Abstract
Background:
Tumour budding has been reported as a promising prognostic marker in many cancers. This meta-analysis assessed the prognostic value of tumour budding in oral squamous cell carcinoma (OSCC).
Methods:
We searched OvidMedline, PubMed, Scopus and Web of Science for articles that studied tumour budding in OSCC. We used reporting recommendations for tumour marker (REMARK) criteria to evaluate the quality of studies eligible for meta-analysis.
Results:
A total of 16 studies evaluated the prognostic value of tumour budding in OSCC. The meta-analysis showed that tumour budding was significantly associated with lymph node metastasis (odds ratio=7.08, 95% CI=1.75–28.73), disease-free survival (hazard ratio=1.83, 95% CI=1.34–2.50) and overall survival (hazard ratio=1.88, 95% CI=1.25–2.82).
Conclusions:
Tumour budding is a simple and reliable prognostic marker for OSCC. Evaluation of tumour budding could facilitate personalised management of OSCC.
Keywords: tumour budding, oral squamous cell carcinoma, prognosis, marker, invasive front, treatment, survival
Oral squamous cell carcinoma (OSCC) is the most common malignancy of the oral cavity and constitutes the majority of head and neck squamous cell carcinomas. According to a recent report, ∼300 000 new cases of oral cancer were diagnosed worldwide in 2012, and with a consequent 145 000 cancer-related deaths (Ferlay et al, 2015). The incidence of OSCC has increased in many countries and especially in young people (Muller et al, 2008; Korvala et al, 2017). In the Western world, the main aetiological factors for OSCC are tobacco and alcohol consumption. Chewing of Areca nuts and the use of snuff are the classic risk factors in the Indian population. The 5-year survival rate of OSCC patients is relatively low, and especially the patients with recurrence have poor outcomes. Identifying cases at risk for recurrence remains challenging.
Many histopathologic prognostic parameters (e.g., tumour grade, depth of invasion, perineural invasion, lymphovascular invasion, lymphocytic host response and mitotic activity) are usually evaluated in haematoxylin- and eosin- (H–E) stained sections. Such information is included in pathology reports to aid in predicting the behaviour of OSCC. This is paramount for planning of an appropriate and successful management. However, some of these parameters (e.g., tumour grade and lymphocytic response) have not been promising prognosticators, especially in early stage OSCC (Chen et al, 2013; Almangush et al, 2015a). Moreover, recent research has introduced several biomarkers for OSCC, but they are not yet eligible to be included in the pathology report (Soland and Brusevold, 2013; Almangush et al, 2017a). In addition, such biomarkers require additional staining procedures which are not routinely used. Therefore, it is important to identify new powerful prognostic markers that are adaptable to conventional H–E staining.
Tumour budding, defined as the presence of single cancer cell(s) or cluster(s) of less than five cancer cells at the invasive front (IF), has been reported in many cancers as a promising prognostic feature (Kadota et al, 2015; Almangush et al, 2016; Rogers et al, 2016). Tumour budding at the IF (Figure 1) indicates the dissociation of invasive cancer cells from the main tumour mass. Several recent studies have evaluated the significance of tumour budding in OSCC. The aim of the current study was to systematically review the studies on tumour budding in OSCC and to present a meta-analysis of the prognostic value of tumour budding in OSCC. We also discuss the shortcomings in the published studies and provide recommendations for further research to standardise the evaluation method of tumour budding in OSCC.
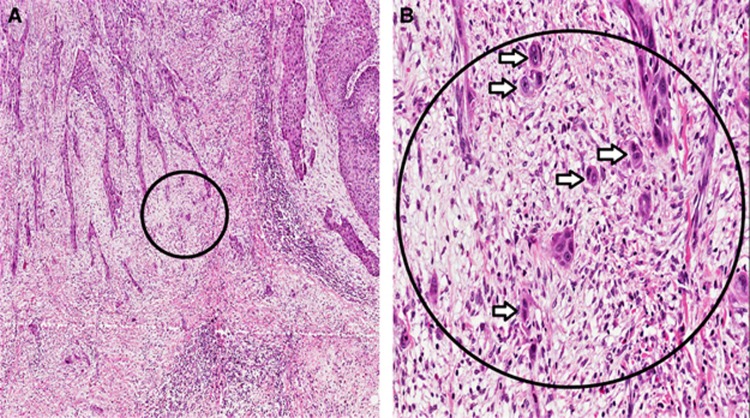
Figure 1.
Tumour budding, defined as single cancer cell or clusters of less than five cells at the invasive front of oral squamous cell carcinoma (OSCC). (A) Low magnification (× 4); and (B) high magnification (× 20) of the area inside the circle.
Materials and methods
Search protocol
OvidMedline, PubMed, Scopus and Web of Science were searched using the following keywords: (‘oral’ or ‘mouth’ or ‘tongue’ or ‘floor of mouth’ or ‘lip’ or ‘gingiva’ or ‘buccal’ or ‘palate’) and (‘tumour budding’). Our search was limited to articles in the English language. The end point of the search was May 2017. To ensure inclusion of all relevant articles, we manually searched the reference lists of all eligible studies. When searching and screening the studies, we followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) (Moher et al, 2009).
Exclusion criteria
We excluded studies in a language other than English, studies on animal samples and conference abstracts.
Quality assessment
We used reporting recommendations for tumour marker prognostic studies (REMARK) guidelines (Altman et al, 2012) to assess the quality of studies that evaluated the prognostic value of tumour budding in OSCC. We summarised the main guidelines in Table 1. Any study that received a score of less than 6 was not included in our meta-analysis.
Table 1. Evaluation criteria that have been used to assess the quality of studies evaluated tumour budding in OSCC (adapted from REMARK).
