Abstract
Parasite-mediated competition can shape community structure and host distribution. If two species compete for resources, parasites may indirectly change the outcome of competition. We tested the role of a trematode parasite in mediating microhabitat use by congeneric isopods Austridotea annectens and Austridotea lacustris. Although both isopods share resources, they rarely co-occur in the same discrete microhabitats. We set up mesocosms with and without competition and/or parasites to examine the role of parasites in host distribution and habitat segregation. Austridotea annectens showed a clear preference for one microhabitat type regardless of competition or parasitic infection. By contrast, A. lacustris showed little habitat selection in the absence of competition, but favoured sandy habitats in the presence of uninfected A. annectens and rocky habitats when competing with infected A. annectens. Our results suggest that parasites in one species affect the distribution of another species, and mediate competition between these species. We demonstrated the impacts of a parasite on the microhabitat use of its host's competitor. This also represents an example of a super-extended phenotype, where a parasite affects the phenotype of a non-host.
Keywords: isopod, extended phenotype, habitat selection, parasite mediation
1. Introduction
Community structure and species distribution are shaped by abiotic and biotic factors, including competition and predation. Parasite mediation can modify the outcomes of these interactions and structure communities [1–3]. Host phenotypes altered by parasites may indirectly affect foraging, habitat preferences and predator avoidance [4,5]. As such, non-lethal effects of parasites can influence important direct interactions, such as competition, and ultimately species distribution and community composition [1,6]. Apparent competition may occur between species that share a parasite, creating indirect competition between host species [1,2]. Variability in parasite susceptibility can result in less resistant species being poorer competitors. The more resistant host species may become the stronger competitor, with the parasite mediating interactions between hosts and altering their outcome [2]. The more resistant host may even exclude its competitor from prime habitats and thus reduce food access. Although parasites can modulate direct competition between species [7,8], few studies have examined the more subtle indirect effects of parasites on host phenotypes and their consequences for interspecific competition [3].
Closely related species are often susceptible to the same parasites, yet prevalence and virulence may vary among them [1,7,8]. In New Zealand freshwaters, the isopods Austridotea annectens and Austridotea lacustris frequently co-occur and both are known to be infected by the trematode parasite Maritrema poulini, yet infection levels differ greatly between the two congeneric hosts. Maritrema poulini uses waterfowl as definitive host. Eggs produced by adult worms pass out with faeces and infect the snail first intermediate host. After asexual multiplication within the snail, cercariae (free-swimming larvae) are released in the water to seek crustacean second intermediate hosts, in which they encyst. The life cycle is completed through predation of infected crustaceans by waterfowl. Generally, M. poulini has a very high prevalence in A. annectens versus less than 1% in A. lacustris; local and temporal variations in infection levels are observed in A. annectens. Infection intensity is also much higher, usually an order of magnitude, in A. annectens than A. lacustris [9].
Although both isopods have similar body sizes, diet and predators, they very rarely occupy the same discrete microhabitats [10]. Austridotea lacustris occurs in rocky habitats along the shore [10], where shelter from predators is available but temperature fluctuations are pronounced, and desiccation possible due to variations in water levels. By contrast, A. annectens lives on sandy substrate, where it is exposed to predators and competition from amphipods.
Although both isopods co-occur within a metre of each other, their striking small-scale segregation by microhabitat types remains unexplained. We hypothesize that parasitism drives habitat use and competition between these species. We experimentally test this hypothesis by quantifying isopod behaviour in the presence and absence of competition and parasites, and use our results to propose the novel concept of a parasite's super-extended phenotype.
2. Methods
Lake Waihola, South Island, New Zealand (46°01′ 14 S, 170°05′05 E) is a shallow, coastal lake with a shoreline including boulder areas, stretches covered by rocks and sandy patches with macrophyte cover. Parts of the shoreline are exposed to air twice daily due to tides, increasing desiccation risk and exposure to extreme temperatures for individuals in these areas.
