Abstract
Objectives:
There is an increasing need to identify reliable biomarkers for distinguishing Crohn’s disease (CD) from other gastrointestinal disorders sharing similar clinical and pathological features. This study aimed at evaluating the diagnostic potential of antibodies to zymogen granule glycoprotein GP2 (aGP2) in a large, well-defined Chinese cohort with a special focus on their role in discriminating CD from intestinal Behçet's disease (BD) and intestinal tubercolosis (ITB).
Methods:
A total of 577 subjects were prospectively enrolled, including 171 patients with CD, 208 patients with ulcerative colitis (UC), 71 with BD, 57 with ITB and 70 healthy controls (HC). aGP2 and anti-Saccharomyces cerevisiae antibodies (ASCA) were determined by ELISA. Perinuclear antineutrophil cytoplasmic antibodies were tested by indirect immunofluorescent assay.
Results:
aGP2 IgG and IgA levels were significantly elevated in patients with CD compared with those in patients with UC, intestinal BD, and ITB and HC. Conversely, ASCA IgG levels were not different between CD and intestinal BD patients, whereas ASCA IgA levels did not discriminate CD from intestinal BD and ITB patients. aGP2 IgA and IgG displayed a better assay performance (larger areas under the curve) over ASCA IgA and IgG in differentiating CD from disease controls (P<0.05). ASCA IgA did not discriminate CD from disease controls. aGP2 IgA and/or IgG was significantly associated with penetrating disease (B3) and ileal CD (L1) (P<0.05), whereas ASCA IgA and/or IgG was not.
Conclusions:
In comparison with ASCA, aGP2 distinguishes CD from intestinal BD or ITB as disease controls more efficiently, aiding in the differential diagnosis of IBD.
Introduction
Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), represents a group of chronic inflammatory disorders in the gastrointestinal (GI) tract.1, 2 IBD have multifactorial etiology, which includes dysregulation of the immune response to the gut microbiota, genetic susceptible polymorphism and environmental risk factors.3
Currently, the diagnosis of CD remains a tremendous challenge to physicians, and most patients with CD suffer from a diagnostic delay (a period from appearance of first symptoms to diagnosis), which may lead to a complicated disease course and increased operation rate.4 As CD covers a series of rather non-specific clinical symptoms including intestinal and extra-intestinal involvements, other disorders affecting the GI with similar clinical manifestations create a diagnostic dilemma. Particularly, intestinal tuberculosis (ITB) and intestinal Behçet's disease (BD) often present similar clinical symptoms and pathology to those seen in CD patients.5, 6 In addition, subclassification of IBD into CD and UC poses another diagnostic dilemma. CD and UC display considerable differences in terms of lesion localization in the GI and histopathologic presentations,1, 2 resulting in significant differences in clinical management and therapy options. Furthermore, stratification of 10–15% of all IBD patients is also challenging in the case of indeterminate colitis due to undiscriminating biopsy data and a certain overlap of colonic inflammation symptoms in IBD patients.7, 8
Serological biomarkers have gained extensive attention over the past decade due to their ready availability and non-invasiveness, and, hence, have been used complementary to endoscopic and histological tests. Anti-saccharomyces cerevisiae antibodies (ASCA) and perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are routinely utilized for screening patients with clinical suspicion of IBD.9 However, it has been suggested that the discriminatory capability of ASCA in CD is far from satisfactory due to its unsatisfactory sensitivity and specificity, especially in Asia.10, 11, 12 For example, studies from our group and others have found that ASCA displayed a poor clinical performance in differentiating CD from ITB,12, 13, 14 highlighting a critical need for developing new biomarkers.
In light of recent research on the antigenic targets of pancreatic autoantibodies (PAB), zymogen granule glycoprotein 2 (GP2) stood out as a major autoantigen of PAB.15, 16, 17 GP2, which is a highly glycosylated protein with two major sites of synthesis (pancreatic, intestinal), is overexpressed at the site of intestinal inflammation in patients with CD, indicating a direct involvement of GP2 in the inflammatory process.15 In fact, anti-GP2 antibodies (aGP2) were present in 21–45% patients with CD and significantly less prevalent in patients with UC.18 Of interest, ASCA-negative CD patients were tested positive for aGP2 IgA and/or IgG, highlighting the potential of aGP2 in the serological diagnosis of CD.19, 20 We have previously demonstrated that IgA/IgG aGP2 were present in 54.3% of Chinese patients with CD and just 14.3% with UC in small cohorts of patients with IBD (35 CD and CU patients, each), supporting the aforementioned diagnostic potential of aGP2.21 However, the small sample size may have introduced analytic bias and, thus, a larger cohort study is badly needed. Furthermore, to our knowledge, no study has assessed the clinical relevance of aGP2 in distinguishing CD from ITB and intestinal BD. Given the high prevalence of intestinal BD or ITB in China and other Asian countries as well as the diagnostic challenge of differentiating those two diseases from CD, it is of paramount importance to assess the clinical significance of aGP2 accordingly. Thus, in the present study, we included a large cohort of IBD patients, including 171 patients with CD and 208 with UC as well as 128 disease controls (71 patients with intestinal BD and 57 patients with ITB) for the evaluation of the diagnostic performance of aGP2.
Materials and methods
Subjects and disease stratification
A total of 577 subjects were prospectively enrolled in this study, including 171 patients with CD, 208 patients with UC, 71 patients with intestinal BD, and 57 patients with ITB and 70 healthy controls (HC). HC included subjects without any signs of infection or inflammation or other apparent illnesses. All patients were diagnosed and managed at the Department of Gastroenterology, Peking Union Medical College Hospital (PUMCH). The diagnosis of IBD was determined based on the Lennard-Jones criteria.22 Accordingly, subjects were diagnosed with CD or UC based on a combination of standard criteria that included clinical symptoms, physical examination, colonoscopy, imaging (bariums studies and CT enterography), and histopathology. Patients with enteric infections, ischemia, non-steroidal anti-inflammatory drug induced ulceration, and radiation colitis were excluded. Clinical phenotypes of IBD patients were determined based on the Montreal Classification 23 (age at diagnosis: A1, below 17 years; A2, between 17 and 40 years; A3, above 40 years, location of disease: L1, ileal; L2, colonic; L3, ileocolonic; L4, upper disease, disease behavior: B1, non-stricturing, non-penetrating; B2, stricturing; B3, penetrating; P, perianal disease modifier). Patients with UC were classified by E classification (E1, proctitis, lesions limited to the rectum; E2, left-sided colitis, lesions below the splenic flexure; E3, pancolitis, lesions exceeded the splenic flexure). The activity of UC was defined by the Simple Clinical Colitis Activity Index (SCCAI) as mild (3–5 scores), moderate (6–11 scores) and severe (above 12 scores). The activity of CD was defined by Crohn's Disease Activity Index (CDAI), as previously described with CDAI scores<150 as symptomatic remission and CDAI scores ≥150 as active disease24, 25. Specifically, the activity of CD was defined as mild (CDAI scores of 150–220), moderate (CDAI scores of 221–450) and severe (CDAI scores of >450). The demographics and clinical characteristics of the CD and UC patients are shown in Table 1. Study protocols were reviewed and approved by the Ethical Committee of PUMCH and informed consents were obtained from all participants. All sera were stored at−20 °C until analysis.