| Checklist | Criteria |
|---|---|
| Introduction | The hypotheses and objectives of the study were clearly explained |
| Cohort description | Retrospective or prospective cohort with a well-defined study population |
| Medical treatment of the cases was explained | |
| Patient data | The basic data such as age, gender, clinical stage and histopathologic grade was provided |
| Evaluation method | Well-described method including the microscopic field/s and the cutoff point. Inter-observer variability was evaluated |
| Prognostic analysis | The survival end point was defined and/or the relationship between the tumour budding and lymph node metastasis was studied |
| Statistical analysis | Estimated effect (e.g., hazard ratio, relative risk with their confidence interval), which reveal the relationship between tumour budding and the survival end point/s |
| The independence of prognostic value was reported by multivariate analysis | |
| Classical prognostic factors | The prognostic value of the classical prognostic factors (e.g., stage and grade) were reported |
| The relationship between tumour budding and classical prognostic factors was reported | |
| Interpretation of the prognostic value and discussion | Comparison of the current findings with other studies |
| Strengths and limitations of the current data | |
| Recommendation for further research |
Statistical methods
The meta-analysis was performed by the ‘meta’ package (version 4.8-1) in statistical software R (version 3.4.0). For each analysis, we carried out an inverse variance-weighted fixed-effects analysis. For completeness, a DerSimonian–Laird random effects analysis (DerSimonian and Laird, 1986) was also performed. We considered the random effects analysis as our main result to account for heterogeneity between the studies. In addition to the meta-analysed effect sizes, our results also included the estimated proportion of variation in effect sizes due to heterogeneity (I2) (Higgins and Thompson, 2002) and the DerSimonian–Laird estimate of the variance of the effect sizes (t2) (DerSimonian and Laird, 1986). We first conducted meta-analyses for each survival end point even if tumour stage, oral subsite or budding cutoff point varied between the studies. To reduce heterogeneity among the included studies, we then conducted additional meta-analyses specifically for studies with early stage cases and for studies from single oral subsite (oral tongue). We also conducted separate meta-analyses for studies with a similar cutoff point of tumour budding.
Results
Search results
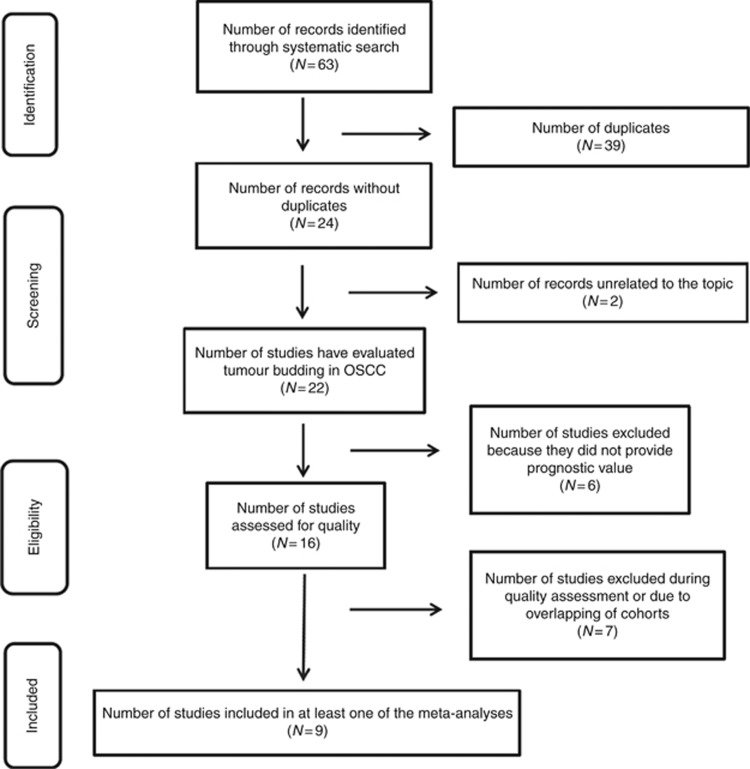
A total of 63 hits were retrieved from searches of databases, and 39 hits were excluded as duplicates. There were 22 studies that had evaluated tumour budding in OSCC (Figure 2). Of these, 16 studies had reported the prognostic value of tumour budding in OSCC (Table 2). The other six studies had evaluated tumour budding in OSCC without providing its prognostic value (Table 3).
Figure 2.
Flow diagram outlining the search strategy and the search results along various steps.
Table 2. Summary of the studies that examined the prognostic value of tumour budding in OSCC.
| (Authors, year) Country | Cases | Stage | Location | Follow up | Primary treatment | Staining | Cutoff | % | Field | Survival analysis | HR (95% CI) | P value | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Wang et al, 2011) China | 133 | I–IV | Tongue | 65 months | Surgery | H–E | 5 buds | 44.4% | × 20 | OS | 3.350 (1.774−6.323) 3.029 (1.535−5.977) | 0.0014 | 8 |
| (Almangush et al, 2014) Finland | 233 | cT1–2N0 | Tongue | 67 months | Surgery | H–E | 5 buds | 34.8% | × 20 | DSS | 2.00 (1.17−3.40) 2.04 (1.17−3.55) | 0.01 | 7 |
| (Manjula et al, 2015) India | 33 | T1–T4 | Gingivo buccal complex | 15 months | Surgery | H–E | 10 buds | 63.6% | NA | DFS | 1.32 (0.59−2.95) | 0.49 | 6 |
| LNM | OR 7.5 (1.49−37.66) | 0.014 | |||||||||||
| (Almangush et al, 2015b) Finland and Brazil | 311 | cT1–2N0 | Tongue | 57 months | Surgery | H–E | 5 buds | 30.9% | × 20 | DSS | 2.59 (1.58−4.26) 1.76 (1.01−3.06) | <0.001 0.044 | 7 |
| DFS | 1.85 (1.21–2.82) 1.80 (1.10−2.93) | 0.005 0.020 | |||||||||||
| OSa | 1.40 (1.01−1.93) 1.62 (1.17−2.25) | 0.042 0.004 | |||||||||||
| (Angadi et al, 2015) India | 75 | T1–T4 | Oral cavity | NA | Surgery | H–E | 10 buds | 45.3% | × 25 | LNM | OR 6.79 (2.28−20.18) | <0.001 0.001 | 7 |
| (Attramadal et al, 2015) Norway | 58 | cT1–2N0 | Oral cavity | 55 months | Surgery | IHC | 5 buds | 51.7% | × 20 | DFS | NA | 0.043 | 5 |
| (Jensen et al, 2015a) Denmark | 199 | T1–T4 | Tongue and floor of mouth | 4.6 years | Surgery | IHC | Median bud count | 50.3% | × 20 (DIA) | LNM | AUC of 0.69 (95% CI 0.61−0.76) | NA | 8 |
| OS | 1.8 (1.3−2.6) 1.6 (1.1−2.3) | 0.01 | |||||||||||
| DFS | 2.1 (1.2−3.6) | <0.01 | |||||||||||
| (Xie et al, 2015) China | 195 (106 with follow up) | cT1–2N0 | Tongue | 56 months | Surgery | IHC | 5 buds | 52.8% | × 20 | Occult LNM | NA | 0.015 | 7 |
| Local relapse | NA | 0.001 | |||||||||||
| OS | 10.44 (2.43−44.88) 5.58 (1.23−25.38) | 0.002 0.026 | |||||||||||
| (Nandita et al, 2016) India | 30 | NA | Oral cavity | NA | NA | H–E | 5 buds | NA | NA | OS | NA | NA | 3 |
