Abstract
We report a 62-year-old white woman with metastatic choroidal melanoma who developed immune checkpoint inhibitor (ICI)-induced enteritis and grade 3 diarrhea refractory to steroids and infliximab. Her diarrhea quickly resolved after infusion of vedolizumab, and the patient was able to taper down steroids. Vedolizumab’s mechanism of action and its gut specificity have the potential to reverse immune-induced enterocolitis without neutralizing or reversing the therapeutic benefit of ICI on the malignancy.
Introduction
Immune checkpoints inhibitors (ICI), such as nivolumab and ipilimumab, have revolutionized treatments of metastatic malignancies, particularly melanoma.1-3 Given their mechanism of action, these drugs can trigger various organ-specific immune-related adverse events, such as enterocolitis mimicking inflammatory bowel disease.4 Discontinuation of ICI therapy and the rapid institution of immunosuppressive therapy (within 5 days) is needed to minimize the risk of complications, such as bowel perforation and death.1 For grade 1 diarrhea, symptomatic treatment with loperamide and rehydration is advised. For grade 2 diarrhea, steroid therapy with either budesonide or 1 mg/kg oral prednisone is recommended. In severe diarrhea (grade 3 or higher), high-dose intravenous (IV) steroids should be given. If no improvement is seen after 3–5 days, a dose of infliximab has been typically and successfully used to treat ICI-induced enterocolitis in most patients.1-4
Case Report
A 62-year-old white woman, diagnosed with choroidal melanoma in 2009 and treated with enucleation and adjuvant sunitinib therapy, presented with recurrent metastatic disease 5 years later. Nivolumab and ipilimumab were started, but ipilimumab was discontinued after 2 doses because of ICI-induced hepatitis, which was treated with a course of prednisone. Twelve weeks after restarting nivolumab, the patient developed severe diarrhea refractory to oral prednisone and to discontinuation of nivolumab. Stool studies, including stool polymerase chain reaction for Clostridium difficile were negative. Her C-reactive protein level was 1.8 mg/L. Colonoscopy revealed normal colonic mucosa but mild erythema in the terminal ileum. The pathology of random colon biopsies was unremarkable, but ileal biopsies showed mild expansion of the lamina propria by chronic inflammatory cells, including plasma cells and acute cryptitis. Upper endoscopy showed severely denuded, fissured, and friable duodenal mucosa (Figure 1). The pathology showed marked acute inflammatory cell infiltrates involving most of the glandular epithelium, as well as crypt abscesses. Duodenal villi were blunted and minimally congested, without granulomas or intranuclear inclusion bodies (Figure 2). T-cell phenotyping showed an increased number of T cells (13%) in the duodenum, and 40% of those were positive for CD3 and CD4. Immunostaining for herpes simplex virus and cytomegalovirus was negative.
Figure 1.
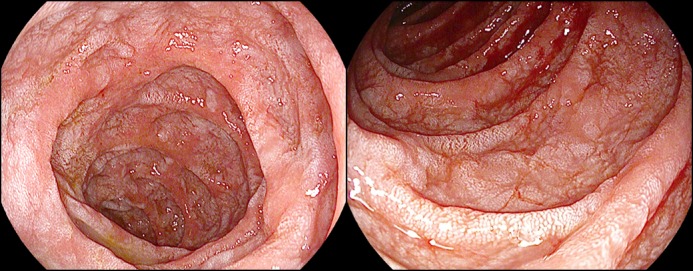
Upper endoscopy showing severely denuded, fissured, and friable duodenal mucosa.
Figure 2.
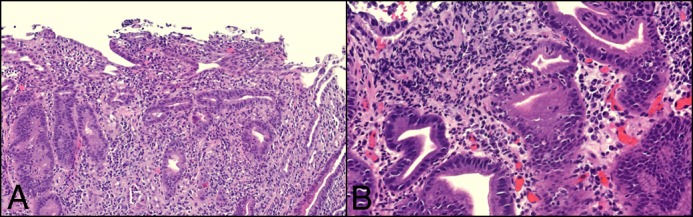
Biopsy slides. (A) Duodenal villi are blunted and minimally congested. (B) Deep crypt infiltration and several crypt abscesses are noted. A significant expansion of the lamina propria is noted in the presence of chronic inflammatory cells, including numerous plasma cells. There is no evidence of granulomas or intranuclear inclusion bodies.
