Abstract
Tropical forest loss and fragmentation are due to increase in coming decades. Understanding how matrix dynamics, especially secondary forest regrowth, can lessen fragmentation impacts is key to understanding species persistence in modified landscapes. Here, we use a whole-ecosystem fragmentation experiment to investigate how bat assemblages are influenced by the regeneration of the secondary forest matrix. We surveyed bats in continuous forest, forest fragments and secondary forest matrix habitats, ~15 and ~30 years after forest clearance, to investigate temporal changes in the occupancy and abundance of old-growth specialist and habitat generalist species. The regeneration of the second growth matrix had overall positive effects on the occupancy and abundance of specialists across all sampled habitats. Conversely, effects on generalist species were negligible for forest fragments and negative for secondary forest. Our results show that the conservation potential of secondary forests for reverting faunal declines in fragmented tropical landscapes increases with secondary forest age and that old-growth specialists, which are often of most conservation concern, are the greatest beneficiaries of secondary forest maturation. Our findings emphasize that the transposition of patterns of biodiversity persistence in island ecosystems to fragmented terrestrial settings can be hampered by the dynamic nature of human-dominated landscapes.
Introduction
Humanity’s global footprint is so ubiquitous and far-reaching that many argue that we now live in a new geological epoch, the Anthropocene1. Habitat loss and fragmentation are pervasive and conspicuous features of this new historical context, which, in combination with other human-related threats, are compelling the planet into a “sixth wave of extinction”2,3.
The scars of the Anthropocene defaunation are being carved deep into the planet’s biodiversity strongholds, the tropical forests4. As large swaths of old-growth forest give way to expanding humanized landscapes, species persisting in forest remnants are left to endure the pervasive consequences of increased isolation and decreased area5. Landscape-wide assemblage dynamics in fragments created in the aftermath of deforestation are dependent, to a large extent, on the nature of the matrix within which forest patches are embedded6. Conservation science has traditionally conceived the modified matrix as a “sea” of hostile habitat, in which fragments act as “islands” and this analogy has guided much of the theory and practice of the field6,7. However, equating forest fragments with island ecosystems, while appropriate in some situations, fails to accommodate the heterogeneous and dynamic nature of most present-day modified landscapes8,9.
Vertebrate assemblage dynamics in tropical land-bridge islands have painted a dire portrait of the consequences of forest fragmentation in true island systems10–12. Mainland studies that also construed fragments as true islands, have arrived at similar pessimistic narratives13,14. However, direct comparisons between these two systems (true islands vs mainland) have revealed that assemblages persisting in forest patches embedded in terrestrial human-dominated landscapes defy the patterns exhibited by their water-embedded analogues9,15.
Second growth nowadays constitutes the predominant type of forest cover across the tropics16, providing myriad services and natural products to human populations worldwide, and key habitat for countless forest-dwelling species17,18. Although some fragmentation-related extinctions can be averted by forest regeneration14,15,19, the role of second growth in biodiversity conservation remains controversial20–22. Central to the debate is the capacity of secondary forest to preserve old-growth specialist species and to buffer the impacts of fragmentation on assemblages living in forest remnants16,17,23.
We surveyed bats, a taxon demonstrably sensitive to habitat modification24, in forest fragments and secondary forest sites, ~15 and ~30 years after forest clearance in the early 1980s at the Biological Dynamics of Forest Fragments Project (BDFFP), the world’s largest and longest-running fragmentation experiment, located in the central Brazilian Amazon25 (Fig. 1). Determining the responses of tropical species to habitat change is often hindered by the rarity of old-growth specialists for which data are often too sparse for reliable inference at the species level. This commonly leads to the exclusion of species captured less frequently (which are often of conservation concern) from the analysis or to several species being lumped together according to group membership (e.g. feeding guilds), thus preventing the detection of species-specific responses. Here, we overcome this difficulty by employing a joint species distribution modelling framework that combines species-specific models into a single hierarchical model that allows the detection of the relationship between environmental variables and species responses simultaneously at the species and community levels26,27. Our aim was to examine the effect of matrix regeneration between ~1996 and ~2011 on old-growth specialist and habitat generalist phyllostomid bat species (and Pteronotus parnellii) across the three main habitats of the BDFFP: continuous primary forest, primary forest fragments and secondary forest matrix. We predicted that the maturation between study periods of the secondary forest surrounding forest fragments would provide extra resources for old-growth specialists, leading to increases in occupancy and abundance in this group both within fragments and the secondary regrowth matrix. Conversely, we expected that the successional advance of the secondary vegetation would have diminished the availability of food resources for generalist bats (many of which feed on early-successional plants), hence reducing their abundance in the same habitats. Additionally, since similarity in structure and floristic composition between secondary and primary forests increases with regeneration time16,28 we predicted bat assemblage similarity between continuous forest and secondary forest to be higher ~30 years after forest clearance (~2011) than half-way through the study period (~15 years after forest clearance; ~1996). Similarly, due to a reduction in fragment-matrix contrast, we predicted that assemblage similarity between forest fragments and continuous forest was going to increase over the same period.
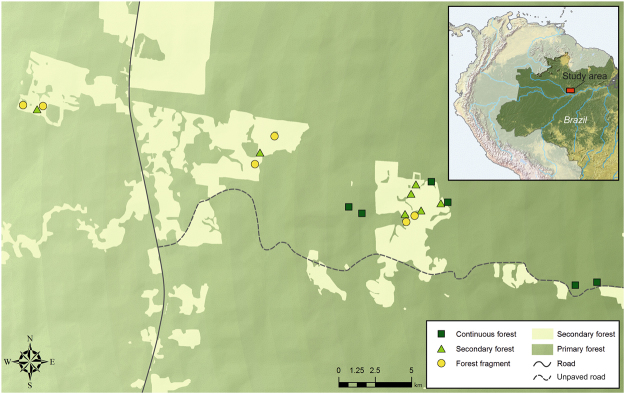
Figure 1.
The Biological Dynamics of Forest Fragments Project (BDFFP), Central Amazon, Brazil. Light green represents secondary forest matrix and dark green continuous forest and forest fragments. Location of the study area within Brazil is shown in the map inset. The map was based on shapefiles provided by the BDFFP management team (http://pdbff.inpa.gov.br/) and was produced in ArcMap 10.3 (http://desktop.arcgis.com/en/arcmap/).