| (Seki et al, 2016) Japan | 91 | T1–T4 | Tongue and floor of mouth (biopsy) | From 4 months to 5 years | Surgery; 47 cases received preoperative CT | IHC | 3 buds | 50.5% | × 20 | LNM | Univariate: NA OR 31 (2.6−331.8) | <0.01 | 6 |
| OS | NA | <0.05 | |||||||||||
| RFS | NA | <0.01 | |||||||||||
| (Xie et al, 2016) China | 100 | T1–T4 | Tongue | 3 years | Surgery | H–E, IHC | 5 buds | 49% | × 20 | OS | 2.23 (0.99−5.01) | 0.046 | |
| LNM | NA | <0.01 | |||||||||||
| (Seki et al, 2017) Japan | 209 | cT1–T4 | Oral cavity (biopsy) | 16-72 months | Surgery; 111 cases received preoperative CT | IHC | 5 buds | 28.7% | × 20 | LNM | Univariate: NA OR 30.05 (10.98−82.23) | <0.01 | 6 |
| RFS | NA | <0.01 | |||||||||||
| OS | NA | 0.01 | |||||||||||
| (Boxberg et al, 2017) Germany | 157 | T1–T4 | Oral cavity | 33.2 months | Surgery | H–E | 5 buds | 26.1% | × 40 | OS | NA | 0.003 | 5 |
| DSS | NA | 0.001 | |||||||||||
| DFS | NA | 0.003 | |||||||||||
| (Pedersen et al, 2017) Denmark | 222 | cT1–2N0 | Oral cavity | 36 months | Surgery | IHC | DIA | NA | DIA | PFS | 7.1 (2.4−20.5) 2.3 (1.5−3.8) | <0.001 | 8 |
| OS | 4.0 (1.9−8.4) 1.6 (1.1−2.2) | 0.01 | |||||||||||
| Occult LNM | AUC of 0.83 (95% CI: 0.78−0.89) | <0.001 | |||||||||||
| (Hori et al, 2017) Japan | 48 | cT1–2N0 | Tongue | 71 months | Surgery | H–E | 5 buds | 27% | × 20 | Neck recurrence | Univariate: NA RR 24.07 (2.27−254.89) | <0.001 <0.01 | 6 |
| (Arora et al, 2017) India | 336 | cT1–2N0 | OSCC | 60 months | Surgery | H–E | 5 buds | 39.6% | × 20 | LNM | OR 1.92 (1.18−3.12)b OR 1.28 (1.09−2.61) | 0.008 0.039 | 8 |
Abbreviations: AUC=area under curve; CI=confidence interval; CT=chemotherapy; DFS=disease-free survival; DIA=digital image analysis; H–E=haematoxylin and eosin staining; HR=hazard ratio; IHC=immunohistochemical staining with cytokeratin or pan-cytokeratin. × 20=refer to × 20 objective lens; LNM=lymph node metastasis; NA=not available; OR=odds ratio; OS=overall survival; %=percentage of cases with high intensity of tumour buddingl; PFS=progression free survival; RFS=relapse free surviva; RR=risk ratio; SCC=squamous cell carcinoma.
We conducted the OS from data of our original study Almangush et al, (2015b) for this meta-analysis.
We computed a univariate OR (with its 95% CI) estimate for tumour budding from study of Arora et al, 2017.
Notes: Wang et al, (2011) and (Xie et al, 2015, 2016) are overlapped.
Almangush et al, (2014) and Almangush et al, (2015b) are overlapped.
Jensen et al, (2015a) and (Pedersen et al, 2017) are overlapped.
Seki et al, (2016) and Seki et al, (2017) are overlapped.
HR, RR, OR and CI in bold are from multivariate analysis.
Table 3. Summary of the studies evaluated tumour budding in OSCC without analysis of its prognostic value.
| (Authors, year) Country | Cases | Stage | Location | Follow up | Primary treatment | Staining | Cutoff | % | Field | Findings related to tumour budding |
|---|---|---|---|---|---|---|---|---|---|---|
| (Marangon Junior et al, 2014) Brazil | 57 | NA | Oral cavity | NA | NA | IHC | 5 buds | 75.4% | × 20 | High intensity tumour budding is associated with higher density of stromal myofibroblasts and higher expression of laminin-5 gamma 2 chain |
| (Sawazaki-Calone et al, 2015) Brazil | 113 | T1–T4 | Oral cavity | 5 years | Surgery | H–E | 5 buds | NA | × 20 | Tumour budding is a parameter of the budding-depth (BD) prognostic model. BD showed a superior prognostic value compared to other histopathologic grading systems |
| (Jensen et al, 2015b) Denmark | 28 | NA | Oral cavity | NA | NA | IHC | NA | NA | NA | A relationship between tumour budding and myofibroblasts was seen but was not a general featureBudding cells have shown low expression of E-cadherin |
| (Zhang et al, 2016) China | 73 | T1–T4 | Tongue | 114 months | CT for 7 cases, RT for 17, and surgery for others | H–E | 5 buds | 75.4% | × 20 | High intensity of tumour budding was more common in tongue cancer (75.4%) compared to high intensity of tumour budding in nasopharyngeal carcinoma (45.5%) |
| (Strieder et al, 2017) Brazil | 53 | T1–T4 | Lip | 159.4 months or 57.4 months | Surgery | H–E | 5 buds | 67.9% | × 20 | Tumour budding is a parameter of the budding-depth (BD) prognostic model. BD showed a high prognostic value for lip cancer |
| (Leao et al, 2017) Brazil | 103 | NA | Oral cavity | NA | NA | H–E; IHC | 5 buds | NA | × 20 | Evaluation of tumour budding by IHC showed higher reproducibility and replicability compared to H–E |
Abbreviations: CT=chemotherapy; H-E=haematoxylin and eosin staining; IHC=immunohistochemical staining with cytokeratin or pan-cytokeratin.
Statistical results
A meta-analysis of the prognostic value of tumour budding for lymph node metastasis, disease-free survival and overall survival is summarised in Figures 3, 4, 5. For each end point, there was at least one meta-analysis of three high-quality studies (according to REMARK guidelines; Table 1) that had reported the necessary statistical values (hazard ratio (HR) or odds ratio (OR) and confidence interval (CI)). According to our analyses, there was strong evidence for tumour budding to be considered as a promising prognostic marker for OSCC.