The patient was diagnosed with grade 3 ICI-induced enteritis and started on 80 mg/day IV methylprednisolone. Because of persistent diarrhea, she received 5 mg/kg infliximab at week 0, 2, 6, and 14, but she remained steroid-dependent. In addition, she developed central venous catheter–induced methicillin-sensitive Staphylococcus aureus bacteremia after her first infliximab infusion. An infliximab trough level drawn 6 weeks after the fourth infusion was 34 μg/mL with no detectable antibody to infliximab. She was therefore deemed to be a primary non-responder to infliximab, and she was maintained on steroids.
Re-staging of her melanoma in December 2016 showed interval disease progression, and she was restarted on nivolumab and ipilimumab. Within 4 weeks, she developed severe diarrhea again, complicated by acute renal failure. Computed tomography of her abdomen with contrast excluded small-bowel metastasis. Three days after the first dose of 300 mg IV vedolizumab, the patient had a dramatic symptomatic improvement. After the second dose of 300 mg IV vedolizumab at week 2, she had 2 formed bowel movements per day, and prednisone was tapered down to 10 mg/day; without additional doses of vedolizumab, she was able to resume nivolumab and remained diarrhea-free at her 6-month follow-up.
Discussion
We describe a case of severe ICI-induced enteritis secondary to combination nivolumab and ipilimumab therapy and refractory to steroid and to infliximab despite therapeutic infliximab trough levels that responded rapidly and completely to vedolizumab.
Our case highlights some additional points in the management of ICI-induced enterocolitis. First, complete evaluation with upper endoscopy and colonoscopy with terminal ileum intubation is recommended to avoid overlooking ICI-induced enteritis. Most reports of ICI-induced gastrointestinal adverse reactions focused on findings of colitis. In our experience, nivolumab often caused an isolated enteritis with normal colonic examination. Second, in patients refractory to a first or second dose of infliximab, the drug trough level should be obtained; this will help determine whether the patient would benefit from dose escalation (if the trough level is low) or from switching to a different class of drug (if an adequate drug level is seen). Third, a multidisciplinary approach involving a gastroenterologist, an oncologist, and a pathologist who are familiar with the disease process and the treatment of ICI-induced enterocolitis is crucial in caring for such patients. Finally, this case highlights the potential role of vedolizumab in refractory ICI enterocolitis.
There are few recent case reports highlighting the use of vedolizumab in ipilimumab-induced colitis.5-7 To our knowledge, this is the first reported case of the use of vedolizumab in ICI-induced enteritis refractory to steroids and to infliximab. Response to steroids can be suboptimal in grade 3 diarrhea, and there is a need for a potent but safe therapeutic alternative. ICI increases the percent of activated CD4+ and CD8+ T cells, which can lead to autoimmune enterocolitis. Vedolizumab is a humanized murine antibody with activity against the α4β7-integrin heterodimer on the surface of CD4+ T cells.8 The α4β7-integrin binds to its ligand MAdCAM-1, which is expressed on the endothelial surface of venules within the gut and its associated lymphoid tissue, facilitating the trafficking of T cells into the gut mucosa. By blocking the interaction between α4β7-integrin and MadCAM-1, vedolizumab prevents T cells from binding and homing into the inflamed bowel mucosa, which can explain its efficacy in ICI-mediated enterocolitis.8 Being gut-specific, vedolizumab has an excellent safety profile, and it is less likely to reverse the therapeutic benefit of ICI.9 Hence, vedolizumab should be considered as a potential first-line biologic in the treatment of patients with ICI-induced enterocolitis refractory to steroid or of steroid-dependent patients.
Disclosures
Author contributions: P. Diana and C. Mankongpaisarnrung wrote and edited the manuscript. MB Atkins, JC Zeck, and A. Charabaty edited the manuscript. A. Charabaty is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Reference
- 1.Graziani G, Tentori L, Navarra P. Ipilimumab: A novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol Res. 2012;65:9–22. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- 4.Rastogi P, Sultan M, Charabaty AJ, Atkins MB, Mattar MC. Ipilimumab-associated colitis: An IpiColitis case series at MedStar Georgetown University Hospital. World J Gastroenterol. 2015;21:4373–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66:581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh AH, Ferman M, Brown MP, Andrews JM. Vedolizumab: A novel treatment for ipilimumab-induced colitis. BMJ Case Rep. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarini AA, Hruz P, Berger CT, et al. Vedolizumab as a successful treatment of CTLA 4-associated autoimmune enterocolitis. J Allergy Clin Immunol. 2017;139:1043–6. [DOI] [PubMed] [Google Scholar]
- 8.McLean LP, Shea-Donohue T, Cross RK. Vedolizumab for the treatment of ulcerative colitis and Crohn's disease. Immunotherapy. 2012;4:883–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut. 2017;66:839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


