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN: The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 8: 3274–3284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Wang P, Wan WW, Xiong SL, Feng H, Wu N: Cancer stem-like cells can be induced through dedifferentiation under hypoxic conditions in glioma, hepatoma and lung cancer. Cell Death Discov 3: 16105, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Qiang L, Wu T, Zhang HW, et al. : HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ 19: 284–294, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Behnan J, Isakson P, Joel M, et al. : Recruited brain tumor-derived mesenchymal stem cells contribute to brain tumor progression. Stem Cells 32: 1110–1123, 2014 [DOI] [PubMed] [Google Scholar]
- 62).Iwadate Y: Epithelial-mesenchymal transition in glioblastoma progression. Oncol Lett 11: 1615–1620, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Roggendorf W, Strupp S, Paulus W: Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol 92: 288–293, 1996 [DOI] [PubMed] [Google Scholar]
- 64).Yi L, Xiao H, Xu M, et al. : Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol 232: 75–82, 2011 [DOI] [PubMed] [Google Scholar]
- 65).Ye XZ, Xu SL, Xin YH, et al. : Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol 189: 444–453, 2012 [DOI] [PubMed] [Google Scholar]
- 66).Könnecke H, Bechmann I: The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin Dev Immunol 914104, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Wei J, Gabrusiewicz K, Heimberger A: The contoversial role of microglia in malignant gliomas. Clin Dev Immunol 2013: 285246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Iwadate Y, Fukuda K, Matsutani T, Saeki N: Intrinsic protective mechanisms of the neuron-glia network against glioma invasion. J Clin Neurosci 41: 33–43, 2016 [DOI] [PubMed] [Google Scholar]
- 69).Rong Y, Durden DL, Van Meir EG, Brat DJ: ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol 65: 529–539, 2006 [DOI] [PubMed] [Google Scholar]
- 70).Evans SM, Judy KD, Dunphy I, et al. : Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res 10: 8177–8184, 2004 [DOI] [PubMed] [Google Scholar]
- 71).Keith B, Simon MC: Hypoxia-inducible factors, stem cells, and cancer. Cell 129: 465–472, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Li Z, Bao S, Wu Q, et al. : Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15: 501–513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Bhagat M, Palanichamy JK, Ramalingam P, et al. : HIF-2α mediates a marked increase in migration and stemness characteristics in a subset of glioma cells under hypoxia by activating an Oct-4/Sox-2-Mena (INV) axis. Int J Biochem Cell Biol 74: 60–71, 2016 [DOI] [PubMed] [Google Scholar]
- 74).Nakazawa MS, Eisinger-Mathason TS, Sadri N, et al. : Epigenetic re-expression of HIF-2α suppresses soft tissue sarcoma growth. Nat Commun 7: 10539, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Shahrzad S, Bertrand K, Minhas K, Coomber BL: Induction of DNA hypomethylation by tumor hypoxia. Epigenetics 2: 119–125, 2007 [DOI] [PubMed] [Google Scholar]
- 76).Baysan M, Woolard K, Bozdag S, et al. : Micro-environment causes reversible changes in DNA methylation and mRNA expression profiles in patient-derived glioma stem cells. PLoS ONE 9: e94045, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Hermes M, Geisler H, Osswald H, Riehle R, Kloor D: Alterations in S-adenosylhomocysteine metabolism decrease O6-methylguanine DNA methyltransferase gene expression without affecting promoter methylation. Biochem Pharmacol 75: 2100–2111, 2008 [DOI] [PubMed] [Google Scholar]
- 78).Prickaerts P, Adriaens ME, Beucken TVD, et al. : Hypoxia increases genome-wide bivalent epigenetic marking by specific gain of H3K27me3. Epigenetics Chromatin 9: 46, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Carmona-Gutierrez D, Ruckenstuhl C, Bauer MA, Eisenberg T, Büttner S, Madeo F: Cell death in yeast: growing applications of a dying buddy. Cell Death Differ 17: 733–734, 2010 [DOI] [PubMed] [Google Scholar]
- 80).Yuen CA, Asuthkar S, Guda MR, Tsung AJ, Velpula KK: Cancer stem cell molecular reprogramming of the Warburg effect in glioblastomas: a new target gleaned from an old concept. CNS Oncol 5: 101–108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).DeBerardinis RJ, Mancuso A, Daikhin E, et al. : Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 104: 19345–19350, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Márquez J, Alonso FJ, Matés JM, Segura JA, Martín-Rufián M, Campos-Sandoval JA: Glutamine addiction in gliomas. Neurochem Res 42: 1735–1746, 2017 [DOI] [PubMed] [Google Scholar]
- 83).Szeliga M, Albrecht J: Glutamine metabolism in gliomas. Adv Neurobiol 13: 259–273, 2016 [DOI] [PubMed] [Google Scholar]
- 84).Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB: Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518: 413–416, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Hjelmeland AB, Wu Q, Heddleston JM, et al. : Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ 18: 829–840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Bierie B, Moses HL: Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 6: 506–520, 2006 [DOI] [PubMed] [Google Scholar]
- 87).Bruna A, Darken RS, Rojo F, et al. : High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 11, 147–160, 2007 [DOI] [PubMed] [Google Scholar]
- 88).Kahlert UD, Nikkhah G, Maciaczyk J: Epithelial-to-mesenchymal (-like) transition as a relevant molecular event in malignant gliomas. Cancer Let 33:131–138, 2013 [DOI] [PubMed] [Google Scholar]
- 89).Mahabir R, Tanino M, Elmansuri A, et al. : Sustained elevation of Snail promotes glial-mesenchymal transition after irradiation in malignant glioma. Neuro-oncology 16: 671–685, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90).Chang AL, Miska J, Wainwright DA, et al. : CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory t cells and myeloid-derived suppressor cells. Cancer Res 76: 5671–5682, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).De I, Steffen MD, Clark PA, et al. : CSF1 overexpression promotes high-grade glioma formation without impacting the polarization status of glioma-associated microglia and macrophages. Cancer Res 76: 2552–2560, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Zhang M, Kleber S, Röhrich M, et al. : Blockade of TGF-β signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res 71: 7155–7167, 2011 [DOI] [PubMed] [Google Scholar]
- 93).Pistollato F, Abbadi S, Rampazzo E, et al. : Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells 28: 851–862, 2010 [DOI] [PubMed] [Google Scholar]
- 94).Kim YH, Yoo KC, Cui YH, et al. : Radiation promotes malignant progression of glioma cells through HIF-1alpha stabilization. Cancer Let 354: 132–141, 2014 [DOI] [PubMed] [Google Scholar]
- 95).Zhou YC, Liu JY, Li J, et al. : Ionizing radiation promotes migration and invasion of cancer cells through transforming growth factor-beta-mediated epithelial-mesenchymal transition. Int J Radiat Oncol Biol Phys 81: 1530–1537, 2011 [DOI] [PubMed] [Google Scholar]
- 96).Hardee ME, Marciscano AE, Medina-Ramirez CM, et al. : Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res 72: 4119–4129, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]