Results
We captured 4,028 bats in the first period (35, 33 and 22 species in continuous forest, forest fragments and secondary forest respectively; 20 species shared between the three habitats) and 2,081 bats in the second period (33, 34 and 35 species in continuous forest, forest fragments and secondary forest respectively; 26 species shared between the three habitats). Twenty-seven species were classified as specialists whereas 23 were classified as habitat generalists (Supplementary Table S1).
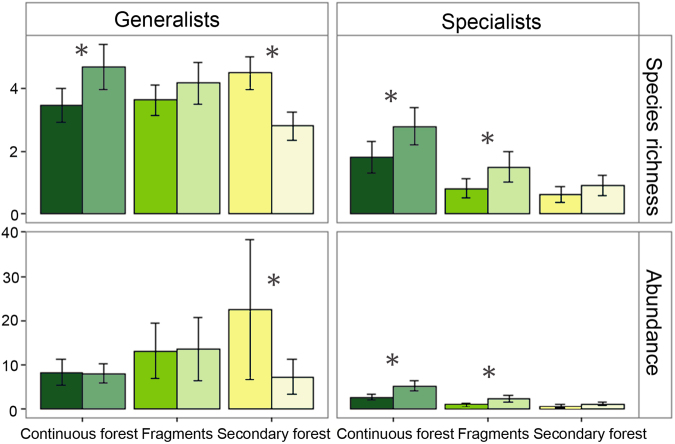
Our modelling results revealed that the regeneration of the matrix between the two periods had overall positive effects on the estimated occupancy and abundance of specialist bats in secondary forest sites and fragments, whereas effects on generalist species were negligible (fragments) or negative (secondary forest) (Fig. 2). Model predictions indicate that for specialist bats the mean number of species expected to be captured during a survey visit nearly doubled in fragments (0.81 in ~1996; 1.5 in ~2011) while remaining virtually unchanged for generalist species (3.63 in ~1996; 4.17 in ~2011). In secondary forest, this figure also increased for specialist bats (0.62 in ~1996; 0.91 in ~2011), while decreasing for generalist species (4.5 in ~1996; 2.81 in ~2011) and in continuous forest increased for both groups (1.81 in ~1996; 2.79 in ~2011 (specialists) and 3.47 in ~1996; 4.7 in ~2011 (generalists)) (Fig. 2). The mean number of individuals captured during a given survey varied little between the first and second period in continuous forest and fragments but decreased by nearly 2/3 in secondary forest (from 23.24 in ~1996 to 8.39 in ~2011) (Supplementary Fig. S2). In this habitat, generalists and specialists exhibited opposite trends between periods, with the mean number of individuals of generalist species declining from 22.55 in ~1996 to 7.3 in ~2011 and the mean number of individuals of specialist species increasing from 0.68 to 1.1 in the same period (Fig. 2).
Figure 2.
Bat species richness and abundance of generalist and specialist bats in continuous forest, fragments and secondary forest, ~15 years (dark-coloured bars) and ~30 years (lighter-coloured bars) after experimental forest clearance. Plotted are the predictions of the mean number of species and the mean number of individuals ( ± posterior standard deviation) captured per survey visit. Capture effort was standardized within each habitat category and thus the results are comparable only between periods but not across habitat types. Asterisks stand for high statistical support (posterior probability > 95%) for the predictions being higher or lower ~30 years after experimental forest clearance (2011–2013) than ~15 years after experimental forest clearance (1996–2002). Species’ habitat affinities are reported in Table S1 (for classification description see Methods) and results for all species combined are provided in Supplementary Fig. S1.
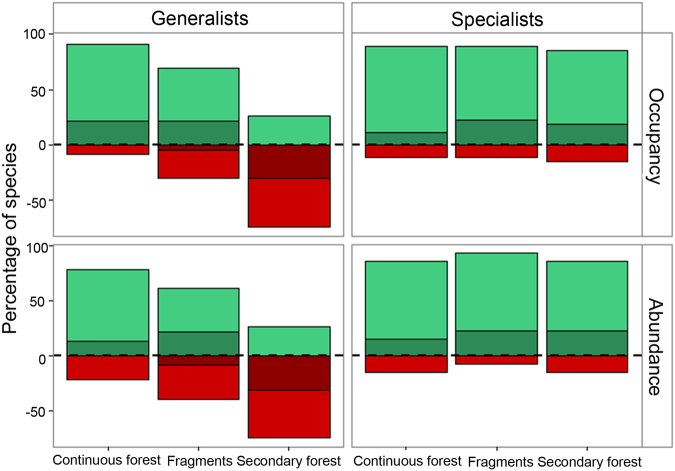
Between ~1996 and ~2011, only 3 and 4 of the 27 species classified as specialists decreased in occupancy respectively in fragments and secondary forest. Furthermore, statistical support for these declines was limited (Fig. 3; Supplementary Table S2). During the same period, out of the 27 specialists, the abundance increased for 24 in fragments and for 23 in secondary forest. In contrast, of the 23 species classified as generalists, 7 declined in occupancy in fragments and 17 in secondary forest (high statistical support for 1 and 7 species, respectively) (Fig. 3; Supplementary Table S2). Seven generalist species declined in abundance in fragments and 17 in secondary forest (Fig. 3).
Figure 3.
Change in species occupancy probability and abundance of generalist and specialist bats in continuous forest, fragments and secondary forest, ~15 years and ~30 years after experimental forest clearance. Plotted is the percentage of species with positive (green) and negative (red) changes in probability of occurrence and mean number of individuals predicted to be captured per survey visit between the first and second period (~15 and ~30 after experimental forest clearance). Dark and light colours represent respectively, percentage of species with high (posterior probability > 95%) and low statistical support (posterior probability < 95%). Predictions account for within-habitat differences in capture effort between the two periods. Species-specific values are reported in Supplementary Table S2; species’ habitat affinities are given in Supplementary Table S1 (for classification description see Methods).