Table 1. Demographics of patients with inflammatory bowel disease and controls.
| CD (n=171) | UC (n=208) | Intestinal BD (n=71) | ITB (n=57) | HC (n=70) | |
|---|---|---|---|---|---|
| Female, n (%) | 45 (26.3) | 95 (45.7) | 32 (45.1) | 34 (59.6) | 38 (54.3) |
| Median age at study (years, max, min) | 33 (85, 10) | 43 (77, 12) | 38 (73,10) | 43 (76, 14) | 45.5 (70, 19) |
| Median duration (years, max, min) | 5 (39, 0.1) | 4 (40, 0.1) | 3.5 (24, 0.1) | 1 (20, 0.1) | N.A. |
| Median age at diagnosis (years, max, min) | 27 (69,7) | 36 (70,12) | 31 (70, 9) | 34.5 (67, 18) | N.A. |
| Age at diagnosis, n (%) | |||||
| Below 17 years (A1) | 29 (17.0) | 9 (4.3) | 9 (12.7) | 0 (0) | N.A. |
| Between 17 and 40 years (A2) | 105 (61.4) | 117(56.3) | 31 (43.7) | 34 (59.6) | N.A. |
| Above 40 years (A3) | 37 (21.6) | 82(39.4) | 31 (43.7) | 23 (40.4) | N.A. |
| Disease location, n (%) | |||||
| Proctitis (E1) | N.A. | 8 (3.8) | N.A. | N.A. | N.A. |
| Left-sided colitis (E2) | N.A. | 49 (23.6) | N.A. | N.A. | N.A. |
| Pancolitis (E3) | N.A. | 151 (72.6) | N.A. | N.A. | N.A. |
| Ileal (L1) | 36 (21.1) | N.A. | 6 (8.5) | 11 (19.3) | N.A. |
| Colonic (L2) | 39 (22.8) | N.A. | 20 (28.2) | 14 (24.6) | N.A. |
| Ileocolonic(L3) | 96 (56.1) | N.A. | 45 (63.4) | 32 (56.1) | N.A. |
| Upper disease, modifier (L4) | 11 (6.4) | N.A. | 9 (12.7) | 0 (0) | N.A. |
| Disease behavior, n (%) | |||||
| Non-stricturing, non-penetrating (B1) | 54 (31.6) | N.A. | N.A. | N.A. | N.A. |
| Stricturing (B2) | 58 (33.9) | N.A. | N.A. | N.A. | N.A. |
| Penetrating (B3) | 31 (18.1) | N.A. | N.A. | N.A. | N.A. |
| Stricturing and penetrating (B2+B3) | 28 (16.4) | N.A. | N.A. | N.A. | N.A. |
| Perianal disease (p) | 65 (38.0) | N.A. | N.A. | N.A. | N.A. |
| Disease severity, n (%) | |||||
| Symptomatic remission | 86 (50.3) | 40 (19.2) | N.A. | N.A. | N.A. |
| Mild | 25 (14.6) | 30 (14.4) | N.A. | N.A. | N.A. |
| Moderate | 44 (25.7) | 65 (31.3) | N.A. | N.A. | N.A. |
| Severe | 16 (9.4) | 73 (35.1) | N.A. | N.A. | N.A. |
| Extraintestinal manifestations | |||||
| Musculoskeletal | 26 (15.2) | 36 (17.3) | N.A. | N.A. | N.A. |
| Dermatologic | 46 (26.9) | 33 (15.9) | N.A. | N.A. | N.A. |
| Ocular | 4 (2.3) | 4 (1.9) | N.A. | N.A. | N.A. |
| Primary sclerosing cholangitis | 1 (0.5) | 2 (1.0) | N.A. | N.A. | N.A. |
| Thrombosis | 1 (0.5) | 3 (1.4) | N.A. | N.A. | N.A. |
| Treatment, n (%) | |||||
| 5-ASA | 100 (58.5) | 161 (77.4) | N.A. | N.A. | N.A. |
| Immunosuppressive | 78 (45.6) | 35 (16.8) | N.A. | N.A. | N.A. |
| Steroids | 106 (62.0) | 132 (63.5) | N.A. | N.A. | N.A. |
| Response | 68 (64.2) | 80 (38.5) | N.A. | N.A. | N.A. |
| Resistance | 15 (14.2) | 24 (11.5) | N.A. | N.A. | N.A. |
| Dependence | 23 (21.7) | 28 (13.5) | N.A. | N.A. | N.A. |
| Previous surgery | 66 (38.6) | 28(13.5) | N.A. | N.A. | N.A. |
| GMA | 0 (0) | 4 (1.9) | N.A. | N.A. | N.A. |
| Anti-TNF therapy | 43 (25.2) | 8 (3.8) | N.A. | N.A. | N.A. |
| Median duration (years, max, min) | 1 (4.5, 0.1) | 0.3 (2.5, 0.1) | N.A. | N.A. | N.A. |
| Response | 33 (76.7) | 5 (2.4) | N.A. | N.A. | N.A. |
| Resistance | 2 (4.7) | 2 (1.0) | N.A. | N.A. | N.A. |
| Secondary non-response | 8 (18.6) | 1 (0.5) | N.A. | N.A. | N.A. |
Abbreviations: BD, Behçet's disease; CD, Crohn's disease; GMA, granulocyte and monocyte adsorption apheresis; HC, health controls; ITB, intestinal tuberculosis; TNF, tumor necrosis factor; UC, ulcerative colitis; 5-ASA, 5-aminosalicylic acid; N.A., not applicable. 5-ASA therapy is the first-line treatment for UC and CD. For steroids treatment, response referred to the patients who experienced reduced symptoms and GI inflammation after steroids treatments and the reduced disease was maintained throughout the whole treatment period. Resistance referred to patients who experienced a primary lack of drug efficacy in reducing their symptoms after steroids treatments. Dependence referred to the patients who experienced reduced symptoms and GI inflammation after steroids treatments, but the disease relapsed when the steroids were withdrawn. For anti-TNF therapy, response referred to the patients who experienced reduced symptoms and GI inflammation after anti-TNF therapy and the reduced disease was maintained throughout the whole treatment period. Resistance referred to patients who experienced a primary lack of drug efficacy in reducing their symptoms and GI inflammation after anti-TNF therapy. Secondary non-response referred to patients who failed to maintain an initial response due to acquired drug resistance.
Serum antibodies determination
Serum aGP2 IgG and IgA were determined by ELISA (Generic Assays, Dahlewitz/Berlin, Germany), according to the manufacturer’s instructions.21 The cutoff value for positivity was set to 15 U/ml for IgG aGP2 and 10 U/ml for IgA aGP2, as recommended by the manufacturer. Serum IgG ASCA and IgA ASCA were determined by ELISA (Inova Diagnostics, San Diego, USA). Values above 25 U/ml were considered as positive according to the manufacturer’s instructions. Serum IgG pANCA and IgA pANCA were tested by indirect immunofluorescent assay (IFA) (Euroimmune, Luebeck, Germany), in accordance with the manufacturer’s instructions. IFA testings were performed starting with an initial dilution of 1/10. Serial dilutions of 1/20, 1/40, 1/80, and 1/160 were further performed for all positive samples. Two experienced technologists interpreted the results.
Discriminatory capability of aGP2 and ASCA in differentiating CD vs. UC and CD vs. disease controls
Receiver operating characteristics (ROC) analysis was utilized to evaluate the discriminatory capability of aGP2 and ASCA in differentiating CD vs. UC and CD vs. disease controls. ROC curves were generated by plotting sensitivity vs. (1-specificity) for IgG aGP2, IgA aGP2, IgG ASCA and IgA ASCA. Areas under the curves (AUCs) with their corresponding 95% confidence intervals (CIs) were determined.
Statistical analysis
All statistical tests were performed by SPSS 20.0 statistical software package (SPSS Inc., Chicago, Illinois, USA), Prism 5.02 (GraphPad Software, San Diego, California, USA) and MedCalc (MedCalc Software, Ostend, Belgium). Quantitative variables were compared with a Kruskal-Wallis test followed by post-hoc analysis by Conover26. Categorical variables were compared with a χ2 test or Fisher’s exact testing for 4 × 4 contingency tables. The clinical relevance of multiple antibodies was assessed with logistic regression models for each clinical variable, and the results are presented as odds ratio with 95% CI. Receiver operator curves (ROCs), which were constructed by logistic regression models, as previously described27, were used to determine the discrimination power of aGP2 and ASCA. Spearman's rank correlation coefficient was used to assess the correlations between multiple autoantibodies and age at diagnosis. P values of less than 0.05 were considered significant.
