Abstract
This paper summarises the past, present and future of asbestos exposure. The future scenarios as to the mesothelioma incidence in countries, where asbestos has been banned, are discussed.
Keywords: Asbestos, environmental, occupational, exposure, mesothelioma
Introduction
The term asbestos covers a group of natural minerals (hydrated silicates) that form long, thin fibres when they crystallize. These fibres can then separate lengthwise forming even thinner fibres: this particular characteristic distinguishes asbestos from other silicates and is responsible for its dangerous properties.
There are two groups of asbestos minerals, the serpentine and amphibole group.
Chrysotile, known as white asbestos, is a magnesium silicate belonging to the serpentine group and is the most common type (about 95% of all asbestos use).
The other asbestos types belong to the amphibole group and include crocidolite, known as blue asbestos, amosite, also called brown asbestos, and anthophyllite. All these types have a stronger mechanical and chemical resistance than chrysotile.
The most commonly used types of asbestos in industry are chrysotile, crocidolite, amosite and anthophyllite available from mining activities, whilst actinolite and tremolite are only natural pollutants.
The past
The history of asbestos dates back thousands of years and some of the names it has been given are particular and fascinating. Indeed, due to its flexibility and spinnability it was associated to linen and Pliny the Elder defined it as “live linen”, whilst Pausanias called it “linen of Karpas”, a locality on the island of Cyprus. When referring to its fireproof and thermal isolation properties, it was associated to the salamander, also considered to be “fireproof” in the early Medieval period and Marco Polo in his “Milione” (The Travels of Marco Polo), used the term salamander to define asbestos. He also described how asbestos was extracted (1).
“Next to the district of Kamul follows that of Chinchitalas, which in its northern part borders on the desert, and is in length sixteen days’ journey. It is subject to the Grand Khan and contains cities and several strong places. …A substance is likewise found of the nature of the salamander, for when woven into cloth, and thrown into the fire, it remains incombustible. The following mode of preparing it I learned from one of my travelling companions, named Curficar, a very intelligent Turkoman, who had the direction of the mining operations of the province for three years. The fossil substance procured from the mountain consists of fibres not unlike those of wool. ...Of the salamander under the form of a serpent, supposed to exist in fire, I could never discover any traces in the eastern regions. It is said that they preserve at Rome a napkin woven from this material, in which was wrapped the sudarium of our Lord, sent as a gift from one of the Tartar princes to the Roman Pontiff”.
Benjamin Franklin also used the term “salamander cotton”, many centuries later. The two terms “salamander and linen” were also used in more modern times. Indeed, the trade name “salamandra” was used by mass producers of the first thermo-resistant mattresses. Even as late as the 1960s, Ernst Baader, a well-affirmed occupational specialist, defined, in his 5th Edition on occupational pathology, this new form of pneumoconiosis as “Bergflachs-lunge”, which literally translated means “mountain linen lung” (2).
The Protoevangelium of James, reports that also Holy Mary risked being exposed to asbestos: “And there was a council of the priests, saying: Let us make a veil for the temple of the Lord. And the priest said: Call to me the undefiled virgins of the family of David. And the priest remembered the child Mary, that she was of the family of David, and undefiled before God. And the priest said: Choose for me by lot who shall spin the gold, and the white, and the fine linen, and the silk, and the blue, and the scarlet, and the true purple. And the true purple and the scarlet fell to the lot of Mary, and she took them, and went away to her house. And Mary took the scarlet, and span it” (3).
St. Augustine in his work The City of God quotes asbestos as an inextinguishable lamp: “…There is a stone found in Arcadia, and called asbestos, because once lit it cannot be put out. …There was or is a temple of Venus in which a candelabrum set in the open air holds a lamp, which burns so strongly that no storm or rain extinguishes it, and which is therefore called, like the stone mentioned above, the asbestos or inextinguishable lamp. …That lamp, therefore, was either by some mechanical and human device fitted with asbestos, or it was arranged by magical art in order that the worshippers might be astonished, or some devil under the name of Venus so signally manifested himself that this prodigy both began and became permanent” (4).