Assemblage similarity between continuous forest and fragments increased slightly with time for generalists when considering both occupancy and abundance but declined for specialists. For secondary forests, occupancy- and abundance-based assemblage similarities relative to continuous forest declined for both groups. However, statistical support for these trends was limited (Table 1; See Supplementary Table S3 for assemblage similarity comparisons for all species combined).
Table 1.
Assemblage similarity between continuous forest and modified habitats (fragments and secondary forest), ~15 years and ~30 years after experimental forest clearance. We defined the similarity between two assemblages as the correlation between model-predicted occurrence probabilities or abundances (both log-transformed). The values in the table show posterior mean similarities between assemblages inhabiting continuous forests and modified habitats for the two study periods, as well as the posterior probability by which the similarities were lower in the first period than in the second.
| Generalists | Fragments | Secondary Forest | ||
|---|---|---|---|---|
| Occupancy | Abundance | Occupancy | Abundance | |
| 1996–2002 | 0.64 | 0.71 | 0.60 | 0.68 |
| 2011–2013 | 0.76 | 0.79 | 0.51 | 0.56 |
| Posterior probability | 0.8 | 0.74 | 0.21 | 0.31 |
| Specialists | ||||
| 1996–2002 | 0.84 | 0.85 | 0.78 | 0.78 |
| 2011–2013 | 0.74 | 0.76 | 0.61 | 0.63 |
| Posterior probability | 0.16 | 0.16 | 0.09 | 0.09 |
Discussion
There is a lack of studies that directly investigate temporal trends in wildlife responses to fragmentation and even fewer that evaluate how matrix use changes through time (but see5,29). Consequently, in contrast to the evaluation of species responses to spatial features, which has received some attention in the bat conservation literature, temporal variation, and in particular how bat responses to fragmentation are shaped by changes in matrix condition, remains little explored24. Here, we show that most phyllostomid bat species and Pteronotus parnellii benefited from the increased permeability of the matrix associated with the maturation of the secondary regrowth surrounding the BDFFP fragments, and that specialist and generalist species exhibited contrasting responses to matrix regeneration across the study landscape.
As hypothesized, we found that the maturation of second growth surrounding the BDFFP fragments lead to a landscape-wide increase in the occupancy and abundance of specialists, while reducing the occupancy and abundance of generalists in secondary forest sites. Our results therefore mirror the recovery documented for beetle30 and bird31 assemblages following the development of secondary vegetation in the matrix at the BDFFP. However, it is worth emphasizing that the BDFFP is surrounded by vast expanses of continuous forest harbouring healthy source populations and is buffered from selective logging, fires, species invasions, and many other ancillary threats plaguing contemporary tropical fragmented landscapes25. The recovery here documented is therefore likely to represent a best-case scenario and patterns reported might be harder to observe under conditions that increasingly characterize the majority of human-modified tropical landscapes.
The sole reliance on mist-netting data precludes a complete overview of the effects of second forest regeneration on the BDFFP chiropteran fauna as a whole since, with the exception of P. parnellii, Amazonian aerial insectivorous bats (a diverse group that includes the families Thyropteridae, Furipteridae, Mormoopidae, Emballonuridae, Vespertilionidae, Molossidae and Natalidae)32 are not effectively sampled with mist-nets33. However, we anticipate that old-growth specialist aerial insectivores are likely to have benefited from the maturation of second growth surrounding the BDFFP fragments in a similar way than their phyllostomid counterparts and to the aerial insectivore P. parnellii.
Our results contrast with the catastrophic faunal declines observed in rodent communities by Gibson et al.11 in the forest islands of the Chiew Larn reservoir in Thailand. Whereas most species of the mega-diverse bat assemblage at the BDFFP increased in occupancy and abundance across the second growth-dominated landscape, in the land-bridge island system in Thailand and, during a similar time window (~20 years, Gibson et al.11; ~15 years, our study), most species became extinct in forest fragments surrounded by a static matrix (water). The recovery observed at the BDFFP was mostly due to the recolonization of previously deforested areas and forest fragments by specialist species, which increased in all sampled habitats during the second period. This recolonization is likely attributable to an increased diversity of food resources in the matrix, allowing to fulfil the energetic requirements of a larger set of species other than generalists. Additionally, many specialist bats at the BDFFP are highly edge-sensitive34–36 and consequently the maturation of the secondary vegetation adjoining fragment edges might have increased habitat suitability by reducing the magnitude of edge effects across the landscape. Notwithstanding major morphological and ecological differences between rodents and bats, the widely different trajectories exhibited by assemblages inhabiting true island systems11 and fragments embedded within a regenerating matrix (this study) highlight the potential of second growth forests to mitigate fragmentation-related extinctions.
In spite of the signs of recovery exhibited by specialist species across our study landscape ~30 years after forest clearance, our results do not support an increase in assemblage similarity between continuous forest and secondary forest over time. This, together with evidence that bat assemblages in smaller fragments (≤10 ha) and secondary forest sites still differ considerably from continuous forest in terms of species richness, evenness, composition and abundance34,37, suggests that the second growth matrix at the BDFFP still acts as an environmental filter. This filtering shapes bat assemblages in a trait-mediated manner, selectively benefiting bat species with a phytophagous diet and reduced body mass38,39. Similar pervasive consequences of forest clearance can still be detected in birds40–42 and primates43 in the BDFFP landscape, highlighting that, although second growth can be of conservation significance, primary forest is of irreplaceable value23,44.
Our results have important implications for the interpretation of land-use change studies using space-for-time approaches. Researchers rarely have the opportunity to collect data prior to the main disturbance events that mould humanized landscapes. Consequently, studies often have to rely on nearby sites where the target impact has not yet taken place and assume that these accurately mimic pre-disturbance conditions45. Here, we show that the species richness of generalists and both the species richness and abundance of specialists have increased in our reference sites in continuous forest, indicating considerable temporal heterogeneity in undisturbed forest assemblages over a period of ~15 years. This suggests that space-for-time results may be undermined not only by confounding effects arising from spatial heterogeneity but also by constraints associated with the temporal heterogeneity of the assemblages inhabiting sites used as spatial surrogates. This shifting baseline somewhat limits our capacity to attribute the observed changes in fragment and secondary forest bat assemblages entirely to the effect of matrix maturation. However, the contrasting temporal trends in the species richness of generalists in continuous forest and secondary forest, i.e. increase in continuous forest vs. decrease in secondary forest, indicate that secondary forest regeneration plays an important role in the assemblage dynamics across the landscape. Yet, our limited knowledge of the extent of spatial and temporal dynamics of generalist and specialist species in continuous forest and how these fluctuations may relate to patterns in fragmented landscapes still precludes a full understanding of these systems and therefore should be a high priority for future research.