Results
Levels and prevalence of CD-related antibodies in patients with CD, UC, intestinal BD, and ITB as well as HC
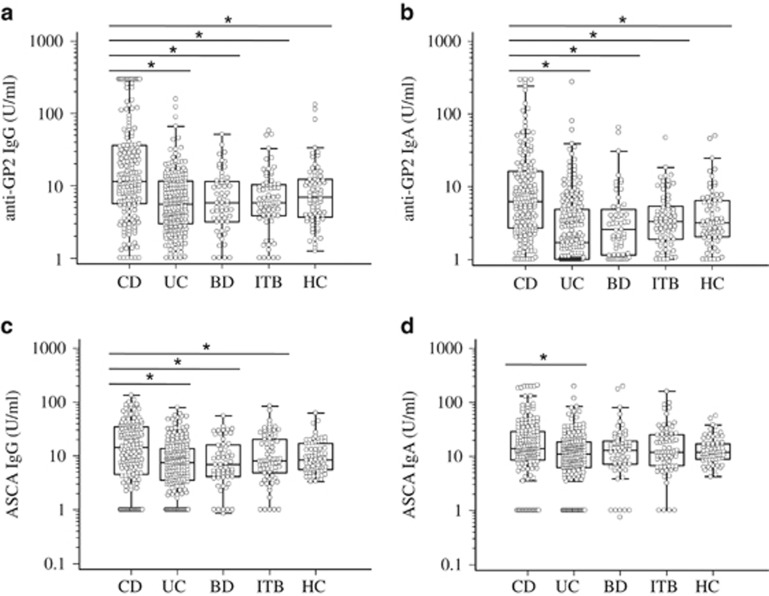
All CD-related antibodies demonstrated significantly different values in patients with CD, UC, intestinal BD, ITB and HC (Kruskal-Wallis test: P<0.05, respectively). The levels of IgG and IgA aGP2 were significantly elevated in patients with CD, compared to those in patients with UC, intestinal BD, and ITB as well as HC (post-hoc analysis, P<0.05) (Figure 1a). The prevalences of IgG aGP2, IgA aGP2, IgA or IgG aGP2 (IgA/G aGP2), or IgA and IgG aGP2 (IgA+G aGP2) in patients with CD were 42.7, 33.9, 49.7, and 26.9%, respectively, which were significantly higher than those in the remaining subjects (Table 2). The levels of IgG ASCA were significantly elevated in patients with CD, compared to those in patients with UC, intestinal BD, and ITB (P<0.05) (Figure 1c). In contrast, the levels of IgA ASCA in patients with CD were only significantly higher compared to those in patients with UC, but not to those in patients with intestinal BD and ITB as well as HC (Figure 1d).
Figure 1.
The levels of aGP2 and ASCA in 171 patients with Crohn’s disease (CD), 208 with ulcerative colitis (UC), 71 with intestinal Behçet's disease (BD), 57 with intestinal tuberculosis (ITB), and 70 healthy controls (HC). Box plots showing the medians along with the interquartile range * P<0.05; Kruskal Walllis test followed by post-hoc analysis in accordance with Conover.
Table 2. Prevalence of multiple autoantibodies in patients with CD, UC, intestinal BD, ITB and in HC.
| CD (n=171) | UC (n=208) | Intestinal BD (n=71) | ITB (n=57) | HC (n=70) | PValue CD vs. UC | PValue CD vs. BD | PValue CD vs. ITB | PValue CD vs. HC | |
|---|---|---|---|---|---|---|---|---|---|
| ASCA IgG, n (%) | 61 (35.7) | 22 (10.6) | 14 (19.7) | 11 (19.3) | 4 (5.7) | <0.001 | 0.015 | 0.021 | <0.001 |
| ASCA IgA, n (%) | 49 (28.7) | 32 (15.4) | 18 (25.4) | 10 (17.5) | 9 (12.9) | 0.002 | 0.601 | 0.097 | 0.009 |
| ASCA either, n (%) | 77 (45.0) | 42 (20.2) | 23 (32.4) | 15 (26.3) | 11 (15.7) | <0.001 | 0.069 | 0.013 | <0.001 |
| ASCA both, n (%) | 33 (19.3) | 12 (5.8) | 9 (12.7) | 6 (10.5) | 2 (2.9) | <0.001 | 0.216 | 0.541 | 0.001 |
| GP2 IgG, n (%) | 73 (42.7) | 29 (13.9) | 10 (14.1) | 10 (17.5) | 14 (20.0) | <0.001 | <0.001 | 0.001 | 0.001 |
| GP2 IgA, n (%) | 58 (33.9) | 23 (11.1) | 11 (15.5) | 8 (14) | 9 (12.9) | <0.001 | 0.004 | 0.004 | 0.001 |
| GP2 either, n (%) | 85 (49.7) | 42 (20.2) | 18 (25.4) | 15 (26.3) | 17 (24.3) | <0.001 | <0.001 | 0.002 | <0.001 |
| GP2 both, n (%) | 46 (26.9) | 10 (4.8) | 3 (4.2) | 3 (5.3) | 6 (8.6) | <0.001 | <0.001 | 0.001 | 0.002 |
| pANCA IgG, n (%) | 20 (11.7) | 116 (58.8) | 9 (12.7) | 5 (8.8) | 0 (0) | <0.001 | N.A. | N.A. | 0.010 |
| pANCA IgA, n (%) | 11 (6.4) | 62 (29.8) | 2 (2.8) | 2 (3.5) | 0 (0) | <0.001 | N.A. | N.A. | 0.037 |
| pANCA either, n (%) | 22 (12.9) | 126 (60.6) | 9 (12.7) | 5 (8.8) | 0 (0) | <0.001 | N.A. | N.A. | 0.006 |
| pANCA both, n (%) | 9 (5.3) | 52 (25.0) | 2 (2.8) | 2 (3.5) | 0 (0) | <0.001 | N.A. | N.A. | 0.062 |
Abbreviations: ASCA, anti-Saccharomyces cerevisiae antibody; BD, Behçet's disease; CD, Crohn's disease; GP2, anti-zymogen granule glycoprotein 2 antibodies; HC, health controls; ITB, intestinal tuberculosis; pANCA, anti-neutrophil cytoplasmic antibodies; UC, ulcerative colitis; N.A., not applicable.
The prevalences of IgG ASCA, IgA ASCA, IgA or IgG ASCA (IgA/G ASCA), or IgA and IgG ASCA (IgA+G ASCA) in patients with CD were 35.7, 28.7, 45.0, and 19.3%, respectively. The prevalence of IgG ASCA was significantly higher in patients with CD than in other subjects (Table 2). In contrast, no significant differences in the prevalence of IgA ASCA, IgA/G ASCA, or IgA and IgA+G ASCA were observed between patients with CD and patients with intestinal BD. In addition, no significant differences in the prevalence of IgA+G ASCA were identified between patients with CD and patients with ITB (Table 2).
IgG pANCA, IgA pANCA, IgA or IgG pANCA (IgA/G pANCA), or IgA and IgG pANCA (IgA+G pANCA) were detected in 58.8, 29.8, 60.6, and 25.0% of patients with UC, respectively, which were significantly higher than those in patients with CD (Table 2).