There was a boom in commercial asbestos mining with chrysotile in the late 19th century: Italy, Canada and Russia were the first between 1866–1890, followed by South Africa and Australia between the two World Wars. More than three quarters of the global market was provided with asbestos from Canada, in Quebec and Russia, in the Urals. The amphibole production began with crocidolite in South Africa in the late 19th century, followed by Australia in the 1930s. Whilst crocidolite mining was stopped in Australia more than 50 years ago, the South-African production of amosite stopped more recently, in 1992, after 80 years of activity in the Pengue mine in Transvaal. Finland’s anthophyllite production stopped in 1975 after 50 years of activity.
The earliest known use of asbestos was in about 2500 B.C. in what is now Finland, where asbestos fibers were mixed with clay to form stronger ceramic utensils and pots.
The use of asbestos as a textile was known as far back as 1,000–1,500 years B.C. in China and Greece.
The mass production of goods in asbestos or containing asbestos, began with textiles, first in Italy and then in Anglo-Saxon countries. At an industrial level, chrysotile was the first type to be used, followed by crocidolite in South Africa and the UK. The first use of asbestos in friction materials (clutches and brake linings) was in 1918; the production of asbestos cement began in the late 19th century Austria, Italy and the USA. The first application of asbestos as an insulating material instead of hemp, dates back to 1882 in the USA. The most recent type of application using spray-on asbestos, was developed in Great Britain in 1931.
Due to the high versatility of asbestos, the 1950s witnessed its commercial use in a wide range of products used in everyday life, like wicks, shoes, cigarette filters, artificial snow in Xmas decorations on film sets and fake logs for gas fires. The peak of asbestos production was in the 1970s, which took a progressively decreasing trend, that has stabilised today to what was present in the year 2000. Indeed, data from the International Ban Asbestos Secretariat report that the world 2015 asbestos production was equal to 2,026,000 tons, which is almost superimposable on that of 2000 (2,070,000 tons) (5,6).
There was a drastic reduction in the use of asbestos in European countries from the 1990s (a drop from 2,500,000 tons in 1990 to 530,000 tons in 2000), as was observed in the USA (from 803,000 tons in 1973 to 15,000 in 2000). However, at the same time, there was an increase in its use in non-EU countries, in Asia and above all in China and India, as well as Africa and in some Latin-American countries like Brazil (6).
Asbestos-related diseases
Lung fibrosis, was the first asbestos-related disease to be reported in the 1920s–1930s, above all by Cooke, the author of the first scientific report, who coined the name asbestosis (7,8). In 1927, Mc Donald described asbestos bodies calling them “curious” (9). The question of asbestos was brought to the attention of the International Labour Office by Gloyne and Merewether in 1938 (10).
Twenty years later, what once had seemed to be exclusively an occupational disease, was put into question as a possible risk factor for the general population. Indeed, it was observed that the Finnish anthophyllite mines had very superficial fibres capable of causing pleural plaques (above all calcified), due to environmental exposure (11).
In 1968, Rubino et al. presented data on pleural plaque prevalence in non-occupationally exposed subjects, living in the neighbourhood of the Balangero Chrysotile mine in the Province of Turin, northern Italy (12). The paper published by Thomson and Graves in 1966 had a strong impact on the scientific community, as it reported that asbestos bodies were present in more than 25% of the lungs of 500 subjects at autopsy in Miami, Florida (13). These data were confirmed with an even higher prevalence, in a study carried out in Turin in1968 (14).
Meanwhile, the International Labour Organization (ILO) promoted the extension of the 30-year-old international radiological classification of pneumoconiosis to include asbestosis, as until that time the classification was restricted to coal-worker’s pneumoconiosis and silicosis. This classification was completed in 1971 when the guideline by the ILO on silicosis and coal-worker’s pneumoconiosis was pooled with that of the Union for International Cancer Control (UICC) on asbestosis. This classification was modified over the years according to the technological advances. The latest revision, based on the traditional chest X-ray radiographs was made in 2000 and then further updated with digital radiological images in 2011.
With a 10-year time-lapse from the first identification of asbestosis, the first reports on the occupational neoplastic effects of asbestos were published. After several case reports, further evidence was obtained in experimental studies in 1941 (15,16).