Despite the controlled, experimental conditions of the BDFFP, our findings add to an increasing body of evidence (e.g.9,15) emphasizing that the transposition of patterns of biodiversity persistence in island ecosystems to fragmented terrestrial settings can be hampered by the dynamic nature of human-dominated landscapes, and consequently predictions under the island biogeographic framework can distort our understanding and misguide conservation strategies. Accordingly, and in light of the contrasting temporal trajectories of specialist and generalist species at the BDFFP, alternative theoretical frameworks, importantly, countryside biogeography46, in which species’ differential habitat affinities can be accommodated, emerge as better suited for forecasting biological changes in human-modified landscapes9.
In spite of some noteworthy regional declines in deforestation rates (e.g. Brazilian rainforests), tropical forest loss has increased by more than 2,000 km2/year since the beginning of the millennium47. Much of these deforested areas will be used to meet the growing demands for food and biofuel of an increasing human population48. However, following forest clearing, some converted areas are allowed to regenerate, giving rise to human-modified landscapes in which secondary forests account for an increasing proportion of total forest cover16. Our results, although contingent on the existence of nearby source populations, add to the evidence that secondary forests offer a tremendous opportunity for both assisted and non-assisted habitat restoration49. Among bats, frugivorous species are effective seed dispersers, especially of pioneer plant species50 and gleaning insectivores play essential roles in the reduction of herbivory levels through trophic control of herbivorous arthropods51. Populations able to persist in primary forest remnants can therefore enhance second growth successional processes and by doing so, aid in maintaining the provision of ecosystem services and improve habitat quality and connectivity in regenerating tropical forests.
To a large extent, the conservation potential of the world’s tropical secondary rainforests depends on the legal framework underpinning their governance. In the Brazilian Amazon, the state of Pará has recently introduced legislation recommending protection of >20-year-old secondary forest (as identified through inspection of satellite images) as well as younger stands depending on the total stand basal area of native trees and palms52. Although legal protection per se does not ensure long-term safeguarding of the services provided by second-growth forests, it represents a critical step towards their management. We therefore urge researchers, practitioners and policy makers to adopt similar protective measures, especially in areas where primary forest is scarce or highly fragmented.
Human-modified tropical landscapes are in continuous flux, with areas of secondary forest being converted to agricultural land and vice-versa. Vegetation disturbances, both anthropogenic and natural (e.g. fire), are irregular in space and time, moulding mosaic landscapes in which the classic split between fragments and matrix is blurred53. The ability of species to persist in such dynamic landscapes will ultimately depend on the interaction between their intrinsic traits (e.g. mobility and life span), interspecific interactions and the availability of habitat capable of meeting their specific resource needs. Although hotly debated23,54,55, the “rescue” potential of secondary forests in these dynamic landscapes is far from negligible16. While adding to mounting evidence that secondary forests are of conservation value, our, and many other long-term studies at the BDFFP (reviewed by Laurance et al.)25 and elsewhere in the tropics (e.g.)17,56 reveal that continuous primary forest and large (>100 ha) forest fragments are of overwhelming importance for the conservation of tropical biodiversity.
Our results show that specialist bats, which occurred at low abundances in secondary regrowth and in forest fragments ~15 years after the experimental clearing, have benefited from the increased permeability of the matrix associated with the maturation of the secondary forest in the matrix during the last 15 years. This suggests that matrix management, and specifically the management of regenerating (secondary) forest can majorly dictate the future of biodiversity in human-modified landscapes, including that remaining in fragments of natural vegetation.
Material and Methods
Study area
Bat surveys took place at the Biological Dynamics of Forest Fragments Project (BDFFP), approximately 80 km north of Manaus (2°30′S, 60°W, 30–125 m above sea level), state of Amazonas, Brazil (Fig. 1). Forest in the ~1,000 km2 study area is non-flooded (terra firme) rainforest with a canopy height of ca. 23 m and emergent trees reaching 55 m57. The forest at the BDFFP is among the most biodiverse in the world (tree species richness often exceeding 280 species/ha)58 and, with the exception of the experimental fragmentation, has been sheltered from anthropogenic disturbances such as logging and fires. The climate is characterized by a dry season between June and October and annual rainfall varies from 1,900 to 3,500 mm. Eleven fragments were isolated from continuous forest by distances of 80–650 m in the early 1980s and are categorized into size classes of 1, 10 and 100 ha. Fragments were originally located within cattle ranches (3,000–5,000 ha each) but poor soils and low productivity dictated the abandonment of livestock activities and fragments became gradually surrounded by secondary forest dominated mainly by Vismia spp. and Cecropia spp.25. Following secondary forest proliferation, fragment isolation was maintained by clearing a 100 m-wide strip of regrowth at intervals of ~10 years around most experimental forest fragments35. During this study fragment re-isolation occurred between 1999 and 2001. For a description of the study landscape experimental manipulation and ecosystem-wide responses see Laurance et al.25.
Bat sampling
In both study periods (1996–2002 and 2011–2013) we sampled bats in forest fragments (six sites, three of 1 ha and three of 10 ha), secondary forest (seven sites) and continuous forest (six sites) (Fig. 1). Sampling started at dusk and nets were deployed until 0:00 am, being revised at intervals of ~20 minutes. Bias in capture rates due to net shyness was avoided by spacing visits to the same site by periods of three to four weeks and sampling was interrupted during heavy rains.