Combination of multiple autoantibodies for distinguishing patients with CD from patients with other disorders
To further assess the role of antibodies in distinguishing patients with CD from patients with other diseases, we calculated specificity and likelihood ratio for each of the antibodies (Table 3). For distinguishing CD from intestinal BD, IgG aGP2, IgA aGP2, IgA/G aGP2, or IgA+G aGP2 displayed a better diagnostic performance than their corresponding ASCA counterparts. Specifically, IgA+G aGP2 exhibited the highest LR+ of 5.83, with a sensitivity of 26.9% and a specificity of 95.4% (Table 3). Similarly, IgG aGP2, IgA aGP2, IgA/G aGP2, or IgA+G aGP2 demonstrated a superior diagnostic performance than their corresponding ASCA counterparts in differentiating CD from ITB. Particularly interesting is the high LR+ value of IgA+G aGP2 (LR+, 10.49) with a sensitivity of 26.9% and a specificity of 97.4% (Table 3).
Table 3. Assay performance parameters of IBD-related antibodies.
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ | LR- | |
|---|---|---|---|---|---|---|
| CD vs. UC | ||||||
| ASCA IgG | 35.7 | 89.4 | 73.5 | 62.8 | 3.37 | 0.72 |
| ASCA IgA | 28.7 | 84.6 | 60.5 | 59.1 | 1.86 | 0.84 |
| ASCA either | 45.0 | 79.8 | 64.7 | 63.8 | 2.23 | 0.69 |
| ASCA both | 19.3 | 94.2 | 73.3 | 58.7 | 3.35 | 0.86 |
| GP2 IgG | 42.7 | 86.1 | 71.6 | 64.6 | 3.06 | 0.67 |
| GP2 IgA | 33.9 | 88.9 | 71.6 | 62.1 | 3.07 | 0.74 |
| GP2 either | 49.7 | 79.8 | 66.9 | 65.9 | 2.46 | 0.63 |
| GP2 both | 26.9 | 95.2 | 82.1 | 61.3 | 5.60 | 0.77 |
| ASCA+/ANCA-a | 41.5 | 92.8 | 82.6 | 65.9 | 5.76 | 0.86 |
| GP2+/ANCA-a | 43.3 | 90.9 | 79.6 | 66.1 | 4.74 | 0.62 |
| GP2+/ASCA+a | 29.8 | 93.8 | 79.7 | 61.9 | 4.77 | 0.75 |
| GP2+/ASCA +/ANCA-a | 28.1 | 97.1 | 88.9 | 62.2 | 9.73 | 0.74 |
| UC vs. CD | ||||||
| ANCA IgG | 55.8 | 88.3 | 85.3 | 37.9 | 4.77 | 0.50 |
| ANCA IgA | 29.8 | 93.6 | 84.9 | 47.7 | 4.63 | 0.75 |
| ANCA either | 60.6 | 87.1 | 85.1 | 35.5` | 4.71 | 0.45 |
| ANCA both | 25.0 | 94.7 | 85.2 | 49.1 | 4.75 | 0.79 |
| ANCA+/ASCA-a | 47.6 | 90.6 | 86.1 | 41.3 | 5.09 | 0.58 |
| ANCA+/ GP2-a | 49.5 | 93.6 | 90.4 | 39.6 | 7.70 | 0.54 |
| ANCA+/ GP2-/ASCA-a | 39.9 | 95.3 | 91.2 | 43.4 | 8.53 | 0.63 |
| CD vs. intestinal BD | ||||||
| ASCA IgG | 35.7 | 80.0 | 82.4 | 32.1 | 1.78 | 0.80 |
| ASCA IgA | 28.7 | 75.4 | 75.4 | 28.7 | 1.16 | 0.80 |
| ASCA either | 45.0 | 67.7 | 78.6 | 31.9 | 1.39 | 0.51 |
| ASCA both | 19.3 | 87.7 | 80.5 | 29.2 | 1.57 | 0.92 |
| GP2 IgG | 42.7 | 84.6 | 88.0 | 35.9 | 2.77 | 0.68 |
| GP2 IgA | 33.9 | 86.2 | 86.6 | 33.1 | 2.45 | 0.77 |
| GP2 either | 49.7 | 75.4 | 84.2 | 36.3 | 2.02 | 0.67 |
| GP2 both | 26.9 | 95.4 | 93.9 | 33.2 | 5.83 | 0.77 |
| CD vs. ITB | ||||||
| ASCA IgG | 35.7 | 84.6 | 91.0 | 23.1 | 2.32 | 0.76 |
| ASCA IgA | 28.7 | 79.5 | 86.0 | 20.3 | 1.40 | 0.90 |
| ASCA either | 45.0 | 74.4 | 88.5 | 23.6 | 1.76 | 0.74 |
| ASCA both | 19.3 | 89.7 | 89.2 | 20.2 | 1.88 | 0.90 |
| GP2 IgG | 42.7 | 84.6 | 92.4 | 25.2 | 2.77 | 0.68 |
| GP2 IgA | 33.9 | 87.2 | 92.1 | 23.1 | 2.65 | 0.76 |
| GP2 either | 49.7 | 74.4 | 89.5 | 25.2 | 1.94 | 0.68 |
| GP2 both | 26.9 | 97.4 | 97.9 | 23.3 | 10.49 | 0.75 |
ASCA +, ASCA either; GP2 +, GP2 either; ANCA +, ANCA either; ASCA -, both ASCA IgA and ASCA IgG negative; GP2 -, both GP2 IgA and GP2 IgG negative; ANCA -, both ANCA IgA and ANCA IgG negative. CD, Crohn's disease; UC, ulcerative colitis; BD, Behçet's disease; ITB, intestinal tuberculosis; ASCA, anti-Saccharomyces cerevisiae antibody; ANCA, anti-neutrophil cytoplasmic antibodies; GP2, anti-zymogen granule glycoprotein 2 antibodies; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR-, negative likelihood ratio; N.A., not applicable.
For the discrimination of CD from UC on a single antibody reactivity basis, IgA+G aGP2 exhibited the highest positive likelihood ratio (LR+) of 5.60, with a sensitivity of 26.9% and a specificity of 95.2%. With regard to the combination of different markers, GP2+/ASCA+/ANCA- displayed the highest LR+ of 9.73, with a sensitivity of 28.1% and a specificity of 97.1%. pANCA demonstrated a good diagnostic performance in distinguishing UC from CD with a sensitivity of 60.6% and a specificity of 87.1% (Table 3).
Discriminatory capacities of aGP2 And ASCA in differentiating A subgroup of CD with ileal involvement from ITB or from intestinal BD
The diagnostic potential of aGP2 and ASCA in differentiating ileal CD from ITB or from intestinal BD were also evaluated. Generally, the levels and prevalences of IgG aGP2, IgA aGP2 and IgG ASCA, but not IgA ASCA, were significantly higher in patients with ileal CD than those from ITB or intestinal BD (supplementary Figure 1, and supplementary Table 1). IgG GP2 displayed the highest LR+ of 3.66 in differentiating ileal CD from intestinal BD, followed by IgA GP2 (LR+, 3.33). IgA GP2 exhibited the highest LR+ of 3.67 in differentiating ileal CD from ITB, followed by IgG GP2 (LR+, 2.94) (supplementary Table 2).
Relationship between aGP2 and ASCA in CD
The distributions and relationships between aGP2 and ASCA in patients with CD were illustrated by a Venn diagram. Of note, 35.1% (60/171) patients with CD were negative for both antibodies. The remaining 64.9% (111/171) patients were positive for at least one marker. Interestingly, only 29.8% (51/171) patients were positive for both antibodies. Importantly, 19.9% (34/171) ASCA negative CD patients were positive for aGP2, while 15.2% (26/171) aGP2-negative CD patients were positive for ASCA (Figure 2).
Figure 2.