In 1947 Merewether, head medical officer of Her Majesty’s Factory Inspectorate, brought to light the gravity of the question in the Anglo-Saxon world—the Commonwealth was, at that time, the largest asbestos producer in the world—and reported a 10:1 ratio between lung cancer in workers affected by asbestosis compared to those affected by silicosis (17). In 1955 Richard Doll’s epidemiological study made a definite confirmation of the causal association between occupational asbestos exposure and lung cancer (18).
The history of mesothelioma, another asbestos-associated neoplastic pathology, which is very rare in the general population, ran a parallel course, even if its evolution took place some 15 years after that of lung cancer. Although sporadic case reports were described in literature as from the late 1940s, Wagner published the first casuistry on subjects with asbestos-related mesothelioma in 1960 (19). These data were extrapolated from the South-African cluster of persons exposed to crocidolite in the Cape District. Wagner’s paper studied 33 mesothelioma cases and only a fraction of them had been professionally exposed in the asbestos mines, whilst some of them were only residents in the mine area.
Some of the females affected had even broken apart the leftover rocks with small hammers to remove any residual crocidolite for personal sale.
This publication was followed by others on peritoneal mesothelioma and by a similar report on the large deposit of crocidolite in Wittenoom, North-west Australia (20,21). The difficulties involved in making a pathologic diagnosis of mesothelioma led the UICC to recommend the set-up of a professional panel on mesothelioma, in 1964. The European Union constituted an international panel in the middle of the 1970s.
To date, even in countries that have banned asbestos mesothelioma still represents a serious problem, for two reasons. Firstly, the long latency period between exposure and the onset of symptoms (on average >40 years); secondly, the persisting diagnostic difficulties that led to the drafting of International Guidelines (22). The pleural localization of the disease has a much higher incidence and is of very difficult diagnosis as it may mimick metastatic involvement of the pleura from malignancies. Signs and symptoms of primary lesions are generally similar to those of secondary diseases, as is diagnostic imaging. Indeed, according to the estimates made on malignant pleural effusion (the most common sign in mesothelioma) by the World Health Organization, it must be emphasized that, in industrialised countries, the ratio between metastatic disease and primary malignancy is 100:1 (23).
Exposure assessment
Airborne dust in the workplace has been measured for at least a century now. The sampling methods and extraction equipment have varied over this period, as have the analytical methods. The first approach was gross filtration on cotton wool filter and the dust was recovered by ignition, succeeded by the “sugar tube”, where the dust was trapped on a bed of sugar granules and assessed by dissolving the sugar in water, filtering and weighing. In the subsequent period impact sampling, using the konimeter, replaced this method using slides (konimetry) or by impingement, i.e., within a liquid substance (usually water) or exploiting the physical attraction phenomenon (thermal or electrostatic precipitation). All these methods are used to obtain from a known volume of environmental air, a dust “deposit” then observed in the analytical phase by an optic microscope.
The modern era began in England in 1965, with a proposal made by the Asbestosis Research Council, to use a membrane filter method, i.e., a solid but porous support medium. The advent of the electronic microscope was when it became necessary to assess also environmental air, that requires not only quantification, but also identification of the airborne fibres.
The rules and regulations governing the concentration threshold limit values (TLVs) of airborne asbestos fibres and the comparison of the results with set measurements, is a more recent event. Neither the type of fibre, nor their morphology was distinguished for the first 30 years or so, until 1968 when the British Occupational Hygiene Society recommended optical counting of fibres on a measured area of the filter. The number of fibres became very small and the volume of reference air adopted was the millilitre. Later distinctions were made between serpentine and amphibole asbestos, applying more severe TLV’s (lower) for the latter, if present. Therefore, the TLV’s set for chrysotile are valid as long as it is the only type of asbestos being used, otherwise, the TLV’s set for the amphibole group are valid “for all types of asbestos”.
Table 1 summarizes the TLV’s proposed over the years by the American Conference of Governmental Industrial Hygienists (ACGIH). These limits were drastically reduced over time as more evidence came to light on the neoplastic effects of asbestos, in particular mesothelioma, which may arise even at very low exposure doses.