During the first sampling period bats were surveyed from January 1996 to June 1999 in forest fragments and continuous forest sites59, and from October 2001 to November 2002 in secondary forest60. The mist-netting protocol consisted of eight (secondary forest sites) and 18 to 24 (fragments and continuous forest sites) ground-level mist nets (12 m × 2.5 m) placed along existing trails. Trails used for sampling forest fragments were located as close as possible to the centre of the fragment. We surveyed fragment and continuous forest sites on seven to 12 nights and secondary forest sites between three to seven nights. Total mist net effort was 8,757, 9,429 and 860 mist-net hours (mnh; 1 mnh equals one 12 m net open for 1 h) for continuous forest, fragments and secondary forests, respectively. Captured bats were identified to species-level and had standard morphometric and demographic data collected. For this first study period, detailed site descriptions, methods and results for fragments and continuous forest can be found in Sampaio61 and Sampaio et al.59 and for secondary forest in Bobrowiec & Gribel60. Our analyses are restricted to ground-level captures in fragment and continuous forest interiors59 and to captures in Vismia- and Cecropia-dominated secondary forest60. Distance between sampling sites ranged from 148 m to 41 km and consequently some level of non-independence between bat assemblages of sites located closer together is plausible.
During the second period we re-surveyed all 19 sites between August 2011 and June 2013. The mist-netting protocol consisted of seven (secondary forest sites) and 14 (fragments and continuous forest sites) ground-level mist nets (12 × 2.5 m) placed at existing trails. Total mist net effort was 4,009, 3,963 and 1,941 mnh for continuous forest, fragments and secondary forests, respectively. Similarly to the first period, captured bats were identified and had standard morphometric and demographic data collected.
Bat capture and handling was conducted following guidelines approved by the American Society of Mammalogists62 and in accordance with Brazilian conservation and animal welfare laws. Sampling guidelines were approved by the ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade) and research was conducted under permit number 26877–2.
We restricted our analyses to phyllostomid bats and Pteronotus parnellii since all other captured species are inadequately sampled with ground-level mist-nets33. Taxonomy follows Gardner63.
Species affinities to primary and secondary forest
We used the statistical approach developed by Chazdon et al.64 to classify species into one of four groups: primary forest specialists, secondary forest specialists, generalists or too rare to classify. Classification was based on the whole dataset of 10,311 captures of 50 species sampled at the BDFFP between 1996 and 2014. Only a sub-set of these captures (6,109) was subsequently used in the joint species distribution models (see below). The method uses a multinominal model based on species relative abundance in both habitats (here defined as continuous primary forest vs forest fragments and secondary forest) and simultaneously minimizes bias due to different sampling effort between habitats and due to insufficient captures of rare species. Classification was conducted in R v.3.0.265 using function clamtest of the vegan package and was based on the super-majority specialization threshold (K = 2/3) and setting a significance level of P = 0.01. We conservatively grouped primary forest specialists and species too rare to classify into a single group and since only two species were assigned to the secondary forest category, they were lumped together with generalists. We therefore considered two functional groups in our analysis: primary forest species and species too rare to classify (hereafter “specialist species”) and generalists and secondary forest specialists (hereafter “generalist species”).
Joint species distribution model
We applied a joint species distribution model27 to relate the bat occurrence data to environmental covariates. As a sampling unit, we considered one mist-netting session in one site (n = 301 mist-netting sessions) (the study design is illustrated in Supplementary Fig. S2). As the data involved a large fraction of zeros (70%), we applied a hurdle model, thus modelling separately presence-absence (model 1), and abundance conditional on presence (model 2). In model 1, the response variable was the vector of presence-absences of all the 50 species, and we assumed a Bernoulli distribution with a probit link-function (Y matrix, Supplementary Fig. S2). In model 2, the response variable was the vector of abundances of those species which were present, whereas species that were absent were considered as missing data (Y matrix, Supplementary Fig. S2). In this case, we assumed an overdispersed Poisson distribution with a log-link function. Abundance was measured as the number of captured individuals, of which we subtracted one to match the range of the assumed distribution (overdispersed Poisson) with the range of the response variable (note that conditional on presence, the smallest value for number of individuals is one, not zero). As explanatory variables, we included habitat type (categorical: continuous forest, fragment, or secondary forest), survey period (first (1996–2002) or second survey (2011–13)), percentage of secondary forest cover within a radius of 500 m from each site and the log-transformed survey effort, measured as mist-net hours (X matrix, Supplementary Fig. S2). We also included an interaction between survey period and habitat type, as well as an interaction between survey period and secondary forest cover. Percent secondary forest cover was measured from a detailed digital map of the BDFFP landscape based on Landsat Thematic Mapper data from 1996 (for the first survey period) and 2011 (for the second survey period) – see Carreiras et al.66 for image classification details. A buffer size of 500 m was selected so as to minimize overlap between neighbouring sites.
To account for repeated measurements at the same sites, we assumed a site-level random effect, implemented at the community level using the latent factor approach of Ovaskainen et al.67. As species traits, we included the classification into habitat generalists and specialists (T matrix, Supplementary Fig. S2). To account for phylogenetic non-independence, we followed Abrego et al.68 to structure the error variance with a phylogenetic correlation matrix, derived from a phylogenetic tree under the diffusion model (C matrix, Supplementary Fig. S2). The phylogenetic tree was taken from Jones et al.69. We fitted the model in the Bayesian framework using the Gibbs sampler of Ovaskainen et al.66,70. We used the hierarchical modelling of species communities (HMSC) software for MatLab (HMSC-MatLab) to fit the model to the data, assuming the default priors described in the Supporting Information of Ovaskainen et al.27. We ran the model for 50,000 iterations out of which 15,000 were discarded as transient.
We used the parameterized model to predict the expected species richness and number of captured individuals (for all species and separately for generalists and specialists) in each habitat class and study period per survey visit. Species richness was computed as the sum (over the species) of the occurrence probabilities predicted by model 1. Number of individuals was computed as the sum (over the species) of species-specific abundances, computed as the product of occurrence probability (from model 1) and abundance conditional on presence (prediction of model 2 plus one). In these predictions, we standardized the mist netting effort to the mean value of a given habitat category across both study periods, and the percentage of secondary forest to the mean value of a particular habitat type during a given survey period. Capture effort was standardized within each habitat category and thus the results are comparable between periods but not across habitat types. Species-level responses were assessed by computing the difference between the occurrence probability and mean number of individuals expected to be captured per survey visit between the first and the second period.