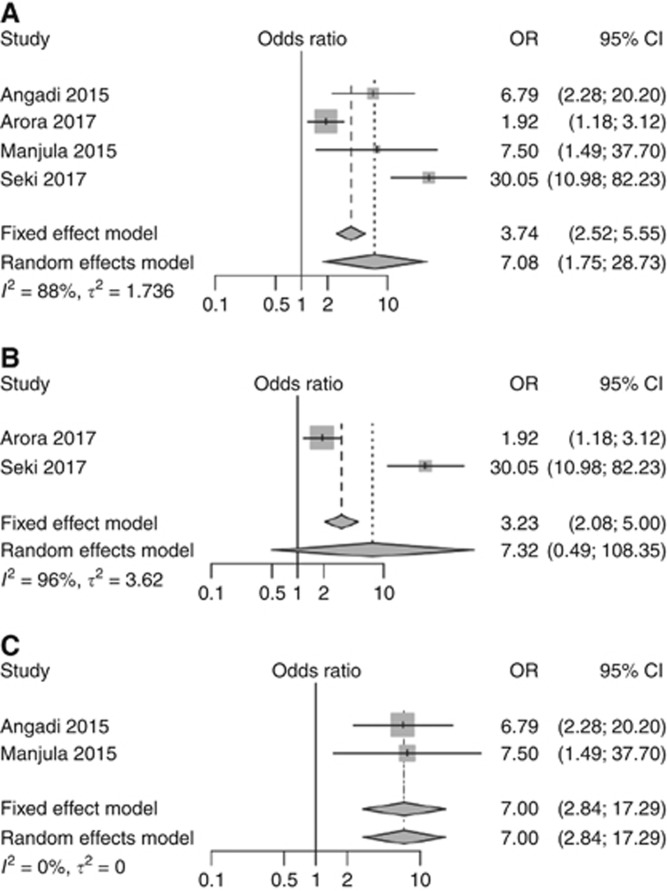
Figure 3.
Forest plots for the pooled analyses of the studies evaluated the prognostic value of tumour budding in assessing lymph node metastasis of OSCC. (A) All eligible studies. (B) Studies used five-bud cutoff point. (C) Studies used 10-bud cutoff point.
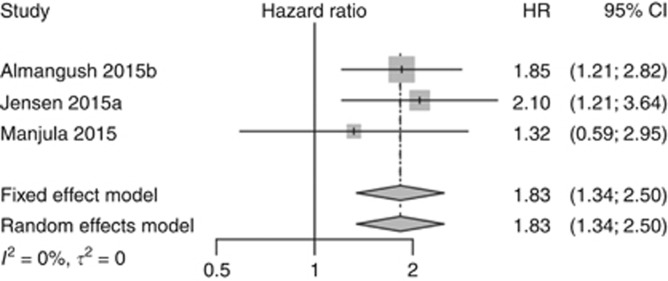
Figure 4.
Pooled analysis for disease-free survival.
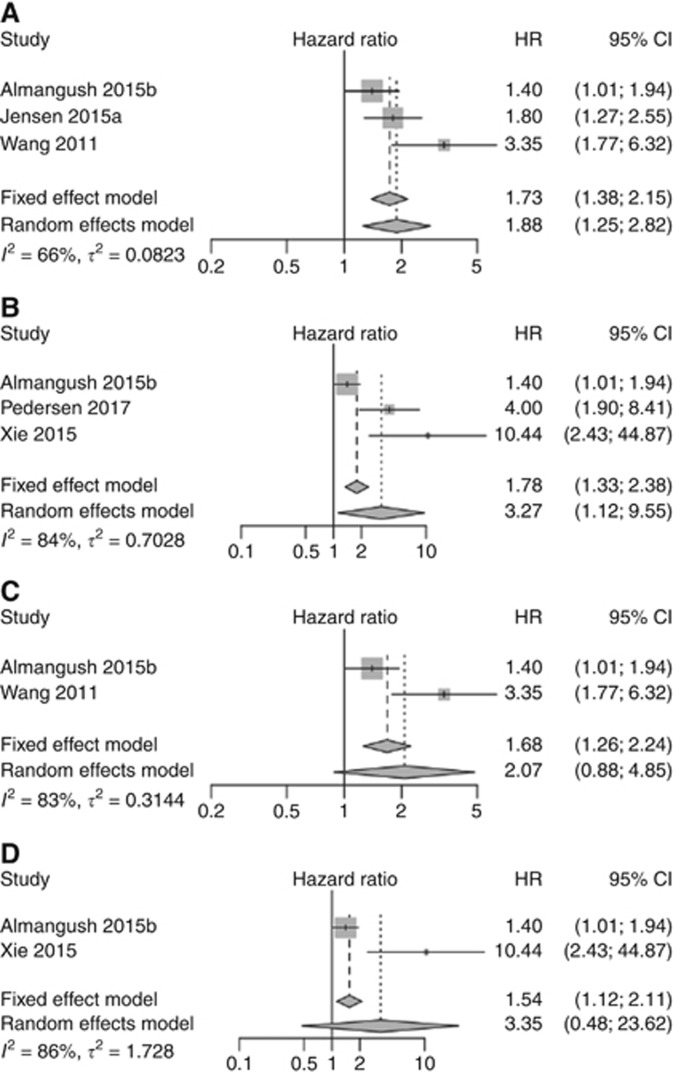
Figure 5.
Pooled analyses for overall survival. (A) All stages of OSCC. (B) Pooled analysis for overall survival of OSCC including studies of early stage only. (C) Pooled analysis for overall survival including only oral tongue cancer studies which used five-bud cutoff point. (D) Pooled analysis for overall survival including early stage oral tongue cancer studies that used five-bud cutoff point.
Our meta-analyses of eligible studies with different budding cutoff points for risk stratification indicated that high-grade tumour budding was significantly associated with the presence of lymph node metastasis (Figure 3A) when compared with low-grade tumour budding (OR=7.08, 95% CI=1.75–28.73). Subsequently, our meta-analysis of studies (Arora et al, 2017; Seki et al, 2017) that used a cutoff point of five buds (Figure 3B) and our pooled analysis of the other studies (Angadi et al, 2015; Manjula et al, 2015) that used a cutoff point of 10 buds (Figure 3C) showed similar odds ratios (OR=7.32, 95% CI=0.49–108.35; and OR=7.0, 95% CI=2.84–17.29, respectively), while the former analysis was more heterogeneous.