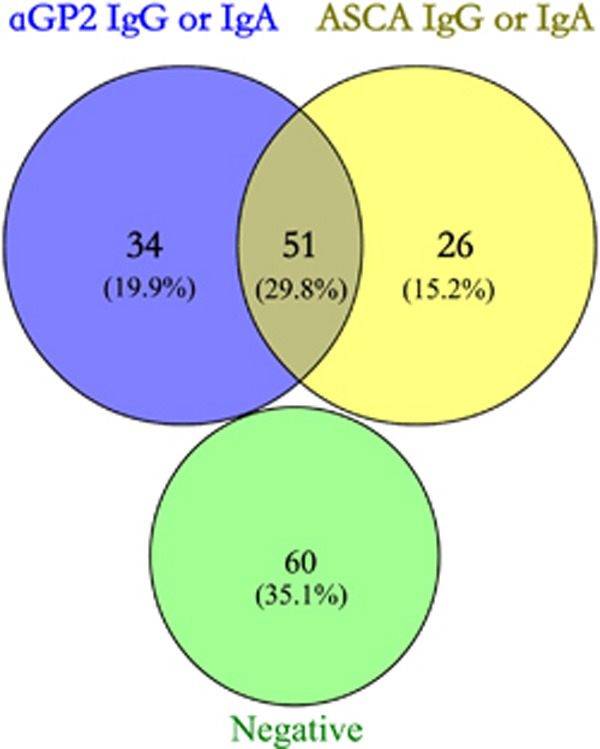
Venn diagram describing the relationships between serological markers (aGP2 and ASCA) in CD cohort (n=171).
Diagnostic power of aGP2 And ASCA in discriminating CD vs. UC and CD vs. disease controls
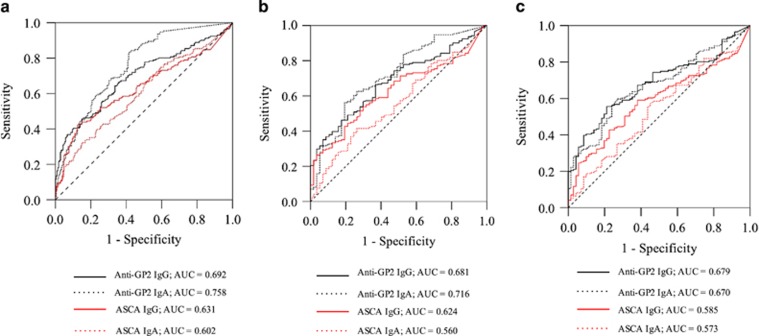
ROC analysis was utilized to evaluate the diagnostic power of aGP2 and ASCA in differentiating CD vs. ITB, CD vs. intestinal BD and CD vs. UC. For differentiating CD vs. ITB, IgA aGP2 displayed the highest AUC of 0.716, which was significantly higher than those of IgG ASCA and IgA ASCA (Figure 3 and Table 4). IgG aGP2 displayed a AUC of 0.681, which was significantly higher than that of IgA ASCA (Figure 3 and Table 4). For differentiating CD vs. intestinal BD, IgA aGP2 and IgG aGP2 showed similar AUC values of 0.670 and 0.679, respectively, which, were significantly higher than those of IgG ASCA and IgA ASCA (Figure 3 and Table 4). For differentiating CD vs. UC, IgA aGP2 exhibited the highest AUC of 0.758, which was significantly higher than the other three markers (P<0.05, respectively). IgG aGP2 also demonstrated a better discriminatory performance over IgA ASCA (P=0.0113) and a tendency to a better performance over IgG ASCA (P=0.0691) (Table 4).
Figure 3.
Receiver-operating characteristics (ROC) analysis of the discrimination power of aGP2 (IgA and IgG) and ASCA (IgA and IgG) in patients with CD (n=171) and UC (n=208) (a), CD (n=171) and disease controls (Behçet's disease, n=71; intestinal tuberculosis, n=57) (b), CD (n=171) and intestinal tuberculosis (n=57) (c), CD (n=171) and intestinal Behçet's disease (n=71) (d). AUC, area under curve.
Table 4. Differences in the areas under receiver-operating characteristics curves (ROC) of Crohn’s Disease (CD)-related antibodies comparing 171 CD patients with 208 Ulcerative Colitis (UC) patients, 71 patients with intestinal Behçet's disease and 57 with intestinal tuberculosis.
| GP2-IgA | ASCA-IgG | ASCA-IgA | |
|---|---|---|---|
| CD vs. UC | |||
| GP2-IgG | 0.0095 | 0.0691 | 0.0113 |
| GP2-IgA | 0.0001 | <0.0001 | |
| ASCA-IgG | 0.2695 | ||
| CD vs. ITB | |||
| GP2-IgG | 0.409 | 0.2346 | 0.0263 |
| GP2-IgA | 0.0368 | 0.0001 | |
| ASCA-IgG | 0.1102 | ||
| CD vs. intestinal BD | |||
| GP2-IgG | 0.8058 | 0.0286 | 0.0016 |
| GP2-IgA | 0.0364 | 0.0003 | |
| ASCA-IgG | 0.1258 | ||
Difference in the areas under ROC curves expressed as P-Value.
Clinical relevance of aGP2 and ASCA with disease characteristics in patients with CD
Patients with CD are heterogeneous in terms of disease presentation, location, behavior, extraintestinal manifestations, and response to treatments. The associations of aGP2 and ASCA with those disease characteristics were evaluated in patients with CD (Table 5). Statistical evaluation by χ2 test revealed a significantly positive correlation of IgG aGP2, IgA aGP2, IgA/G aGP2, IgA+G aGP2, IgG ASCA, or IgA+G ASCA with a more complicated penetrating disease (B3) (P<0.05). Consistently, patients that were negative for both markers were less likely to develop B3 behavior (P=0.009). In addition, IgA ASCA were positively correlated with stricturing disease (B2) (P=0.018). IgG aGP2 or IgA+G aGP2 were positively correlated with perianal disease modifier (P) (P<0.02). Of note, IgA aGP2 and IgA/G aGP2 were positively correlated with ileal location (L1) (P=0.022), while ASCA-/aGP2- were negatively correlated with L1 (P=0.003). Further, IgA+G ASCA and ASCA+/aGP2- were positively correlated with ileocolonic location (L3) (P<0.05), whereas IgA aGP2 were negatively correlated with L3 (P=0.033). We did not identify any significant associations between the positivity of aGP2 and disease activity in patients with CD (data not shown). Interestingly, IgG aGP2 and IgA ASCA were negatively correlated with age at diagnosis, indicating that patients diagnosed at younger age were more likely to have those autoantibodies. Additionally, IgG aGP2, IgA/G aGP2, IgA/G ASCA, ASCA+/aGP2- were negatively correlated with A3, while ASCA-/aGP2- were positively correlated with A3, suggesting that the CD patients diagnosed after 40 years old were less likely to have ASCA or aGP2.