We collected naturally infected isopods of both species from the shoreline. Isopods were caught using dip-nets and returned to the laboratory. Individuals of both species were obtained between August 2016 and March 2017, and always maintained in laboratory tanks for a week prior to trials. Additional A. annectens were collected in November 2016 to establish a laboratory population. Female A. annectens carrying eggs were isolated and their young grown for three months. As A. annectens has a natural prevalence of M. poulini close to 100%, a laboratory population was needed to ensure a supply of parasite-free individuals. Laboratory conditions matched lake conditions as closely as possible (food, substrate and water from the sampling site and containing natural chemical cues; etc.). Additionally, field-caught individuals were also maintained in laboratory tanks under the same conditions for a week before trials. Observations prior to experiments strongly indicated that field-caught and laboratory-reared individuals behaved similarly in terms of speed of movement, time spent hidden/under cover versus exposed, etc., as well as having similar mortality rates (see electronic supplementary material).
We allocated our trial mesocosms to five treatments. Three treatments consisted of a single species: uninfected A. lacustris, uninfected A. annectens, or infected A. annectens. Two were competitive treatments with both isopods together, one with infected A. annectens and one with uninfected A. annectens. As we found no infected A. lacustris, we had no mesocosms with infected individuals of this species. Owing to availability of individuals, treatments had uneven numbers of replicates: three were parasite-free laboratory-raised A. annectens alone, eight were parasite-free laboratory-raised A. annectens and field-caught A. lacustris, 17 were field-caught parasitized A. annectens alone, 21 were field-caught parasitized A. annectens and A. lacustris, and 14 were field-caught A. lacustris alone. See the electronic supplementary material for further details.
Each mesocosm had six size-matched individuals, either of the same species or half of one species and half of the other, depending on treatment; densities matched those observed in Lake Waihola (electronic supplementary material). Each mesocosm consisted of an opaque plastic tank filled with aerated lake water and containing two microhabitats, one made of sand and small rocks, and the other of sand, small and large rocks (electronic supplementary material, figure S1). Isopods were fed commercial fish pellets and allowed to move freely. After 6 days, individual final locations (on rocks, on sand or under sand) were recorded. Although isopods exhibit some movement in search of resources, they consistently re-settle on or very near their prior location. Thus, final location reliably indicates microhabitat choice. Each individual was measured, sexed [9] and dissected for parasite abundance.
Analyses were performed in JMP® 12 [11]. We compared the frequency of final locations between treatments using contingency analysis. The relationship between parasite abundance and final location was examined using a logistic regression. We tested for differences in parasite abundance and size between mesocosms within treatment using ANOVA. The relationship between the individual final location and their size in each treatment was examined using logistic regression. We used contingency analysis to compare prevalence between final locations.
3. Results
A total of 334 isopods (184 A. annectens, 150 A. lacustris) survived the trials out of 378. Prevalence of M. poulini in field A. annectens was 86% with a mean abundance (±s.e.) of 7.9 ± 0.7. Maritrema poulini was absent in A. lacustris.