The pooled analysis for disease-free survival (Figure 4) also showed that high-grade tumour budding was correlated with poorer survival (HR=1.83, 95% CI=1.34–2.50). For overall survival, tumour budding was associated with poor survival when all stages (Figure 5A) were included (HR=1.88, 95% CI=1.25–2.82) and also when a meta-analysis of early stage (Figure 5B) cases only was performed (HR=3.27, 95% CI=1.12–9.55). The pooled analyses for overall survival of the studies that evaluated tumour budding in oral tongue cancers using five buds as a cutoff point also showed similar results (Figure 5C) when advanced stage was included (HR=2.07, 95% CI=0.88–4.85), as well as when studies of early stage cases (Figure 5D) were analysed separately (HR=3.35, 95% CI=0.48–23.62).
We observed potential heterogeneity (I2⩾66%) between the studies for two analyses of lymph node metastasis (Figure 3A and B) and for overall survival meta-analyses (Figure 5), but we could not assess statistical significance of heterogeneity due to the small number of studies. Of note, for one meta-analysis of lymph node metastasis (Figure 3C), as well as for disease-free survival meta-analysis (Figure 4), we did not observe heterogeneity between the studies (I2=0).
Discussion
The invasive tumour front of OSCC has been an area of research interest in recent decades. Cancer cells at the IF behave aggressively compared with cancer cells in the superficial or central regions of the main tumour mass (Bryne et al, 1992; Jensen et al, 2015a). In addition, cancer cells at the IF may undergo epithelial–mesenchymal transition, which is an important step in progression of tumour metastasis (Christofori, 2006). Tumour budding that may be involved in development of metastasis has been reported at the IF and evaluated in several studies on OSCC (Table 2). Here, we performed a meta-analysis on the results of such studies. Our meta-analysis shows that tumour budding is a promising prognostic marker for OSCC.
The importance of tumour budding in cancer prognosis has been studied widely particularly in colorectal cancer (Rogers et al, 2016; Lugli et al, 2017), where it is recognised as an additional prognostic marker (Koelzer et al, 2014). In oesophageal cancer (Almangush et al, 2016), pancreatic cancer (Karamitopoulou, 2012), breast cancer (Gujam et al, 2015) and lung cancer (Kadota et al, 2014), tumour budding has been reported as a promising prognostic marker. A significant correlation between high tumour budding count and the presence of lymph node metastases is one of the most important findings observed in OSCC (Figure 3) and in many other cancers (Yamaguchi et al, 2010; Landau et al, 2014; Salhia et al, 2015; Cappellesso et al, 2017). Such a finding might indicate that tumour budding is an early step en route to metastasis. A correlation between tumour budding and occult lymph node metastasis was reported in early stage OSCC (Xie et al, 2015). As occult metastasis is the most common reason for relapse and poor prognosis in early stage cases, it is of great importance to validate this correlation in other large multicentre cohorts.
Simplicity, reproducibility and low cost are important characteristics when considering a new marker for clinical application. The published studies in OSCC and in other cancers repeatedly reported these advantages for tumour budding (Wang et al, 2011; Graham et al, 2015; Almangush et al, 2015b). Another advantage of the studies of tumour budding in OSCC is that their results are consistent with those from the first study that evaluated budding in OSCC (Wang et al, 2011). Conversely, controversial findings were reported for the prognostic biomarkers identified for OSCC (Soland and Brusevold, 2013; Almangush et al, 2017a).
When considering a new prognostic marker for clinical application, the marker should also have a significant prognostic value independent from classical markers. Interestingly, for tumour budding, most of the studies that provided multivariate analysis (Wang et al, 2011; Angadi et al, 2015; Almangush et al, 2015b; Seki et al, 2016; Hori et al, 2017; Pedersen et al, 2017) reported that tumour budding has a superior prognostic value compared to other classical markers such as TNM stage, depth of invasion or WHO tumour grade. However, in one study, (Manjula et al, 2015), tumour thickness (5-mm cutoff point) showed superior prognostic value compared with tumour budding, and the same was observed for depth of invasion in the study by Arora et al. (2017). In another study (Jensen et al, 2015a), advanced stage was associated with a poorer prognosis than in cases with high-grade budding. Of note, in the latter two studies (Jensen et al, 2015a; Arora et al, 2017) tumour budding was also reported as an independent prognostic marker in multivariate analysis. Therefore, multivariate analysis of published studies indicates that high-intensity tumour budding, either independently or in addition to the advanced stage, deeply invaded tumour or both, is associated with poor prognosis of OSCC. Only in the study by Manjula et al. (2015), tumour budding was not a prognostic marker in multivariate analysis. However, Manjula et al. used a 10-bud cutoff point to stratify cases into risk scores, and it is possible that some cases with ⩾five buds were included in the low-grade budding group, which subsequently reduced the prognostic value of tumour budding in this cohort.
Different methods have been introduced for the evaluation of tumour budding (Koelzer et al, 2014). However, a traditional method was widely used in the studies on OSCC. In this method, the IF is scanned under low magnification (× 4), and the field with the highest budding number is counted under high magnification (× 20) and used for the score (Wang et al, 2011). The evaluation of intra-tumoural budding was not reported in OSCC. Of note, intra-tumoural budding was shown as a valid method in colorectal cancer (Lugli et al, 2011). In only a few studies, evaluation of the prognostic value of tumour budding at the IF was carried out in biopsy specimens of OSCC (Seki et al, 2016, 2017; Almangush et al, 2017b). However, the IF area might not be included in a biopsy specimen. In such cases, another form of tumour budding, the intra-tumoural budding (i.e., tumour budding between tumour islands) might be more applicable. The latter approach may be of great importance from a clinical point of view for treatment planning of OSCC, and should be further evaluated. In addition, intraoperative evaluation of tumour budding (i.e., using fresh-frozen sections) should also be considered in future studies.
Diverse cutoff points were suggested for stratification of cases into low-grade and high-grade tumour budding (Table 2). In the present studies on OSCC, five-bud cutoff point was the most commonly used (low grade <5 vs high grade ⩾5). We conducted meta-analysis for studies that used different cutoff points (Figure 3A), and then, we conducted separate meta-analyses for studies that used a five-bud cutoff point (Figure 3B) and for studies that used a 10-bud cutoff point (Figure 3C). Interestingly, these meta-analyses show that tumour budding is a useful prognostic marker for OSCC cases. As the risk of poor prognosis begins at the presence of five buds, we suggest considering both five-bud and 10-bud cutoff points in further studies to determine which one of these cutoff points is more predictive of poor prognosis and should therefore be used in clinical practice.