Table 5. Clinical relevance of aGP2 and ASCA with disease characteristics in patients with Crohn’s disease.
| Parameters | IgG aGP2 | IgA aGP2 | aGP2 either | aGP2 both | IgG ASCA | IgA ASCA | ASCA either | ASCA both | ASCA+\aGP2- a | ASCA-\aGP2+ a | ASCA-\aGP2- a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis b | 0.039 | 0.014 | |||||||||
| A3 | 0.011 0.4 (0.2, 0.8) | 0.045 0.5 (0.2, 1.0) | 0.035 0.4(0.2, 1.0) | 0.041 0.4 (0.1, 0.9) | 0.019 2.4 (1.1, 5.0) | ||||||
| L1 | 0.022 2.4 (1.1, 5.0) | 0.022 2.4(1.1, 5.2) | 0.003 0.2 (0.1, 0.6) | ||||||||
| L3 | 0.033 0.5 (0.3, 0.9) | 0.011 2.9(1.2, 7.0) | 0.041 2.0 (1.0, 3.8) | ||||||||
| B2 | 0.018 0.4 (0.2, 0.9) | ||||||||||
| B3 | 0.027 2.1 (1.1, 3.9) | 0.042 2.0 (1.0, 3.8) | 0.032 2.0 (1.1, 3.8) | 0.026 2.2 (1.1, 4.4) | 0.020 2.2 (1.1, 4.2) | 0.022 2.4(1.1, 5.3) | 0.009 0.4 (0.2, 0.8) | ||||
| P | 0.003 2.6 (1.4, 4.8) | 0.015 2.2 (1.2, 4.1) | |||||||||
| Dermatologic | 0.030 0.3 (0.1, 0.9) | ||||||||||
| Steroids resistance | 0.047 3.1(1.0, 10.0) |
Clinical relevance of the markers with disease characteristics in patients with Crohn’s disease expressed as P-Values and OR (95% confidence interval, CI). Only significant relevance for a given parameter by χ2 test is shown. Positive associations are indicated in bold and negative associations in italic (P-Values, odds ratio and range). A represents age at diagnosis (A3, above 40 years), L represents the location of disease (L1, ileal; L3, ileocolonic), and B represents disease behavior (B2, stricturing; B3, penetrating; p, perianal disease modifier) based on the Montreal Classification. ASCA, anti-Saccharomyces cerevisiae antibody; aGP2, anti-zymogen granule glycoprotein 2 antibodies.
ASCA +, ASCA either; aGP2 +, aGP2 either; ASCA -, both ASCA IgA and ASCA IgG negative; aGP2 -, both aGP2 IgA and aGP2 IgG negative.
The correlation between age at diagnosis with the antibodies was assessed with Spearman's rank correlation coefficient.
Furthermore, a significant negative association was found between the combination of ASCA+/aGP2- and dermatologic involvement, indicating that patients with ASCA+/aGP2- were less likely to have dermatologic involvement (P=0.030). In addition, patients with IgA+G ASCA were more likely to display steroids resistance (P=0.047).
As CD patients displayed wide variations in terms of age at diagnosis, disease duration and other factors, a logistic regression model was utilized to assess how those confounding factors affected the diagnostic characteristics of aGP2 (Table 6). Only IgG aGP2 remained an independent risk factor for the presence of B3 along with the confounding factors gender, indicating that males with IgG aGP2 positivity had a higher risk for B3. Further, IgG aGP2 was a predictor for severe disease. The negative association for IgG ASCA with regard to the latter outcome did not reach significance (P<0.05). Other confounding factors were age (older patients) and short disease duration. Further, IgG ASCA was a significant predictor for the response to steroids in patients receiving 5-ASA without stenosing behavior and shorter duration of disease. Patients with dermatologic extraintestinal manifestations having been prescribed no anti-TNF treatment revealed IgA ASCA as a predictor.
Table 6. Logistic regression analyses for autoantibodies in patients with Crohn’s disease.
| Coefficient | Std.Error | Odds ratio | 95% CI | P Value | |
|---|---|---|---|---|---|
| B3 | |||||
| IgG aGP2 | 0.005 | 0.002 | 1.005 | 1.002, 1.009 | 0.0044 |
| Gender | 0.978 | 0.430 | 2.660 | 1.144, 6.184 | 0.0230 |
| Severe disease | |||||
| IgG aGP2 | 0.011 | 0.003 | 1.012 | 1.005, 1.018 | 0.0002 |
| IgG ASCA | −0.035 | 0.020 | 0.965 | 0.928, 1.005 | 0.0822 |
| Age | 0.053 | 0.022 | 1.055 | 1.011, 1.100 | 0.0134 |
| Duration | −0.126 | 0.061 | 0.881 | 0.781, 0.994 | 0.0397 |
| Steroids response | |||||
| IgG ASCA | 0.015 | 0.008 | 1.016 | 1.001, 1.031 | 0.0426 |
| 5-ASA | 1.168 | 0.364 | 3.215 | 1.574, 6.566 | 0.0013 |
| B2 | −0.592 | 0.355 | 0.553 | 0.276, 1.109 | 0.0953 |
| Duration | −0.088 | 0.034 | 0.915 | 0.857, 0.978 | 0.0083 |
| Dermatologic | |||||
| IgA ASCA | 0.008 | 0.004 | 1.008 | 1.000, 1.016 | 0.0461 |
| Anti-TNF | −1.307 | 0.517 | 0.271 | 0.098, 0.746 | 0.0116 |
B represents disease behavior (B2, stricturing; B3, penetrating; p, perianal disease modifier) based on the Montreal Classification. 5-ASA, therapy with 5-aminosalicylic acid; aGP2, anti-zymogen granule glycoprotein 2 antibodies; ASCA, anti-Saccharomyces cerevisiae antibody; Anti-TNF, therapy with anti-TNF; Duration, disease duration; L,location of disease (L1, ileal); age, age at diagnosis;
Significant relationships between one dichotomous dependent variable (various clinical outcomes or treatment variants) and one or more independent variables including aGP2 and ASCA, gender, age, disease duration, surgery are shown (P<0.003 respectively). (only significant correlations are shown).
Discussion
In this study, we evaluated the diagnostic potential of aGP2 in a large, well-defined Chinese cohort with a special focus on the role of aGP2 in distinguishing CD from intestinal BD and ITB. Altogether, aGP2 displayed a better discriminatory capability over ASCA in differentiating CD from UC, CD from intestinal BD, and CD from ITB. In addition, aGP2 was significantly associated with ileal disease. Further, aGP2 was linked to a higher risk for developing a more aggressive disease phenotype (B3), suggesting that the presence of aGP2 may predict individuals who are particularly susceptible to the development of complicated CD behavior. Degenhardt et al. reported the association of aGP2 with the need for surgical intervention which supports our findings.28 The identification of patients at risk would allow an early or more aggressive therapeutic intervention. Taken together, our data suggest that the inclusion of both IgA and IgG aGP2 testing into the routine screen test panels may enhance the overall performance of serological tests for diagnosis of CD, especially in countries with high prevalence of intestinal BD or ITB. In light of recent data proposing IgA aGP2 as a severity and cancer marker in primary sclerosing cholangitis that could be associated with IBD, this recommendation is further supported.29
In this study, the prevalence of aGP2 was similar to our previous study on a small cohort of patients with IBD (49.7 vs. 54.3%),21 was higher than those previously reported from Europe (20.7–36%).30, 31, 32, 33 As the pathogenesis of CD involves a combination of genetic, immunologic, and environmental risk factors, the relatively high prevalence of aGP2 in Chinese patients with CD might be due to the differences in those factors. Interestingly, we noticed that 35% (60/171) CD patients were negative for both aGP2 and ASCA. Among those patients, 47 patients (78.3% B1: 24 patients out of 54 patients, B2: 23 patients out of 58 patients) belonged to either B1 or B2 phenotype, and the rest patients (21.6%, 13/60; B2/3: 5 patients out of 28 patients, B3: 8 patients out of 31 patients) belonged to either B2/3 or B3 phenotype. Thus, other biomarkers34, which are associated with B1 and B2 phenotype, are needed.
Intestinal BD and ITB share many similarities in their clinical presentations and pathology with CD, which renders the diagnosis of CD challenging.5, 6 In this study, we demonstrated that aGP2 exhibited a good discriminatory performance in distinguishing CD from intestinal BD and ITB. This is an important finding because it may provide clues related to the clinical utility of aGP2 in distinguishing CD from other GI disorders that share similar clinical and pathological features. In fact, our study represents the first assessment of the diagnostic potential of aGP2 in distinguishing CD from intestinal BD and ITB. The significantly higher percentage of aGP2 in CD compared to ITB may be due to the different pathogenic mechanism between CD and ITB. GP2 is expressed as a membrane-anchored receptor on M cells in intestinal Peyer's patches (PP). During the CD inflammation in the gut, GP2 is overexpressed and contributes to the generation of aGP215. However, in terms of ITB, GP2 may not overexpressed and thereby, few aGP2 is generated, despite of ileal involvement. Taken together, these results supported aGP2 as promising biomarkers in the diagnosis of CD, especially when it comes to distinguishing CD from intestinal BD or ITB.