Turnover metrics are considered better suited to quantify biodiversity change in local assemblages through time than simple temporal trends of within-sample diversity (temporal α diversity)71. To characterize assemblage turnover, we computed assemblage similarity between the different habitat categories as well as between the two study periods. Assemblage similarity was defined as the correlation between model-predicted occurrence probabilities or abundances (both log-transformed)72 We performed these calculations for all species, and separately for generalists and specialists only.
The data used in this study are archived at 10.6084/m9.figshare.5907322.v2.
Electronic supplementary material
Acknowledgements
We would like to thank the multitude of volunteers and field assistants who helped collecting the data, the BDFFP management team for logistic support and João M.B. Carreiras for providing secondary forest layers for our study area. Funding was provided by the Portuguese Foundation for Science and Technology to C.F.J.M. (PTDC/BIA-BIC/111184/2009), R.R. (SFRH/BD/80488/2011) and A.L.-B. (PD/BD/52597/2014). F.Z.F. and P.E.D.B. were supported by a CAPES fellowship. OO was supported by the Academy of Finland (Grants no. 250444 and 273253) and the Research Council of Norway (CoE grant no. 223257). MC was also supported by the Academy of Finland (Grant no. 257686). This is publication number 733 in the BDFFP technical series.
Author Contributions
C.F.J.M, J.M.P., M.C. and R.R. conceived the ideas; R.R., A.L.-B., F.Z.F, E.S, P.E.D.B., J.M.P. and C.F.J.M collected the data; R.R. and O.O. analysed the data and RR led the writing. All authors discussed the results and commented on the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21999-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Waters, C. N. et al. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science351 (2016). [DOI] [PubMed]
- 2.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345:401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 3.Ceballos G, et al. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Science Advances. 2015;1:e1400253. doi: 10.1126/sciadv.1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malhi Y, Gardner TA, Goldsmith GR, Silman MR, Zelazowski P. Tropical forests in the Anthropocene. Annu. Rev. Environ. Resour. 2014;39:125–159. doi: 10.1146/annurev-environ-030713-155141. [DOI] [Google Scholar]
- 5.Haddad NM, et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Science Advances. 2015;1:e1500052. doi: 10.1126/sciadv.1500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watling JI, Nowakowski AJ, Donnelly MA, Orrock JL. Meta‐analysis reveals the importance of matrix composition for animals in fragmented habitat. Glob. Ecol. Biogeogr. 2011;20:209–217. doi: 10.1111/j.1466-8238.2010.00586.x. [DOI] [Google Scholar]
- 7.He F, Hubbell SP. Species-area relationships always overestimate extinction rates from habitat loss. Nature. 2011;473:368–371. doi: 10.1038/nature09985. [DOI] [PubMed] [Google Scholar]
- 8.Driscoll DA, Banks SC, Barton PS, Lindenmayer DB, Smith AL. Conceptual domain of the matrix in fragmented landscapes. Trends. Ecol. Evolut. 2013;28:605–613. doi: 10.1016/j.tree.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Mendenhall CD, Karp DS, Meyer CF, Hadly EA, Daily GC. Predicting biodiversity change and averting collapse in agricultural landscapes. Nature. 2014;509:213–217. doi: 10.1038/nature13139. [DOI] [PubMed] [Google Scholar]
- 10.Meyer CFJ, Kalko EKV. Assemblage-level responses of phyllostomid bats to tropical forest fragmentation: land-bridge islands as a model system. J. Biogeogr. 2008;35:1711–1726. doi: 10.1111/j.1365-2699.2008.01916.x. [DOI] [Google Scholar]
- 11.Gibson L, et al. Near-complete extinction of native small mammal fauna 25 years after forest fragmentation. Science. 2013;341:1508–1510. doi: 10.1126/science.1240495. [DOI] [PubMed] [Google Scholar]
- 12.Benchimol M, Peres CA. Predicting local extinctions of Amazonian vertebrates in forest islands created by a mega dam. Biol Cons. 2015;187:61–72. doi: 10.1016/j.biocon.2015.04.005. [DOI] [Google Scholar]
- 13.Ferraz G, et al. Rates of species loss from Amazonian forest fragments. Proc. Natl. Acad. Sci. USA. 2003;100:14069–14073. doi: 10.1073/pnas.2336195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wearn OR, Reuman DC, Ewers RM. Extinction debt and windows of conservation opportunity in the Brazilian Amazon. Science. 2012;337:228–232. doi: 10.1126/science.1219013. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe JD, Stouffer PC, Mokross K, Powell LL, Anciães MM. Island vs. countryside biogeography: an examination of how Amazonian birds respond to forest clearing and fragmentation. Ecosphere. 2015;6:art295. doi: 10.1890/ES15-00322.1. [DOI] [Google Scholar]
- 16.Chazdon, R. L. Second Growth: The Promise Of Tropical Forest Regeneration In An Age Of Deforestation. (University of Chicago Press, 2014).
- 17.Barlow JOS, Overal WL, Araujo IS, Gardner TA, Peres CA. The value of primary, secondary and plantation forests for fruit-feeding butterflies in the Brazilian Amazon. J. Appl. Ecol. 2007;44:1001–1012. doi: 10.1111/j.1365-2664.2007.01347.x. [DOI] [Google Scholar]
- 18.Gardner TA, et al. Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 2009;12:561–582. doi: 10.1111/j.1461-0248.2009.01294.x. [DOI] [PubMed] [Google Scholar]
- 19.Stouffer PC, Johnson EI, Bierregaard RO, Jr., Lovejoy TE. Understory bird communities in Amazonian rainforest fragments: species turnover through 25 years post-isolation in recovering landscapes. PLoS ONE. 2011;6:e20543. doi: 10.1371/journal.pone.0020543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melo FPL, Arroyo-Rodríguez V, Fahrig L, Martínez-Ramos M, Tabarelli M. On the hope for biodiversity-friendly tropical landscapes. Trends. Ecol. Evolut. 2013;28:462–468. doi: 10.1016/j.tree.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Arroyo-Rodríguez V, et al. Multiple successional pathways in human-modified tropical landscapes: new insights from forest succession, forest fragmentation and landscape ecology research. Biol. Rev. 2017;92:326–340. doi: 10.1111/brv.12231. [DOI] [PubMed] [Google Scholar]
- 22.Sloan S, Sayer JA. Forest Resources Assessment of 2015 shows positive global trends but forest loss and degradation persist in poor tropical countries. Forest Ecol. Manag. 2015;352:134–145. doi: 10.1016/j.foreco.2015.06.013. [DOI] [Google Scholar]
- 23.Gibson L, et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature. 2011;478:378–381. doi: 10.1038/nature10425. [DOI] [PubMed] [Google Scholar]
- 24.Meyer, C. F. J., Struebig, M. J. & Willig, M. R. In Bats In The Anthropocene: Conservation Of Bats In A Changing World (eds C. Christian Voigt & Tigga Kingston) 63-103 (Springer International Publishing, 2016).