Most studies evaluated tumour budding using H–E staining. Interestingly, a recent study on OSCC concluded that evaluation of tumour budding by immunohistochemistry with pan-cytokeratin antibodies (clones AE1/AE3) showed a better reproducibility of results than those with H–E staining (Leao et al, 2017). However, standardisation of the evaluation method and cutoff point is still necessary. A recent international consensus conference on tumour budding (Lugli et al, 2017) made several statements (including definition, evaluation method and others) for reporting tumour budding in colorectal cancer. Such statements are still necessary to allow inclusion of tumour budding in a pathology report for OSCC cases.
The combination of squamous cell carcinoma (SCC) from different subsites of the oral cavity was a common disadvantage among the studies that evaluated tumour budding in OSCC. Therefore, we recommend a separate analysis for each subsite when reporting tumour budding in future studies. Despite a small number of studies available, we conducted a meta-analysis for overall survival of studies that evaluated tumour budding in oral tongue SCC (Figure 5C and D), which is the most common SCC of the oral cavity. The results of this meta-analysis suggest, although without strong statistical evidence, that cases of oral tongue cancer with a high budding index have a poorer overall survival. This is consistent with the other meta-analyses where the subsites were mixed. Another combination that was also common among the included studies was mixing of early stage and late-stage cancers in the same analysis. We conducted a meta-analysis for the two studies that included only early stage cancers (Figure 5D), and the result suggests that tumour budding in such early stage cases has a prognostic value, but given the wide confidence intervals, this result lacks strong statistical evidence and requires further studies for validation.
Tumour budding in OSCC has also been evaluated using digital pathology (Jensen et al, 2015a; Pedersen et al, 2017). Digital image analysis has been used increasingly in recent research and it has shown better accuracy and reproducibility compared with the conventional method as it allows truly quantitative scores (Riber-Hansen et al, 2012). Moreover, it will be easier to standardise the scoring method using digital image analysis (Pedersen et al, 2017). Therefore, digital image analysis of tumour budding in OSCC should be used to validate results in large cohorts.
Few studies have examined the biological background of tumour budding in OSCC. Immunohistochemical analysis showed that tumour budding is associated with reduced expression of E-cadherin and overexpression of vimentin (Wang et al, 2011). Regarding interactions with the surrounding stroma, high-grade budding was associated with a higher density of stromal myofibroblasts and higher expression of laminin-5 gamma 2 chain (Marangon Junior et al, 2014). In genetic profiling, decreased expression of miR-200a, miR-200b and miR-200c was reported in cancer cells of tumour budding (Jensen et al, 2015a). However, molecular analyses in other cancers have provided more details about the genetic background of tumour budding (Zlobec and Lugli, 2010; Galvan et al, 2015; Bradley et al, 2016; Miyake et al, 2017), and similar analyses in OSCC are still necessary to better understand this phenomenon.
The main limitation of the current meta-analyses is the small number of the original studies. Accordingly, it was difficult to statistically evaluate the heterogeneity between the studies. To avoid bias due to any potential heterogeneity, we focused on a random effects model that is known as an effective method to combine heterogeneous studies (Guolo and Varin, 2017). In addition, for each meta-analysis (Figures 3, 4, 5), we also reported results of a fixed effect model and they were consistent with a random effects model. Moreover, our meta-analyses addressed three different end points (metastasis, overall survival and disease-free survival), and our results regarding the common effect of tumour budding as a negative prognostic marker are valid based on meta-analyses of these different end points. Of note, this effect is also consistent across published studies. Inclusion of different subsites of the oral cavity or mixing of different stages in analysis of the same cohort was another limitation, as mentioned above. The absence of prospective studies was also noted.
Despite these shortcomings, there is sufficient evidence to suggest that OSCCs with high-grade tumour budding are at high risk of poor prognosis. This evidence was prominent and validated in many studies. Similar evidence has also accumulated on the prognostic value of tumour budding in other cancers (Almangush et al, 2016; Rogers et al, 2016; Lugli et al, 2017). To the best of our knowledge, this is the first meta-analysis on the prognostic value of tumour budding in OSCC. We conclude that tumour budding has a prominent prognostic power for OSCC even at early stages of the disease. Future research on OSCC should compare the different evaluation methods with the goal of standardising the assessment method for pathology reports. In addition, understanding the genetic background of tumour budding may facilitate identification of treatment targets in OSCC.
Acknowledgments
This work was financially supported by the Finnish Dental Society (Alhadi Almangush), the Rauha Ahokas Foundation (Alhadi Almangush), the Academy of Finland (288509 and 294050 to Matti Pirinen), the Research Funds of the University of Helsinki (Matti Pirinen), the Helsinki University Hospital Research Fund (Antti A Mäkitie and Tuula Salo), the Sigrid Juselius Foundation (Tuula Salo), the Finnish Cancer Society (Tuula Salo) and the Maritza and Reino Salonen Foundation (Ilmo Leivo).