We have previously shown that GP2 is overexpressed at the site of inflammation in patients with CD but not in patients with UC.15 Indeed, aGP2 also exhibited a good clinical performance in distinguishing CD from UC. IgA aGP2 demonstrated a significantly higher AUC than IgG ASCA and IgA ASCA, indicating that IgA aGP2 may be more useful in distinguishing CD from UC in China. More importantly, the combination of aGP2+ASCA+ANCA- greatly increased the discriminatory capability in differentiating CD from UC with the highest LR+ of 9.73, further highlighting the promising potential of aGP2 in subclassification of IBD.
It has been demonstrated that GP2 is specifically expressed on the apical plasma membrane of M cells, where it functions as a transcytotic receptor for mucosal antigens including FimH-positive bacteria.35, 36 Interestingly, M cells are predominantly present in the small intestine and particularly in the ileum, whereas they are almost absent in the large intestine.35, 37 In our study, the presence of aGP2 was significantly associated with ileal location but not colonic location, which is consistent with other studies and supports the association with the M cell location.19, 20, 38 In addition, we found that aGP2 were associated with a penetrating disease phenotype (B3), suggesting that the presence of aGP2 may identify patients at risk of developing complicated CD behavior. Logistic regression analysis confirmed IgG aGP2 as a predictor for the occurrence of B3 in patients with CD. Interestingly, previous studies reported that aGP2 were correlated with stricturing phenotype (B2)20, 39, 40. However, a recent study also found a significant association between aGP2 and the penetrating phenotype (B3)41. The discrepancy might be due to differences in sample size and ethnic/geographic backgrounds. Further, IgG GP2 was identified as a predictor of severe CD with several confounding factors supporting the association of IgG aGP2 with a severe clinical CD phenotype as reviewed recently.18
A recent study showed that IgA aGP2 were present in approximately 50% patients with primary sclerosing cholangitis (PSC).29 Of note, IgA aGP2 displayed a similar prevalence in PSC patients without concomitant IBD compared with PSC patients with IBD. This suggests that IgA aGP2 may be also associated with the inflammation in bile ducts apart from the one in small intestine.
Interestingly, we also found that aGP2 were negatively associated with age at diagnosis, which was consistent with other studies.20, 40 In particular, IgG aGP2 and IgA/G aGP2 were negatively correlated with A3, indicating that the presence of aGP2 might be associated with an early disease onset.
It has been shown that GP2 displays anti-inflammatory effects by decreasing proliferation, apoptosis, and activation of intestinal epithelial cells and mucosal and peripheral T-cell, as well as inhibiting pro-inflammatory chemokine CXCL8 and upregulating anti-inflammatory cytokine TGF-β1.42 Thus, IgG aGP2 may block the suppressive effect of GP2, thereby promoting intestinal inflammation. In addition, IgA aGP2 may bridge FimH-positve pathogen bound-GP2 with M cell surface GP2, resulting in elevated transcytosis of GP2-covered pathogens.43 Our finding that IgG and IgA aGP2 as well as combinations thereof were correlated with a more aggressive disease phenotype supports the hypothesis that aGP2 indeed can play a pathogenic role in exacerbation or perpetuation of CD inflammation.
In conclusion, our data suggest that aGP2 displayed a better discriminatory performance over ASCA in differentiating CD from UC, CD from intestinal BD, and CD from ITB. The presence of aGP2 could identify CD patients with ileal location, a more complicated penetrating disease behavior and early disease onset. Taken together, our data help to delineate further the clinical utility of aGP2 in the diagnosis of CD, especially when it comes to distinguishing CD from intestinal BD or ITB.
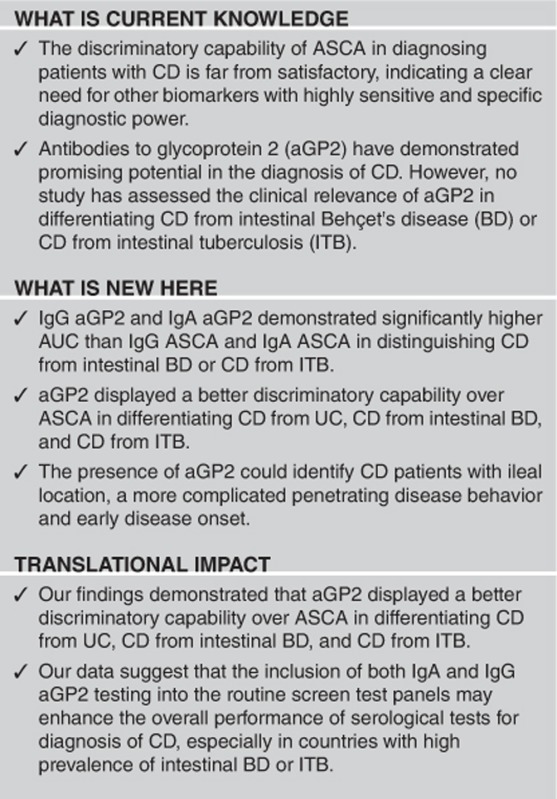
Study Highlights
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisditional claims in published maps and institutional affiliations.
Guarantor of the Article: Shulan Zhang, MD and Yongzhe Li, MD.
Financial support: This work was supported in part by the National Natural Science Foundation of China Grants No. 81373188, 81671618 (to YL), 81771661 (to SZ), Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (CAMS-I2M) No. 2017-I2M-3-001 (to SZ and YL), The National Key Research and Development Program of China No. 2016YFC0903900 (to YL).
Potential competing interests: DR is shareholder of Medipan and GA Generic Assays GmbH. All other authors have no conflicts of interest to disclose.
Specific author contributions: SZ and JL designed and performed the study, and drafted the manuscript. ZW and JL performed the study. DRo designed the study, and contributed by critical revision of the manuscript. PS and Dre critically revised the manuscript and participated in the statistical evaluation of the data. JQ and YL designed the study and drafted the manuscript. All authors were responsible for data analysis and interpretation.