Table 1. Chronology of ACGIH values for asbestos.
| Years | Asbestos types | Adopted | Intended change | |||
|---|---|---|---|---|---|---|
| Mppcf | Fibres/mL | Mppcf | Fibres/mL | |||
| 1946–1967 | All | 5 | [30–180]* | – | – | |
| 1968–1969 | All | 5 | [30–180]* | 2 | 12 | |
| 1970–1973 | All | 5 | [30–180]* | – | 5 | |
| 1972 | – | – | – | – | 5 | |
| 1974–1979 | All | 5 | – | – | ||
| 1978 | Amosite & tremolite | – | – | – | 0.5 | |
| Crocidolite | – | – | – | 0.2 | ||
| Chrysotile & other forms | – | – | – | 2 | ||
| 1980–1986 | Amosite & tremolite | – | 0.5 | – | – | |
| Crocidolite | – | 0.2 | – | – | ||
| Chrysotile & other forms | – | 2 | – | – | ||
| 1987–1997 | Amosite & tremolite | – | 0.5 | – | – | |
| Crocidolite | – | 0.2 | – | – | ||
| Chrysotile & other forms | – | 2 | – | – | ||
| 1991 | Amosite & tremolite | – | – | – | 0.2 | |
| Crocidolite | – | – | – | 0.2 | ||
| Chrysotile & other forms | – | – | – | 0.2 | ||
| 1997 | Amosite & tremolite | – | – | – | 0.1 | |
| Crocidolite | – | – | – | 0.1 | ||
| Chrysotile & other forms | – | – | – | 0.1 | ||
| 1998 | Amosite & tremolite | – | 0.1 | – | – | |
| Crocidolite | – | 0.1 | – | – | ||
| Chrysotile & other forms | – | 0.1 | – | – | ||
*, estimated value. Mppcf, million particles per cubic foot; ACGIH, American Conference of Governmental Industrial Hygienists.
The first limitations on the use of asbestos applied to spray on use, especially in the UK which also later banned crocidolite. Afterwards, over the course of time, other total/partial bans were made in other countries.
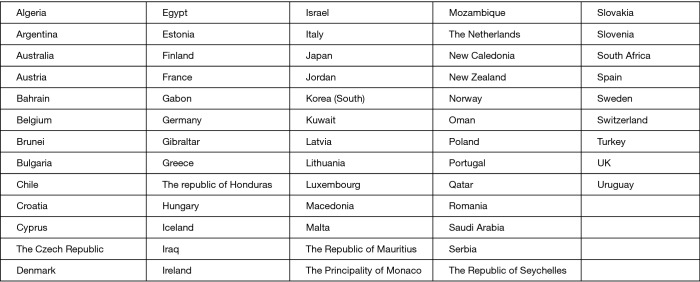
On the basis of the data published by the International Ban Asbestos Secretariat in July 2017 (24), the countries listed in Figure 1 banned the use of asbestos.
Figure 1.
Current asbestos bans
So as to propose a reference, albeit approximate, the average estimated values of concentrations of asbestos fibres in the various workplace environments over the last decades can be compared. These values greatly exceeded the 100 fibres/mL limit before the 1930s, at 100 fibres/mL in 1935–1950. They were at 20 fibres/mL in the 60s, 5 fibres/mL in 1975 and dropped to 1 fibre/mL in the 80s.
The future
When making a hypothesis as to the future effects of asbestos, it must firstly be defined which of its effects one is referring to, whether this is a near or long-term future and lastly, which type of exposure is to be considered. In order to do so, the aspects of the individual countries that have banned asbestos must be taken into consideration as well as those who have not yet done so.
For those who banned, the scenario mainly covers: (I) the residual effects of previous occupational asbestos exposure when its use had not yet been banned; (II) the effects of the remaining asbestos products, the so-called asbestos in situ and their repercussions on the general environment.
Countries that did not ban, are to take into consideration all the possible diseases exposure associated diseases, the only difference compared to the past being the availability of more advanced preventive measures, that, when coupled to the know-how as to the gravity of the possible resulting pathologies, should, in theory, contain the phenomenon to a certain extent.
As to point (I) for those countries who banned, both the entity of the fibre dose retained and the latency period considered necessary for clinical manifestation of the pathology or cause of death take on a particular importance. The pathologies that declined were mainly asbestosis and lung cancer, which are, though variable, clearly dose-dependent and have a relatively short latency period. The trend of pleural pathologies, plaques and mesothelioma, deserve a separate consideration.