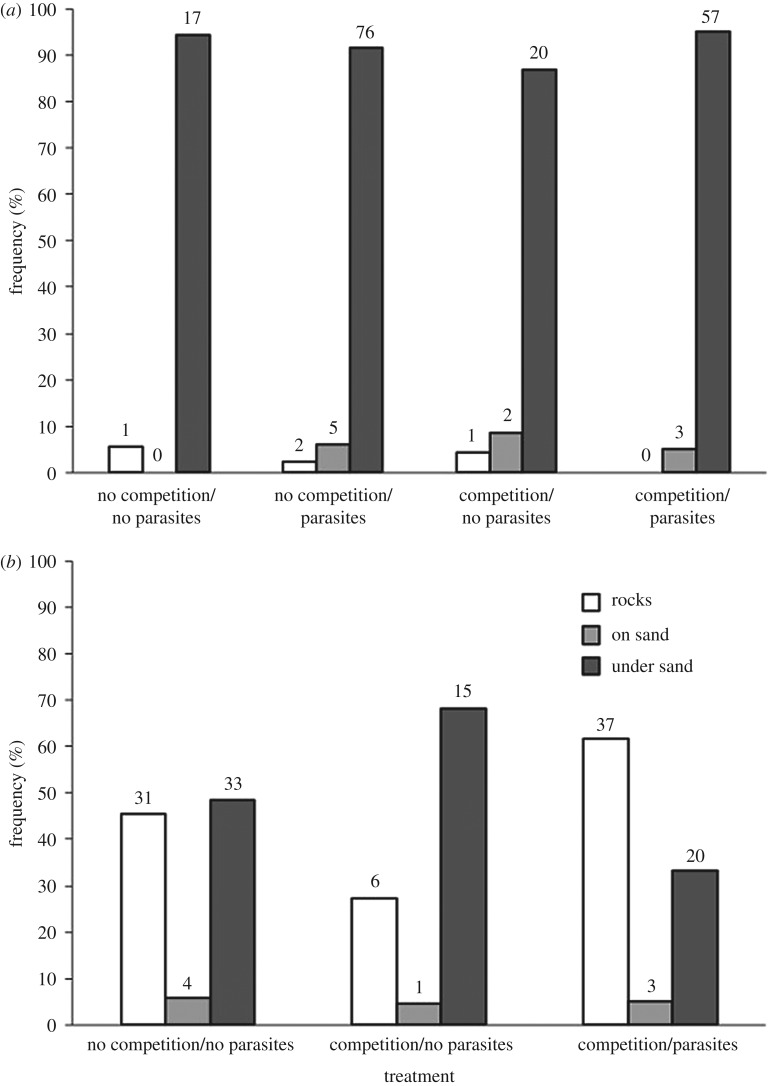
The final location of A. annectens did not differ among treatments and individuals showed a preference for being under the sand in all treatments (figure 1a, see the electronic supplementary material). Laboratory-raised and field-caught individuals exhibited identical habitat preferences. In treatments with and without competition, uninfected A. annectens location preference did not differ (χ2 = 2.8, p = 0.25). Without competition, both infected and uninfected A. annectens preferred the same location (χ2 = 2.4, p = 0.30). With competition, there was no difference in microhabitat selection between infected and uninfected A. annectens (χ2 = 0.38, p = 0.14). Finally, there was no difference between treatments with uninfected A. annectens under competition and treatment with only infected A. annectens (χ2 = 0.87, p = 0.65).
Figure 1.
Frequency of isopods, (a) Austridotea annectens and (b) Austridotea lacustris in final locations in the presence or the absence of competition and parasites. Sample sizes are above each bar.
The final location of A. lacustris varied among treatments, particularly between competition trials with infected versus uninfected A. annectens (figure 1b). Under competition, the final location of A. lacustris varied depending on whether A. annectens individuals were infected (χ2 = 7.1, p = 0.029); A. lacustris preferred rocky substrate when competing with infected A. annectens, but preferentially buried under sand when competing with uninfected individuals. The final location of A. lacustris did not vary between treatments with competition with uninfected A. annectens and without competition (χ2 = 2.1, p = 0.34), although A. lacustris seemed to equally prefer rocks and sand in the absence of competition. There was no difference in final location between treatments with competition with infected A. annectens and treatments without competition (χ2 = 3.1, p = 0.21).
Final location of isopods only varied among the mesocosm replicates when A. lacustris was alone (χ2 = 47.5, p = 0.0013). In other treatments, the final location of either species did not vary among replicates (all p > 0.05). Therefore, data from different mesocosms were combined for further analysis.