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
References
- Almangush A, Bello IO, Coletta RD, Makitie AA, Makinen LK, Kauppila JH, Pukkila M, Hagstrom J, Laranne J, Soini Y, Kosma VM, Koivunen P, Kelner N, Kowalski LP, Grenman R, Leivo I, Laara E, Salo T (2015. a) For early-stage oral tongue cancer, depth of invasion and worst pattern of invasion are the strongest pathological predictors for locoregional recurrence and mortality. Virchows Arch 467(1): 39–46. [DOI] [PubMed] [Google Scholar]
- Almangush A, Bello IO, Keski-Santti H, Makinen LK, Kauppila JH, Pukkila M, Hagstrom J, Laranne J, Tommola S, Nieminen O, Soini Y, Kosma VM, Koivunen P, Grenman R, Leivo I, Salo T (2014) Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck 36(6): 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almangush A, Coletta RD, Bello IO, Bitu C, Keski-Santti H, Makinen LK, Kauppila JH, Pukkila M, Hagstrom J, Laranne J, Tommola S, Soini Y, Kosma VM, Koivunen P, Kowalski LP, Nieminen P, Grenman R, Leivo I, Salo T (2015. b) A simple novel prognostic model for early stage oral tongue cancer. Int J Oral Maxillofac Surg 44(2): 143–150. [DOI] [PubMed] [Google Scholar]
- Almangush A, Heikkinen I, Makitie AA, Coletta RD, Laara E, Leivo I, Salo T (2017. a) Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer 117(6): 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almangush A, Karhunen M, Hautaniemi S, Salo T, Leivo I (2016) Prognostic value of tumour budding in oesophageal cancer: a meta-analysis. Histopathology 68(2): 173–182. [DOI] [PubMed] [Google Scholar]
- Almangush A, Leivo I, Siponen M, Sundquist E, Mroueh R, Makitie AA, Soini Y, Haglund C, Nieminen P, Salo T (2017. b) Evaluation of the budding and depth of invasion (BD) model in oral tongue cancer biopsies. Virchows Arch; epub ahead of print 2 August 2017; doi:10.1007/s00428-017-2212-1. [DOI] [PubMed]
- Altman DG, McShane LM, Sauerbrei W, Taube SE (2012) Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med 10: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angadi PV, Patil PV, Hallikeri K, Mallapur MD, Hallikerimath S, Kale AD (2015) Tumor budding is an independent prognostic factor for prediction of lymph node metastasis in oral squamous cell carcinoma. Int J Surg Pathol 23(2): 102–110. [DOI] [PubMed] [Google Scholar]
- Arora A, Husain N, Bansal A, Neyaz A, Jaiswal R, Jain K, Chaturvedi A, Anand N, Malhotra K, Shukla S (2017) Development of a new outcome prediction model in early-stage squamous cell carcinoma of the oral cavity based on histopathologic parameters with multivariate analysis: the aditi-nuzhat lymph-node prediction score (ANLPS) system. Am J Surg Pathol 41(7): 950–960. [DOI] [PubMed] [Google Scholar]
- Attramadal CG, Kumar S, Boysen ME, Dhakal HP, Nesland JM, Bryne M (2015) Tumor budding, EMT and cancer stem cells in T1-2/N0 oral squamous cell carcinomas. Anticancer Res 35(11): 6111–6120. [PubMed] [Google Scholar]
- Boxberg M, Jesinghaus M, Dorfner C, Mogler C, Drecoll E, Warth A, Steiger K, Bollwein C, Meyer P, Wolff KD, Kolk A, Weichert W (2017) Tumor budding activity and cell nest size determine patient outcome in oral squamous cell carcinoma: Proposal for an adjusted grading system. Histopathology 70(7): 1125–1137. [DOI] [PubMed] [Google Scholar]
- Bradley CA, Dunne PD, Bingham V, McQuaid S, Khawaja H, Craig S, James J, Moore WL, McArt DG, Lawler M, Dasgupta S, Johnston PG, Van Schaeybroeck S (2016) Transcriptional upregulation of c-MET is associated with invasion and tumor budding in colorectal cancer. Oncotarget 7(48): 78932–78945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryne M, Koppang HS, Lilleng R, Kjaerheim A (1992) Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol 166(4): 375–381. [DOI] [PubMed] [Google Scholar]
- Cappellesso R, Luchini C, Veronese N, Mele ML, Rosa-Rizzotto E, Guido E, De Lazzari F, Pilati P, Farinati F, Realdon S, Solmi M, Fassan M, Rugge M (2017) Tumor budding as a risk factor for nodal metastasis in Pt1 colorectal cancers: a meta-analysis. Hum Pathol 65: 62–70. [DOI] [PubMed] [Google Scholar]
- Chen TC, Wang CP, Ko JY, Yang TL, Hsu CW, Yeh KA, Chang YL, Lou PJ (2013) The impact of perineural invasion and/or lymphovascular invasion on the survival of early-stage oral squamous cell carcinoma patients. Ann Surg Oncol 20(7): 2388–2395. [DOI] [PubMed] [Google Scholar]
- Christofori G (2006) New signals from the invasive front. Nature 441(7092): 444–450. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3): 177–188. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5): E359–E386. [DOI] [PubMed] [Google Scholar]
- Galvan JA, Zlobec I, Wartenberg M, Lugli A, Gloor B, Perren A, Karamitopoulou E (2015) Expression of E-cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour-budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Br J Cancer 112(12): 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Graham RP, Vierkant RA, Tillmans LS, Wang AH, Laird PW, Weisenberger DJ, Lynch CF, French AJ, Slager SL, Raissian Y, Garcia JJ, Kerr SE, Lee HE, Thibodeau SN, Cerhan JR, Limburg PJ, Smyrk TC (2015) Tumor budding in colorectal carcinoma: confirmation of prognostic significance and histologic cutoff in a population-based cohort. Am J Surg Pathol 39(10): 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujam FJ, McMillan DC, Mohammed ZM, Edwards J, Going JJ (2015) The relationship between tumour budding, the tumour microenvironment and survival in patients with invasive ductal breast cancer. Br J Cancer 113(7): 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guolo A, Varin C (2017) Random-effects meta-analysis: the number of studies matters. Stat Methods Med Res26(3): 1500–1518. [DOI] [PubMed]
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11): 1539–1558. [DOI] [PubMed] [Google Scholar]
- Hori Y, Kubota A, Yokose T, Furukawa M, Matsushita T, Takita M, Mitsunaga S, Mizoguchi N, Nonaka T, Nakayama Y, Oridate N (2017) Predictive significance of tumor depth and budding for late lymph node metastases in patients with clinical n0 early oral tongue carcinoma. Head Neck Pathol 11(4): 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen DH, Dabelsteen E, Specht L, Fiehn AM, Therkildsen MH, Jonson L, Vikesaa J, Nielsen FC, von Buchwald C (2015. a) Molecular profiling of tumour budding implicates TGFbeta-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J Pathol 236(4): 505–516. [DOI] [PubMed] [Google Scholar]
- Jensen DH, Reibel J, Mackenzie IC, Dabelsteen E (2015. b) Single cell migration in oral squamous cell carcinoma - possible evidence of epithelial-mesenchymal transition in vivo. J Oral Pathol Med 44(9): 674–679. [DOI] [PubMed] [Google Scholar]