Point (II) is characterized by the importance of the low, or very low, typical concentrations may assume, with a few exceptions i.e., where there is a specific cause of environmental pollution which are able to affect mainly pleural pathologies.
However, in both points the relevant question is the incidence of new mesothelioma cases that, from an epidemiological point of view have the main characteristic of being proportional to the 3rd or 4th power of time from first exposure, whatever age the subject had at 1st exposure and despite there being no further exposure (25).
Such a time-dependant trend implies, on an individual basis, that once a subject has reached a critical exposure dose, even if not quantifiable, the hope of a longer survival, due to removal or absence of a competitive cause of death, leaves the possibility of developing a mesothelioma open. This implies that it cannot be supposed that such an effect will have a short-term positive outcome, even in the presence of a total ban.
Effects of previous occupational exposure
According to a publication by English epidemiologists in the 90s, there would have been an early rapid increase in the number of mesothelioma deaths in the UK, up to a peak in the years 2020–2030, i.e., until about 40 years after a maximum import/use of asbestos into the UK (26). These forecasts turned out to be quite accurate, except for some countries that reached their peak before 2020–2030.
A recent pooled analysis on six occupational cohorts and two high environmental exposure cohorts, showed a log-linear relationship between pleural mesothelioma incidence and latency from the start of exposure i.e., at least for the 1st 45/50 years, whilst, this effect seems to taper out over long periods of time. This analysis did not demonstrate a correlation between mesothelioma incidence and duration of exposure (27). Similar results on the role of latency were obtained by another pooled analysis on several Italian cohorts (28).
Although some cohort studies suggest a risk reduction after 20/30 years from cessation of exposure, other data do not seem to confirm this phenomenon (29). This discrepancy may be explained by the relatively small study sample with a prolonged time lapse from cessation of exposure, competitive mortality, or the biological effects which differ between the serpentine and amphibole groups.
A recent international analysis on mesothelioma mortality reports a continuous upward trend in subjects that were exposed in the past when there was an extensive use of asbestos. Conversely, subjects who started work later had a drastic reduction in the probability of exposure (30).
Consequences of environmental exposure
One may wonder at an international level, if no longer using asbestos is associated not only with the “residual effect” at long term, but also with the so-called “third wave of asbestos disease”, a term coined at the New York Conference in 1990 (31). In the broadest sense of its meaning, this definition covers the effects of external pollution in urban/extra-urban areas. These range from external industrial emissions in countries without banning or where banning has been legislated but asbestos not yet removed and, above all the release of airborne fibres into external environments, or even more so into internal ones or other friable insulation asbestos materials. This type of exposure may affect unsuspectable categories not included in the classical list of at-risk professions, where the problem of airborne dispersion of fibres from asbestos containing materials remain, above all during routine maintenance work or natural degradation.
International literature reports very variable reference levels for the general environment that might even differ in orders of magnitude. However, the air quality WHO document (Table 2) may be a useful tool, even if this document cannot be taken as being an absolute reference for each category (32).
Table 2. Asbestos fibres in environmental ambient air.
| Occurrence in air |
| Rural areas (remote from asbestos emission sources): below 100 F/m3 |
| Urban areas: general levels may vary from below 100 to 1,000 F/m3 |
| Near various emission sources the following figures have been measured as yearly averages: |
| (I) Downwind from an asbestos-cement plant at 300 m: 2,200 F/m3; at 700m: 800 F/m3; at 1,000 m: 600 F/m3 |
| (II) At a street crossing with heavy traffic, 900 F/m3 |
| (III) On an express-way, up to 3,300 F/m3 |
| Indoor air: |
| (I) In buildings without specific asbestos sources, concentrations are generally below 1,000 F/m3 |
| (II) In buildings with friable asbestos, concentrations vary irregularly; usually less than 1,000 F*/m3 are found, but in some cases exposure reaches 10,000 F*/m3 |
*, fibres counted with an optical microscope.
It is no easy feat to understand what role the remote occupational/environmental exposure before banning play within a hypothesis of a future mesothelioma epidemic, compared to the low, very low exposure due to the current environmental asbestos pollution.