Supplementary Material
References
- Laass MW, Roggenbuck D, Conrad K. Diagnosis and classification of crohn's disease. Autoimmun Rev 2014; 13: 467–471. [DOI] [PubMed] [Google Scholar]
- Conrad K, Roggenbuck D, Laass MW. Diagnosis and classification of ulcerative colitis. Autoimmun Rev 2014; 13: 463–466. [DOI] [PubMed] [Google Scholar]
- Abraham C, Cho JH. Inflammatory bowel disease. The New England journal of medicine 2009; 361: 2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer AM, Dehlavi MA, Fournier N et al. Diagnostic delay in crohn's disease is associated with a complicated disease course and increased operation rate. The American journal of gastroenterology 2013; 108: 1744–1753 quiz 54. [DOI] [PubMed] [Google Scholar]
- Almadi MA, Ghosh S, Aljebreen AM. Differentiating intestinal tuberculosis from crohn's disease: A diagnostic challenge. The American journal of gastroenterology 2009; 104: 1003–1012. [DOI] [PubMed] [Google Scholar]
- Valenti S, Gallizzi R, De Vivo D et al. Intestinal behcet and crohn's disease: Two sides of the same coin. Pediatric rheumatology online journal 2017; 15: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindi M, Riddell RH. Indeterminate colitis. J Clin Pathol 2004; 57: 1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AD, McMillan I, Price AB et al. Natural history of indeterminate colitis. Br J Surg 1991; 78: 179–181. [DOI] [PubMed] [Google Scholar]
- Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011; 140: 1817–1826 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prideaux L, Kamm MA, De Cruz P et al. Inflammatory bowel disease serology in asia and the west. World journal of gastroenterology 2013; 19: 6207–6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrance IC, Murray K, Hall A et al. A prospective comparative study of asca and panca in chinese and caucasian ibd patients. The American journal of gastroenterology 2004; 99: 2186–2194. [DOI] [PubMed] [Google Scholar]
- Zhang S, Luo J, Li J et al. Retrospective evaluation of the clinical utility of serological biomarkers in chinese patients with inflammatory bowel disease: 2-year clinical experience. Clin Chem Lab Med 2017; 55: 865–875. [DOI] [PubMed] [Google Scholar]
- Makharia GK, Sachdev V, Gupta R et al. Anti-saccharomyces cerevisiae antibody does not differentiate between crohn's disease and intestinal tuberculosis. Digestive diseases and sciences 2007; 52: 33–39. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kim YH, Kim WH et al. Diagnostic utility of anti-saccharomyces cerevisiae antibody (asca) and interferon-gamma assay in the differential diagnosis of crohn's disease and intestinal tuberculosis. Clinica chimica acta; international journal of clinical chemistry 2011; 412: 1527–1532. [DOI] [PubMed] [Google Scholar]
- Roggenbuck D, Hausdorf G, Martinez-Gamboa L et al. Identification of gp2, the major zymogen granule membrane glycoprotein, as the autoantigen of pancreatic antibodies in crohn's disease. Gut 2009; 58: 1620–1628. [DOI] [PubMed] [Google Scholar]
- Roggenbuck D, Bogdanos D, Conrad K. Loss of tolerance to one or two major targets in crohn's disease or just cross-reactivity? J Crohns Colitis 2013; 7: e273–e274. [DOI] [PubMed] [Google Scholar]
- Komorowski L, Teegen B, Probst C et al. Autoantibodies against exocrine pancreas in crohn's disease are directed against two antigens: The glycoproteins cuzd1 and gp2. J Crohns Colitis 2013; 7: 780–790. [DOI] [PubMed] [Google Scholar]
- Roggenbuck D, Reinhold D, Baumgart DC et al. Autoimmunity in crohn's disease-a putative stratification factor of the clinical phenotype. Adv Clin Chem 2016; 77: 77–101. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Romanidou O, Roggenbuck D et al. Ileal inflammation may trigger the development of gp2-specific pancreatic autoantibodies in patients with crohn's disease. Clinical & developmental immunology 2012; 2012: 640835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanos DP, Roggenbuck D, Reinhold D et al. Pancreatic-specific autoantibodies to glycoprotein 2 mirror disease location and behaviour in younger patients with crohn's disease. BMC Gastroenterol 2012; 12: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wu Z, Luo J et al. Diagnostic potential of zymogen granule glycoprotein 2 antibodies as serologic biomarkers in chinese patients with crohn disease. Medicine (Baltimore) 2015; 94: e1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard-Jones JE. Classification of inflammatory bowel disease. Scandinavian journal of gastroenterology Supplement 1989; 170: 2–6 discussion 16-9. [DOI] [PubMed] [Google Scholar]
- Silverberg MS, Satsangi J, Ahmad T et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a working party of the 2005 montreal world congress of gastroenterology. Canadian journal of gastroenterology=Journal canadien de gastroenterologie 2005; 19 (Suppl A): 5A–36A. [DOI] [PubMed] [Google Scholar]
- Best WR, Becktel JM, Singleton JW et al. Development of a crohn's disease activity index. National cooperative crohn's disease study. Gastroenterology 1976; 70: 439–444. [PubMed] [Google Scholar]
- Zhang S, Wu Z, Li J et al. Peripheral differentials by cytodiff flow cytometric system predict disease activity in chinese patients with inflammatory bowel disease. Clin Chim Acta 2017; 471: 17–22. [DOI] [PubMed] [Google Scholar]
- Conover WJ. Practical nonparametric statistics. John Wiley & Sons: New York, 1999. [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- Degenhardt F, Dirmeier A, Lopez R et al. Serologic anti-gp2 antibodies are associated with genetic polymorphisms, fibrostenosis, and need for surgical resection in crohn's disease. Inflamm Bowel Dis 2016; 22: 2648–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrek ST, Gotthardt D, Nitzsche T et al. Anti-gp2 iga autoantibodies are associated with poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Gut 2017; 66: 137–144. [DOI] [PubMed] [Google Scholar]
- Roggenbuck D, Reinhold D, Wex T et al. Autoantibodies to gp2, the major zymogen granule membrane glycoprotein, are new markers in crohn's disease. Clinica chimica acta; international journal of clinical chemistry 2011; 412: 718–724. [DOI] [PubMed] [Google Scholar]
- Op De Beeck K, Vermeire S, Rutgeerts P et al. Antibodies to gp2, the major zymogen granule membrane glycoprotein, in inflammatory bowel diseases. Gut 2012; 61: 162–164 author reply 4-5. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Forbes A, Bogdanos DP. Antibodies to glycoprotein 2 (gp2) in patients with inflammatory bowel diseases from uk. Clinica chimica acta; international journal of clinical chemistry 2011; 412: 1163–1164. [DOI] [PubMed] [Google Scholar]
- Bonaci-Nikolic B, Spuran M, Andrejevic S et al. Autoantibodies to gp2, the major zymogen granule membrane glycoprotein, in patients with gluten-sensitive enteropathy: A possible serological trap. Clinica chimica acta; international journal of clinical chemistry 2012; 413: 822–823. [DOI] [PubMed] [Google Scholar]
- Prideaux L, De Cruz P, Ng SC et al. Serological antibodies in inflammatory bowel disease: A systematic review. Inflamm Bowel Dis 2012; 18: 1340–1355. [DOI] [PubMed] [Google Scholar]
- Mabbott NA, Donaldson DS, Ohno H et al. Microfold (m) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal immunology 2013; 6: 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierack P, Rodiger S, Kolenda R et al. Species-specific and pathotype-specific binding of bacteria to zymogen granule membrane glycoprotein 2 (gp2). Gut 2015; 64: 517–519. [DOI] [PubMed] [Google Scholar]
- Hase K, Kawano K, Nochi T et al. Uptake through glycoprotein 2 of fimh(+) bacteria by m cells initiates mucosal immune response. Nature 2009; 462: 226–230. [DOI] [PubMed] [Google Scholar]
- Somma V, Ababneh H, Ababneh A et al. The novel crohn's disease marker anti-gp2 antibody is associated with ileocolonic location of disease. Gastroenterology research and practice 2013; 2013: 683824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels MA, Jendrek ST, Korf T et al. Pancreatic autoantibodies against cuzd1 and gp2 are associated with distinct clinical phenotypes of crohn's disease. Inflamm Bowel Dis 2015; 21: 2864–2872. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Komorowski L, Teegen B et al. Diagnostic and clinical significance of crohn's disease-specific pancreatic anti-gp2 and anti-cuzd1 antibodies. Clin Chem Lab Med 2016; 54: 249–256. [DOI] [PubMed] [Google Scholar]
- Papp M, Sipeki N, Tornai T et al. Rediscovery of the anti-pancreatic antibodies and evaluation of their prognostic value in a prospective clinical cohort of crohn's patients: The importance of specific target antigens [gp2 and cuzd1]. J Crohns Colitis 2015; 9: 659–668. [DOI] [PubMed] [Google Scholar]
- Werner L, Paclik D, Fritz C et al. Identification of pancreatic glycoprotein 2 as an endogenous immunomodulator of innate and adaptive immune responses. Journal of immunology 2012; 189: 2774–2783. [DOI] [PubMed] [Google Scholar]
- Roggenbuck D, Reinhold D, Werner L et al. Glycoprotein 2 antibodies in crohn's disease. Adv Clin Chem 2013; 60: 187–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.