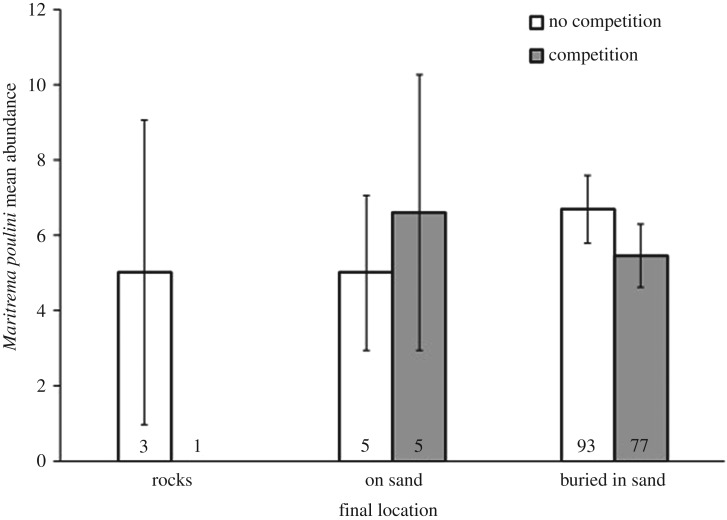
Final location of isopods of both species was not influenced by their sex, size or abundance of parasites they harboured, in any treatment (figure 2; electronic supplementary material).
Figure 2.
Mean abundance (±s.e.) of Maritrema poulini in Austridotea annectens based on final location and the presence of competition. Sample sizes are at the bottom of each bar.
4. Discussion
Parasite-mediated competition can alter microhabitat use by competing species and may shape species distributions and relative abundances. Here, the impact of parasites on microhabitat use by isopods appeared unidirectional, with A. lacustris being indirectly influenced by the trematode infection of its competitor. On its own, A. lacustris showed no preference for either microhabitat. However, in the presence of the competitor A. annectens, the distribution of A. lacustris became biased towards one microhabitat, though microhabitat selection depends on whether the competitor was parasitized or not. By contrast, microhabitat use by A. annectens did not vary as a function of competition or parasite load.
Differences in microhabitat use by A. lacustris in competitive environments may be due to behavioural alterations in their competitor, A. annectens, when infected with M. poulini. Indeed, A. annectens is more active when infected [9]. The activity increase in A. annectens may deter A. lacustris from venturing onto the sand [9]. Additionally, infection may increase the aggressiveness of A. annectens, leading A. lacustris to use rocks as an escape from direct aggression. Further, infected A. annectens have reduced evasive responses to predatory stimuli compared with uninfected individuals [12]. In the absence of predators, behavioural modifications targeting antipredator responses may in turn alter competition between species. The change in habitat use by A. lacustris when competing with infected versus non-infected A. annectens may have other subtle consequences. For example, alteration in habitat choice can affect risk of predation and desiccation, as well as parasite transmission rates.
The direct impacts of parasites on host behaviour, particularly intermediate hosts, are well documented [5]. However, the focus has been on direct consequences for host behaviour or its interactions with conspecifics. What we show here is an effect of a parasite not on its host, but on the behaviour of another species. The natural distribution of the two isopods matches that seen in the competition mesocosms involving infected A. annectens, strongly suggesting that parasites mediate their interactions in nature and shape the spatial distribution of a non-infected species. Further, parasite-induced changes in host behaviour are classic examples of extended phenotypes [13]. Our system may also be an example of a super-extended phenotype, where the parasite is affecting the phenotype of a non-host. Parasite genes have been selected for their effect on host phenotype; it is conceivable that selection may also favour their effects on non-hosts if these benefit the parasite. In our system, A. lacustris moving to rocky habitats not only decreases competition for the parasite's host, but also removes alternative prey from sandy substrates. This very likely increases predation rates by waterfowl on A. annectens, the sole remaining isopod on exposed sand, thereby enhancing parasite transmission to definitive hosts. Selection should favour this super-extended phenotypic effect on a non-host. The super-extended phenotype of M. poulini may shape species distributions and subsequent interactions within this ecosystem [14]. Further study of indirect mediation by parasites is crucial to our growing understanding of factors influencing community structure, population dynamics and species distribution.
Supplementary Material
Supplementary Material
Acknowledgements
Field assistance by A. Filion, A. Friesen, K. Karl, B. Presswell, A. Stumbo and Z. Tobias. Thanks to the Otago Parasitology Research Group for comments. We are also grateful to three anonymous reviewers for their constructive comments and suggestions on previous versions of the manuscript.
Data accessibility
Dataset is available as the electronic supplementary material.