This is really stony ground, as we have to deal with the indiscriminate use of statistic models to extrapolate the results obtained in the past in industrial settings, where exposure was three to four orders of magnitude higher than the current level of airborne fibres in the environment (33). Moreover, there is the clinical presentation of patients without any occupational exposure and only a suspicion of an environmental one.
There was a certain degree of agreement amongst the numerous agencies/authorities as to the fact that there is a lifetime risk (<80 years of age) of one additional case of mesothelioma per 100,000 subjects in the general population, at the level of 1 fibre/litre (34).
However, the US National Research Council has established that at a concentration of 0.4 fibres/litre, i.e., less than half of 1 fibre/litre, the lifetime risk could be that of 15.6 cases for 100,000 subjects (35). Therefore, so as to obtain the lifetime risk of one case/100,000 subjects, we would have to contain the exposure at 9 fibres/m3, i.e., 0.009 fibres/litre. This value is less than the concentration we could find in rural areas where there has been no use of asbestos. It goes without saying that it is not common sense to make this kind of forecast, questioning who is right and who is wrong (36).
Reference values for asbestos
The question of biological references remains difficult: for clinical and forensic medicine purposes the fibre and/or asbestos body burden in the lung take on a particular importance to distinguish between occupational and environmental exposure, at an individual level. Obviously, when there is the need to make a differential diagnosis between pulmonary pathologies e.g., idiopathic pulmonary fibrosis versus asbestosis, a burden with environmental characteristics would lead to the exclusion of an occupational etiology. Conversely, a high fibre and/or asbestos body burden would confirm an occupational etiology.
As to mesothelioma, whatever the level, there is no question as to its etiology, but fibre and/or asbestos body count will allow for the distinction between an occupational or environmental origin.
To this aim, the application of the so-called Helsinki Criteria is useful to identify subjects that, in all probability, have had an occupational asbestos exposure (37,38). It provides the following indications: over 0.1 million amphibole fibres (>5 µm) per gram of dry lung tissue or over 1 million amphibole fibres (>1 µm) per gram of dry lung tissue as measured by electron microscopy in a qualified laboratory, or over 1,000 asbestos bodies per gram of dry lung tissue (100 asbestos bodies per gram of wet tissue) or over one asbestos body per millilitre of bronchoalveolar lavage fluid, as measured by light microscopy in a qualified laboratory.
However, other aspects are involved when dealing with airborne fibres in the general environment. As aforementioned, firstly due to the uncertainty of the estimates available to define a risk as being simply environmental, any attempts to establish limit values, recommended values or safe environmental levels on these bases are to be considered with caution. This clearly evidences the need to establish environmental reference levels for the future, on the assumption that such values will represent basic concentrations in the absence of specific sources of internal/external emission in consideration of the inevitable concentrations due to the ubiquitous presence of asbestos fibres.
However, at this point, some parameters as to environmental fibres are to be redefined, such as “worn fibres”, which are often very short and/or very thin. That is why the classical parameters (diameter <3 µm, length >5 µm, aspect-ratio 3:1), as foreseen by the laws in force also for the environment, loose most of their significance when it comes to the identification of fibres that could be defined as “significative” or “representative” in the general environment.
Conclusions
The history of the use and biological effects of asbestos may be summed up as follows:
The past. It has its roots in the ancient past, with almost magical and mystical connotations, to arrive at an important industrial use, starting from the 19th century, when it was put to many and varied uses thanks to its physical-chemical properties. The increased use of asbestos went hand-in-hand with the dramatic onset of a series of diseases, mainly affecting the respiratory tract, that include lung fibrosis (asbestosis) and lung cancer and mesothelioma. These negative effects led to the progressive adoption of preventive measures and/or the banning of asbestos in many countries.
The present. Two scenarios are present at a world level: those countries that have banned asbestos and those that continue to produce and use it. In the former, from a healthcare point of view, it is evident that the residual effects of exposure before banning are to be managed. Due to the long latency of mesothelioma, we now have cases that can be traced back to occupational exposure in the 1970s–1980s. In countries that have not adopted banning, all the asbestos-related diseases have to be considered, also in the knowledge that even if asbestos were to be banned, the mesothelioma epidemic could well last for more than 4 decades.