- Kadota K, Nitadori J, Woo KM, Sima CS, Finley DJ, Rusch VW, Adusumilli PS, Travis WD (2014) Comprehensive pathological analyses in lung squamous cell carcinoma: single cell invasion, nuclear diameter, and tumor budding are independent prognostic factors for worse outcomes. J Thorac Oncol 9(8): 1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota K, Yeh YC, Villena-Vargas J, Cherkassky L, Drill EN, Sima CS, Jones DR, Travis WD, Adusumilli PS (2015) Tumor budding correlates with the protumor immune microenvironment and is an independent prognostic factor for recurrence of stage i lung adenocarcinoma. Chest 148(3): 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamitopoulou E (2012) Tumor budding cells, cancer stem cells and epithelial-mesenchymal transition-type cells in pancreatic cancer. Front Oncol 2: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelzer VH, Langer R, Zlobec I, Lugli A (2014) Tumor budding in upper gastrointestinal carcinomas. Front Oncol 4: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korvala J, Jee K, Porkola E, Almangush A, Mosakhani N, Bitu C, Cervigne NK, Zandonadi FS, Meirelles GV, Leme AF, Coletta RD, Leivo I, Salo T (2017) MicroRNA and protein profiles in invasive versus non-invasive oral tongue squamous cell carcinoma cells in vitro. Exp Cell Res 350(1): 9–18. [DOI] [PubMed] [Google Scholar]
- Landau MS, Hastings SM, Foxwell TJ, Luketich JD, Nason KS, Davison JM (2014) Tumor budding is associated with an increased risk of lymph node metastasis and poor prognosis in superficial esophageal adenocarcinoma. Mod Pathol 27(12): 1578–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao PL, Marangon Junior H, Melo VV, Caixeta AB, Souza PE, de Aguiar MC, Horta MC (2017) Reproducibility, repeatability and level of difficulty of two methods for tumor budding evaluation in oral squamous cell carcinoma. J Oral Pathol Med 46(10): 949–955. [DOI] [PubMed] [Google Scholar]
- Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Flejou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimaki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P (2017) Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 30(9): 1299–1311. [DOI] [PubMed] [Google Scholar]
- Lugli A, Vlajnic T, Giger O, Karamitopoulou E, Patsouris ES, Peros G, Terracciano LM, Zlobec I (2011) Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair-deficient colorectal cancer patients. Hum Pathol 42(12): 1833–1840. [DOI] [PubMed] [Google Scholar]
- Manjula BV, Augustine S, Selvam S, Mohan AM (2015) Prognostic and predictive factors in gingivo buccal complex squamous cell carcinoma: role of tumor budding and pattern of invasion. Indian J Otolaryngol Head Neck Surg 67(Suppl 1): 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangon Junior H, Rocha VN, Leite CF, de Aguiar MC, Souza PE, Horta MC (2014) Laminin-5gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med 43(3): 199–204. [DOI] [PubMed] [Google Scholar]
- Miyake M, Hori S, Morizawa Y, Tatsumi Y, Toritsuka M, Ohnishi S, Shimada K, Furuya H, Khadka VS, Deng Y, Ohnishi K, Iida K, Gotoh D, Nakai Y, Inoue T, Anai S, Torimoto K, Aoki K, Tanaka N, Konishi N, Fujimoto K (2017) Collagen type IV alpha 1 (COL4A1) and collagen type XIII alpha 1 (COL13A1) produced in cancer cells promote tumor budding at the invasion front in human urothelial carcinoma of the bladder. Oncotarget 8(22): 36099–36114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535. [PMC free article] [PubMed] [Google Scholar]
- Muller S, Pan Y, Li R, Chi AC (2008) Changing trends in oral squamous cell carcinoma with particular reference to young patients: 1971-2006. The Emory University experience. Head Neck Pathol 2(2): 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandita KP, Boaz K, Srikant N, Lewis AJ, Manaktala N (2016) Tumour budding: a promising parameter in oral squamous cell carcinoma. Res J Pharm Biol Chem Sci 7(5): 2059–2063. [Google Scholar]
- Pedersen NJ, Jensen DH, Lelkaitis G, Kiss K, Charabi B, Specht L, von Buchwald C (2017) Construction of a pathological risk model of occult lymph node metastases for prognostication by semi-automated image analysis of tumor budding in early-stage oral squamous cell carcinoma. Oncotarget 8(11): 18227–18237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riber-Hansen R, Vainer B, Steiniche T (2012) Digital image analysis: a review of reproducibility, stability and basic requirements for optimal results. APMIS 120(4): 276–289. [DOI] [PubMed] [Google Scholar]
- Rogers AC, Winter DC, Heeney A, Gibbons D, Lugli A, Puppa G, Sheahan K (2016) Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer 115(7): 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhia B, Trippel M, Pfaltz K, Cihoric N, Grogg A, Ladrach C, Zlobec I, Tapia C (2015) High tumor budding stratifies breast cancer with metastatic properties. Breast Cancer Res Treat 150(2): 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawazaki-Calone I, Rangel A, Bueno AG, Morais CF, Nagai HM, Kunz RP, Souza RL, Rutkauskis L, Salo T, Almangush A, Coletta RD (2015) The prognostic value of histopathological grading systems in oral squamous cell carcinomas. Oral Dis 21(6): 755–761. [DOI] [PubMed] [Google Scholar]
- Seki M, Sano T, Yokoo S, Oyama T (2016) Histologic assessment of tumor budding in preoperative biopsies to predict nodal metastasis in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck 38(Suppl 1): E1582–E1590. [DOI] [PubMed] [Google Scholar]
- Seki M, Sano T, Yokoo S, Oyama T (2017) Tumour budding evaluated in biopsy specimens is a useful predictor of prognosis in patients with cN0 early stage oral squamous cell carcinoma. Histopathology 70(6): 869–879. [DOI] [PubMed] [Google Scholar]
- Soland TM, Brusevold IJ (2013) Prognostic molecular markers in cancer - quo vadis? Histopathology 63(3): 297–308. [DOI] [PubMed] [Google Scholar]
- Strieder L, Coutinho-Camillo CM, Costa V, da Cruz Perez DE, Kowalski LP, Kaminagakura E (2017) Comparative analysis of three histologic grading methods for squamous cell carcinoma of the lip. Oral Dis 23(1): 120–125. [DOI] [PubMed] [Google Scholar]
- Wang C, Huang H, Huang Z, Wang A, Chen X, Huang L, Zhou X, Liu X (2011) Tumor budding correlates with poor prognosis and epithelial-mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med 40(7): 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie N, Wang C, Liu X, Li R, Hou J, Chen X, Huang H (2015) Tumor budding correlates with occult cervical lymph node metastasis and poor prognosis in clinical early-stage tongue squamous cell carcinoma. J Oral Pathol Med 44(4): 266–272. [DOI] [PubMed] [Google Scholar]
- Xie N, Wang C, Zhuang Z, Hou J, Liu X, Wu Y, Liu H, Huang H (2016) Decreased miR-320a promotes invasion and metastasis of tumor budding cells in tongue squamous cell carcinoma. Oncotarget 7(40): 65744–65757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Ishii G, Kojima M, Yoh K, Otsuka H, Otaki Y, Aokage K, Yanagi S, Nagai K, Nishiwaki Y, Ochiai A (2010) Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J Thorac Oncol 5(9): 1361–1368. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang L, Liu H, Zhao L, Li Y, Shen JX, Liu Q, Liu MZ, Xi M (2016) Clinicopathologic characteristics and prognosis of tongue squamous cell carcinoma in patients with and without a history of radiation for nasopharyngeal carcinoma: a matched case-control study. Cancer Res Treat 49(3): 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlobec I, Lugli A (2010) Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 1(7): 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]