Authors' contributions
O.C.F., R.P. and C.L. designed the experiments. O.C.F. conducted fieldwork, performed experiments, analysed the data and wrote the manuscript; the other authors provided editorial advice and critical revision. Additionally, all authors approved the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
No competing interests.
Funding
Project was funded by the Zoology Department, University of Otago.
References
- 1.Park T. 1948. Interspecies competition in populations of Tribolium confusum Duval and Tribolium castaneum Herbst. Ecol. Monogr. 18, 265–307. ( 10.2307/1948641) [DOI] [Google Scholar]
- 2.Hudson PJ, Greenman JV. 1998. Competition mediated by parasites: biological and theoretical progress. Trends Ecol. Evol. 13, 387–390. ( 10.1016/S0169-5347(98)01475-X) [DOI] [PubMed] [Google Scholar]
- 3.MacNeil C, Dick JTA. 2011. Parasite-mediated intraguild predation as one of the drivers of co-existence and exclusion among invasive and native amphipods (Crustacea). Hydrobiologia 665, 247–256. ( 10.1007/s10750-011-0627-2) [DOI] [Google Scholar]
- 4.Minchella DJ, Scott ME. 1991. Parasitism: a cryptic determinant of animal community structure. Trends Ecol. Evol. 6, 250–254. ( 10.1016/0169-5347(91)90071-5) [DOI] [PubMed] [Google Scholar]
- 5.Lefèvre T, Lebarbenchon C, Gauthier-Clerc M, Missé D, Poulin R, Thomas F. 2009. The ecological significance of manipulative parasites. Trends Ecol. Evol. 24, 41–48. ( 10.1016/j.tree.2008.08.007) [DOI] [PubMed] [Google Scholar]
- 6.Mouritsen KN, Poulin R. 2005. Parasites boosts biodiversity and changes animal community structure by trait-mediated indirect effects. Oikos 108, 344–350. ( 10.1111/j.0030-1299.2005.13507.x) [DOI] [Google Scholar]
- 7.Thomas F, Renaud F, Rousset F, Cezilly F, De Meeüs T. 1995. Differential mortality of two closely related host species induced by one parasite. Proc. R. Soc. Lond. B 260, 349–352. ( 10.1098/rspb.1995.0103) [DOI] [Google Scholar]
- 8.Yan G, Stevens L, Goodnight CJ, Schall JJ. 1998. Effects of a tapeworm parasite on the competition of Tribolium beetles. Ecology 79, 1093–1103. ( 10.1890/0012-9658(1998)079[1093:EOATPO]2.0.CO;2) [DOI] [Google Scholar]
- 9.Friesen OC, Poulin R, Lagrue C. 2017. Differential impacts of shared parasites on fitness components among competing hosts. Ecol. Evol. 7, 4682–4693. ( 10.1002/ece3.3062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufour CM, Engels NM, Burns CW. 2007. Distribution, substrate preference and habitat enhancement of the isopod Austridotea lacustris in Tomahawk Lagoon, Otago, New Zealand. New Zeal. J. Mar. Freshw. Res. 41, 299–307. ( 10.1080/00288330709509917) [DOI] [Google Scholar]
- 11.SAS Institute 2015. Cary, NC: SAS Institute. See https://www.jmp.com/en_us/home.html.
- 12.Hansen EK, Poulin R. 2005. Impact of a microphallid trematode on the behaviour and survival of its isopod intermediate host: phylogenetic inheritance? Parasitol. Res. 97, 242–246. ( 10.1007/s00436-005-1435-2) [DOI] [PubMed] [Google Scholar]
- 13.Dawkins R. 1982. The extended phenotype: The gene as the unit of selection, 1st edn Oxford, UK: Oxford University Press. [Google Scholar]
- 14.Whitman TG, et al. 2003. Community and ecosystem genetic: a consequence of the extended phenotype. Ecology 84, 559–573. ( 10.1890/0012-9658(2003)084[0559:CAEGAC]2.0.CO;2) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Dataset is available as the electronic supplementary material.