The future. The problem for those who have not yet banned asbestos is, above all, ethical and involves the control of all the associated diseases. Whilst those who have adopted banning are still left with the question of mesothelioma, even if the international data on this disease show an increasing trend for those who started work many years ago when there was a higher risk of asbestos exposure (30). Conversely, the younger subjects, who have not had occupational exposure and are most likely exposed to decreasing industrial/occupational levels, show lower incidences. In terms of public health, not only should the banning of asbestos be discussed, but also whether future radical steps should or not be taken to remove existing asbestos products that pose a potential risk for asbestos in situ e.g., the total removal of covering in asbestos fibre-cement as decreed by the Netherlands within January 2024 (39).
Acknowledgements
None.
Footnotes
Conflicts of Interest: E Pira and G Discalzi reported personal fees for the defence from several law firms in asbestos litigations, outside the submitted work. F Donato and L Maida have no conflicts of interest to declare.
References
- 1.Marco Polo. The Travel of Marco Polo the Venetian. London: JM Dent, 1908. [Google Scholar]
- 2.Baader EW. Klinische Grundlagen der Sechsundvierzig Meldepfichtigen Berufskrankheiten. Munich and Berlin: Urban and Schwarzenberg, 1960. [Google Scholar]
- 3.New Advent. The Protoevangelium of James. Available online: http://www.newadvent.org/fathers/0847.htm
- 4.Saint Augustine. The City of God. Edited by Rev Marcus Dods. Edinburgh: T & T Clark, 1871. [Google Scholar]
- 5.International Ban Asbestos Secretariat. Graphics: Charts and Maps. Available online: http://www.ibasecretariat.org/graphics_page.php
- 6.Vogel L. Asbestos in the World. HESA Newsletter 2005:27:7-21. [Google Scholar]
- 7.Cooke WE. Fibrosis of the lungs due to the inhalation of asbestos dust. Br Med J 1924;2:147. 10.1136/bmj.2.3317.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooke WE. Pulmonary asbestosis. Br Med J 1927;2:1024-5. 10.1136/bmj.2.3491.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald S. Histology of pulmonary asbestosis. Br Med J 1927;2:1025-6. 10.1136/bmj.2.3491.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gloyne SR, Merewether ER. Asbestos. Occupation and Health: encyclopedia of hygiene, pathology, and social welfare. Ginevra: International Labour Office 1938;S1-15.
- 11.Kiviluoto R. Pleural calcification as a roentgenologic sign of non-occupational endemic anthofillite-asbestosis. Acta Radiol Suppl 1960;194:1-67. [PubMed] [Google Scholar]
- 12.Rubino GF, Concina E, Scansetti G, et al. Ricerca nella popolazione delle placche pleuriche calcifiche come segno radiologico di esposizione all’asbesto (crisotilo). Atti del Convegno di Studi sulla Patologia da Asbesto. Torino: 21 Giugno 1968;63-76.
- 13.Thomson JG, Graves WM., Jr Asbestos as an urban air contaminant. Arch Pathol 1966;81:458-64. [PubMed] [Google Scholar]
- 14.Donna A. Corpuscoli dell’asbestosi nel polmone umano reperiti nel comune materiale autoptico. Atti del Convegno di Studi sulla Patologia da Asbesto. Torino: 21 Giugno, 1968;49-61.