- 25.Laurance, W. et al. An Amazonian rainforest and its fragments as a laboratory of global change. Biol. Rev. 10.1111/brv.12343 (2017). [DOI] [PubMed]
- 26.Warton DI, et al. So many variables: joint modeling in community ecology. Trends. Ecol. Evolut. 2015;30:766–779. doi: 10.1016/j.tree.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Ovaskainen O, et al. How to make more out of community data? A conceptual framework and its implementation as models and software. Ecol. Lett. 2017;20:561–576. doi: 10.1111/ele.12757. [DOI] [PubMed] [Google Scholar]
- 28.DeWalt SJ, Maliakal SK, Denslow JS. Changes in vegetation structure and composition along a tropical forest chronosequence: implications for wildlife. Forest Ecol. Manag. 2003;182:139–151. doi: 10.1016/S0378-1127(03)00029-X. [DOI] [Google Scholar]
- 29.Youngentob KN, Wood JT, Lindenmayer DB. The response of arboreal marsupials to landscape context over time: a large-scale fragmentation study revisited. J. Biogeogr. 2013;40:2082–2093. doi: 10.1111/jbi.12158. [DOI] [Google Scholar]
- 30.Quintero I, Roslin T. Rapid recovery of dung beetle communities following habitat fragmentation in Central Amazonia. Ecology. 2005;86:3303–3311. doi: 10.1890/04-1960. [DOI] [Google Scholar]
- 31.Stouffer PC, Strong C, Naka LN. Twenty years of understorey bird extinctions from Amazonian rain forest fragments: consistent trends and landscape‐mediated dynamics. Divers. Distrib. 2009;15:88–97. doi: 10.1111/j.1472-4642.2008.00497.x. [DOI] [Google Scholar]
- 32.López-Baucells, A. et al. Field Guide to Amazonian Bats. 1-174 (Editora INPA, 2016).
- 33.Silva CR, Bernard E. Bioacoustics as an important complementary tool in bat inventories in the Caatinga drylands of Brazil. Acta Chiropter. 2017;19:409–418. doi: 10.3161/15081109ACC2017.19.2.017. [DOI] [Google Scholar]
- 34.Rocha R, et al. Consequences of a large-scale fragmentation experiment for Neotropical bats: disentangling the relative importance of local and landscape-scale effects. Landscape Ecol. 2017;32:31–45. doi: 10.1007/s10980-016-0425-3. [DOI] [Google Scholar]
- 35.Rocha R, et al. Design matters: An evaluation of the impact of small man-made forest clearings on tropical bats using a before-after-control-impact design. Forest Ecol. Manag. 2017;401:8–16. doi: 10.1016/j.foreco.2017.06.053. [DOI] [Google Scholar]
- 36.Ferreira DF, et al. Season-modulated responses of Neotropical bats to forest fragmentation. Ecol. Evol. 2017;7:4059–4071. doi: 10.1002/ece3.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocha R, et al. Does sex matter? Gender-specific responses to forest fragmentation in Neotropical bats. Biotropica. 2017;49:881–890. doi: 10.1111/btp.12474. [DOI] [Google Scholar]
- 38.Farneda FZ, et al. Trait-related responses to habitat fragmentation in Amazonian bats. J. Appl. Ecol. 2015;52:1381–1391. doi: 10.1111/1365-2664.12490. [DOI] [Google Scholar]
- 39.Farneda FZ, et al. Functional recovery of Amazonian bat assemblages following secondary forest succession. Biol. Cons. 2018;218:192–199. doi: 10.1016/j.biocon.2017.12.036. [DOI] [Google Scholar]
- 40.Figueira L, Tella JL, Camargo UM, Ferraz G. Autonomous sound monitoring shows higher use of Amazon old growth than secondary forest by parrots. Biol. Cons. 2015;184:27–35. doi: 10.1016/j.biocon.2014.12.020. [DOI] [Google Scholar]
- 41.Powell LL, Wolfe JD, Johnson EI, Stouffer PC. Forest recovery in post-pasture Amazonia: Testing a conceptual model of space use by insectivorous understory birds. Biol. Cons. 2016;194:22–30. doi: 10.1016/j.biocon.2015.11.025. [DOI] [Google Scholar]
- 42.Powell LL, Zurita G, Wolfe JD, Johnson EI, Stouffer PC. Changes in habitat use at rain forest edges through succession: a case study of understory birds in the Brazilian Amazon. Biotropica. 2015;47:723–732. doi: 10.1111/btp.12253. [DOI] [Google Scholar]
- 43.Lenz BB, Jack KM, Spironello WR. Edge effects in the primate community of the Biological Dynamics of Forest Fragments Project, Amazonas, Brazil. Am. J. Phys. Anthropol. 2014;155:436–446. doi: 10.1002/ajpa.22590. [DOI] [PubMed] [Google Scholar]
- 44.Barlow J, et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. USA. 2007;104:18555–18560. doi: 10.1073/pnas.0703333104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.França F, et al. Do space-for-time assessments underestimate the impacts of logging on tropical biodiversity? An Amazonian case study using dung beetles. J. Appl. Ecol. 2016;53:1098–1105. doi: 10.1111/1365-2664.12657. [DOI] [Google Scholar]
- 46.Pereira HM, Daily GC. Modeling biodiversity dynamics in countryside landscapes. Ecology. 2006;87:1877–1885. doi: 10.1890/0012-9658(2006)87[1877:MBDICL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Hansen MC, et al. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850–853. doi: 10.1126/science.1244693. [DOI] [PubMed] [Google Scholar]
- 48.Laurance WF, Sayer J, Cassman KG. Agricultural expansion and its impacts on tropical nature. Trends. Ecol. Evolut. 2014;29:107–116. doi: 10.1016/j.tree.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Chazdon RL, Guariguata MR. Natural regeneration as a tool for large-scale forest restoration in the tropics: prospects and challenges. Biotropica. 2016;48:716–730. doi: 10.1111/btp.12381. [DOI] [Google Scholar]