- 15.Nordmann M. Der Berufskrebs der Asbestarbeiter. Z Krebsforsch 1938:47:288-302. 10.1007/BF01621585 [DOI] [Google Scholar]
- 16.Nordman M, Sorge A. Lungenkrebs durch Asbeststaub in Tierversuch. Z. Krebsforsch 1941;51:168-82. 10.1007/BF01620849 [DOI] [Google Scholar]
- 17.Merewether ER. Asbestosis and carcinoma of the lung. Annual Report of the Chief Inspector of Factories for the Year 1947. London: HMSO, 1949;79-81. [Google Scholar]
- 18.Doll R. Mortality from lung cancer in asbestos workers. Br J Ind Med 1955;12:81-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner JC, Sleggs CA, Marchand P, et al. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960;17:260-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keal EE. Asbestosis and abdominal neoplasms. Lancet 1960;2:1211-6. 10.1016/S0140-6736(60)92413-2 [DOI] [PubMed] [Google Scholar]
- 21.Mc Nulty JC. Malignant pleural mesothelioma in an asbestos worker. Med J Aust 1962;49:953-4. [DOI] [PubMed] [Google Scholar]
- 22.Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647-67. 10.5858/arpa.2012-0214-OA [DOI] [PubMed] [Google Scholar]
- 23.Travis WD, Brambilla E, Burke AP, et al. Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Kazan-Allen L. Current Asbestos Bans. International Ban Asbestos Secretariat. Available online: http://ibasecretariat.org/alpha_ban_list.php
- 25.Peto J, Seidman H, Selikoff IJ. Mesothelioma mortality in asbestos workers: implications for models of carcinogenesis and risk assessment. Br J Cancer 1982;45:124-35. 10.1038/bjc.1982.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peto J, Hodgson JT, Matthews FE, et al. Continuing increase in mesothelioma mortality in Britain. Lancet 1995;345:535-9. 10.1016/S0140-6736(95)90462-X [DOI] [PubMed] [Google Scholar]
- 27.Reid A, de Klerk NH, Magnani C, et al. Mesothelioma risk after 40 years since first exposure to asbestos: a pooled analysis. Thorax 2014;69:843-50. 10.1136/thoraxjnl-2013-204161 [DOI] [PubMed] [Google Scholar]
- 28.Ferrante D, Chellini E, Merler E, et al. Italian pool of asbestos workers cohorts: mortality trends of asbestos-related neoplasms after long time since first exposure. Occup Environ Med 2017. Dec;74:887-98. 10.1136/oemed-2016-104100 [DOI] [PubMed] [Google Scholar]
- 29.Pira E, Romano C, Violante FS, et al. Updated mortality study of a cohort of asbestos textile workers. Cancer Med 2016;5:2623-8. 10.1002/cam4.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boffetta P, Malvezzi M, Pira E, et al. International Analysis of Age-Specific Mortality Rates From Mesothelioma on the Basis of the International Classification of Diseases. 10th revision. J Glob Oncol. Available online: http://ascopubs.org/doi/full/10.1200/JGO.2017.010116 [DOI] [PMC free article] [PubMed]
- 31.Landrigan PJ. The third wave of asbestos disease: Exposure to Asbestos in place: public health control. Ann N Y Acad Sci 1991;643:xv-xvi. 10.1111/j.1749-6632.1991.tb24438.x [DOI] [PubMed] [Google Scholar]
- 32.WHO. Regional Office for Europe. Air Quality Guidelines for Europe. 2nd edition. Bilthoven, Netherlands: WHO Regional Publications, 2000. [Google Scholar]
- 33.Piolatto G. Valori di riferimento e valori limite per l'asbestosi. I valori di riferimento e i valori limite nella prevenzione ambientale e occupazionale. In: Aprea C, Sciarra G, Fiorentino ML, et al. editors. Milano: Morgan Ed. Tecniche, 1996:153-61. [Google Scholar]
- 34.Mossman BT. Fibre carcinogenesis and environmental risks. In: Environment and Prevention, Ellis Horwood Ltd 1991;241-55. [Google Scholar]
- 35.U.S. National Research Council of the National Academy of Science. Asbestiform fibres: Non-occupational health risks. Washington, DC: National Academy Press, 1984. [Google Scholar]
- 36.Valic F. lnfluence of exposure conversions and activity-specific exposure-response relationships on the chrysotile asbestos risk assessment. In: Gibbs GW, Dunnigan J, Kido M, Higashi T. editors. Health Risks from Exposure to Mineral Fibres: An International Perspective. North York, Ontario: Captus University Publications, 1993;29-135. [Google Scholar]
- 37.Tossavainen A. Asbestos, asbestosis, and cancer: the Helsinki criteria for diagnosis and attribution. Consensus Report. Scand J Work Environ Health 1997;23:311-6. 10.5271/sjweh.226 [DOI] [PubMed] [Google Scholar]
- 38.Wolff H, Vehmas T, Oksa P, et al. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health 2015;41:5-15. 10.5271/sjweh.3462 [DOI] [PubMed] [Google Scholar]
- 39.Kazan-Allen L. Chronology of asbestos bans and restrictions. International Ban Asbestos Secretariat. Available online: http://ibasecretariat.org/chron_ban_list.php