- 50.de la Peña-Domene M, Martínez-Garza C, Palmas-Pérez S, Rivas-Alonso E, Howe HF. Roles of birds and bats in early tropical-forest restoration. PLoS ONE. 2014;9:e104656. doi: 10.1371/journal.pone.0104656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maas B, et al. Bird and bat predation services in tropical forests and agroforestry landscapes. Biol. Rev. 2016;91:1081–1101. doi: 10.1111/brv.12211. [DOI] [PubMed] [Google Scholar]
- 52.Vieira I, Gardner T, Ferreira J, Lees A, Barlow J. Challenges of governing second-growth forests: a case study from the Brazilian Amazonian State of Pará. Forests. 2014;5:1737–1752. doi: 10.3390/f5071737. [DOI] [Google Scholar]
- 53.del Castillo RF. A conceptual framework to describe the ecology of fragmented landscapes and implications for conservation and management. Ecol. Appl. 2015;25:1447–1455. doi: 10.1890/14-1964.1. [DOI] [PubMed] [Google Scholar]
- 54.Wright SJ, Muller-Landau HC. The uncertain future of tropical forest species. Biotropica. 2006;38:443–445. doi: 10.1111/j.1744-7429.2006.00177.x. [DOI] [Google Scholar]
- 55.Gardner TA, Barlow J, Parry LW, Peres CA. Predicting the Uncertain Future of Tropical Forest Species in a Data Vacuum. Biotropica. 2007;39:25–30. doi: 10.1111/j.1744-7429.2006.00228.x. [DOI] [Google Scholar]
- 56.Martin, P. A., Newton, A. C. & Bullock, J. M. Carbon pools recover more quickly than plant biodiversity in tropical secondary forests. Proc. R. Soc. Lond. B Biol. Sci. 280, (2013). [DOI] [PMC free article] [PubMed]
- 57.Bierregaard Jr, R., Gascon, C., Lovejoy, T. & Mesquita, R. The biological dynamics of forest fragments project in Lessons From Amazonia (eds RO Bierregaard J, Gascon C, Lovejoy TE, Mesquita R) 31-42 (New Haven, 2001).
- 58.Oliveira A. d. & Mori, S. A central Amazonian terra firme forest. I. High tree species richness on poor soils. Biodivers. Conserv. 1999;8:1219–1244. doi: 10.1023/A:1008908615271. [DOI] [Google Scholar]
- 59.Sampaio EM, Kalko EK, Bernard E, Rodríguez-Herrera B, Handley CO. A biodiversity assessment of bats (Chiroptera) in a tropical lowland rainforest of Central Amazonia, including methodological and conservation considerations. Stud. Neotrop. Fauna Environ. 2003;38:17–31. doi: 10.1076/snfe.38.1.17.14035. [DOI] [Google Scholar]
- 60.Bobrowiec P, Gribel R. Effects of different secondary vegetation types on bat community composition in Central Amazonia, Brazil. Anim. Conserv. 2010;13:204–216. doi: 10.1111/j.1469-1795.2009.00322.x. [DOI] [Google Scholar]
- 61.Sampaio, E. Effects Of The Forest Fragmentation On The Diversity And Abundance Patterns Of Central Amazonian Bats. (Logos-Verlag, 2001).
- 62.Sikes RS, Gannon WL, Mammalogists ACAUCOTAS. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011;92:235–253. doi: 10.1644/10-MAMM-F-355.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gardner, A. Mammals of South America Volume 1: Marsupials, Xenarthrans, Shrews and Bats (The University of Chicago Press, 2007).
- 64.Chazdon RL, et al. A novel statistical method for classifying habitat generalists and specialists. Ecology. 2011;92:1332–1343. doi: 10.1890/10-1345.1. [DOI] [PubMed] [Google Scholar]
- 65.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ (2013).
- 66.Carreiras JMB, Jones J, Lucas RM, Gabriel C. Land use and land cover change dynamics across the Brazilian Amazon: insights from extensive time-series analysis of remote sensing data. PLoS ONE. 2014;9:e104144. doi: 10.1371/journal.pone.0104144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ovaskainen O, Abrego N, Halme P, Dunson D. Using latent variable models to identify large networks of species-to-species associations at different spatial scales. Methods Ecol. Evol. 2016;7:549–555. doi: 10.1111/2041-210X.12501. [DOI] [Google Scholar]
- 68.Abrego N, Dunson D, Halme P, Salcedo I, Ovaskainen O. Wood-inhabiting fungi with tight associations with other species have declined as a response to forest management. Oikos. 2016;126:269–275. [Google Scholar]
- 69.Jones KE, Purvis A, MacLarnon ANN, Bininda-Emonds ORP, Simmons NB. A phylogenetic supertree of the bats (Mammalia: Chiroptera) Biol. Rev. 2002;77:223–259. doi: 10.1017/S1464793101005899. [DOI] [PubMed] [Google Scholar]
- 70.Ovaskainen O, Roy DB, Fox R, Anderson BJ. Uncovering hidden spatial structure in species communities with spatially explicit joint species distribution models. Methods Ecol. Evol. 2016;7:428–436. doi: 10.1111/2041-210X.12502. [DOI] [Google Scholar]
- 71.Dornelas M, et al. Assemblage time series reveal biodiversity change but not systematic loss. Science. 2014;344:296–299. doi: 10.1126/science.1248484. [DOI] [PubMed] [Google Scholar]
- 72.Ovaskainen O, Soininen J. Making more out of sparse data: hierarchical modeling of species communities. Ecology. 2011;92:289–295. doi: 10.1890/10-1251.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.