Abstract
Social immunization (SI) is a horizontal transfer of immunity that protects naive hosts against infection following exposure to infected nestmates. While mainly documented in eusocial insects, non-social species also share similar ecological features which favour the development of group-level immunity. Here, we investigate SI in Tenebrio molitor by pairing naive females with a pathogen-challenged conspecific for 72 h before measuring a series of immune and fitness traits. We found no evidence for SI, as beetles who cohabited with a live pathogen-challenged conspecific were not better protected against bacterial challenge. However, exposure to a heat-killed-bacteria-challenged conspecific appeared to increase pathogen tolerance, which manifested in differential fitness investment. Our results together suggest that T. molitor do respond to immune-related cues in the social environment, despite not showing a classic immunization response as predicted.
Keywords: insect immunity, social immunity, Tenebrio molitor, Staphylococcus aureus, tolerance, fitness
1. Introduction
Social insects are particularly vulnerable to pathogenesis owing to high population density and homozygosity [1] and have developed additional protection in the form of group-level social immune defences such as allogrooming and shared antimicrobial secretions [2]. One such defence is social immunization (SI), a horizontal transfer of immunity where social contact with infected nestmates causes an immune upregulation in immunologically naive individuals, enhancing their protection against the same parasite after secondary exposure [3]. Recent evidence for SI in Tribolium castaneum [4] suggests it may not be confined to eusocial insects. Despite selection acting primarily through direct fitness in non-social insects [5], SI should benefit conspecifics in highly connected populations [6] and group-level defences could evolve provided fitness benefits outweigh the costs for the acting individual. Empirical data testing these predictions are warranted.
Here, we examine SI in Tenebrio molitor L. (Coleoptera: Tenebrionidae), which live in large groups within permanent food stores: factors predicted to favour the development of external defences [7]. Immune stimulation is known to alter signalling pheromones in this species [8,9], and females can discriminate between healthy and immune-challenged males [8–10]. We measure the effect of cohabitation with a pathogen-challenged conspecific on a series of immune and fitness traits [11] in otherwise immunologically naive beetles.
2. Material and methods
(a). Insect maintenance
Final-instar T. molitor larvae (Live Foods UK) were maintained under standard laboratory conditions [12,13] and sexed, weighed and isolated upon pupation. Upon eclosion, imagoes were provided ad libitum food and water until experimental treatment, 8–10 days later.
(b). Bacterial culturing
Staphylococcus aureus is a Gram-positive bacterium found in tenebrionid food stores [14], which is known to induce an immune response in T. molitor [12]. A stock of erythromycin-resistant S. aureus (strain SH1000) [12] was used to prepare inoculates (see below and electronic supplementary material, §S1), which ensured repeatable selective culturing using antibiotic-infused medium and excluded non-target bacteria from bacterial load estimates. All inoculum doses were selected based on a preliminary survival analysis (electronic supplementary material, S1).
(c). Experimental treatments
In two separate experiments, pairs of female beetles were housed together in 50 mm Petri dishes for 72 h with ad libitum access to food and water. Each pair consisted of one naive focal beetle and one treated cohabitant (electronic supplementary material, S2). In experiment 1, the effect of SI on immune traits in focal beetles was compared using four cohabitant treatments, where cohabitants either were injected with 2.5 × 104 colony-forming units (CFU) of (i) live or (ii) heat-killed bacteria suspended in 5 µl sterile PBS, (iii) were injected with 5 µl sterile PBS as a wounding control, or (iv) received no treatment. Using live and heat-killed bacterial treatments offered potential for both active (immune upregulation through exposure to an infectious individual) and passive (immune upregulation though transfer of immune effectors from conspecifics) modes of SI [15]. After 72 h, focal beetles were assigned to either an antibacterial activity or a survival assay (§2d). In experiment 2, the effect of SI on fitness traits in focal beetles was compared using three cohabitant treatments: (i) injection of 2.5 × 106 CFU of heat-killed bacteria, plus the same (ii) PBS and (iii) no treatment controls. After 72 h, focal beetles had their fitness measured via reproductive output and survival assays (§2e).
(d). Experiment 1
In vivo antibacterial activity assay: 76 focal beetles were injected with 2.5 × 106 CFU of live bacteria and perfusion bled 8 h later [12]. Haemolymph samples were plated onto erythromycin-infused agar (to ensure recovery of the injected bacteria only) and incubated at 37°C for 48 h, and CFUs enumerated using OpenCFU [16] (electronic supplementary material, S3). Survival assay: 126 focal beetles were injected with 5 × 107 CFU, provided ad libitum food and water, and their survival monitored for 37 days. A further sample of 32 untreated, non-cohabited beetles of the same age were also monitored as a control group (electronic supplementary material, S2a).
(e). Experiment 2
Fitness assays: 224 focal beetles were injected with 2.5 × 106 CFU of heat-killed bacteria; 224 remained unchallenged. All were subsequently housed with an age-matched, unchallenged male and allowed to mate for 24 h. Males were removed and females provided ad libitum food and water, and their survival monitored for 40 days (electronic supplementary material, S2b). Eggs were collected and counted every 3 days, and egg-laying rate per beetle calculated as (total egg count)/(lifespan in days). A subset of three eggs from each collection was photographed to estimate egg volume (electronic supplementary material, S4).
(f). Statistical analysis
All data were analysed in R [17]. We used a negative binomial generalized linear model for CFU counts using the mass package [18], a linear model for egg-laying rates and a linear mixed-effects model (with beetle identity nested within day as the random term) for egg volume measurements, using the nlme package [19]. Survival data in both experiments were assessed using the Cox proportional hazard regression using the survival package [20]. Focal beetle pupal weight (closely correlated with adult weight and size in holometabolous insects [21]) was included as a covariate in models of CFU count data (F1,71 = 4.41, p = 0.04; electronic supplementary material, S5.1) and egg volume data (F1,1261 = 8.62, p = 0.003; electronic supplementary material, S5.9) as it significantly improved their fit.
3. Results
(a). Experiment 1
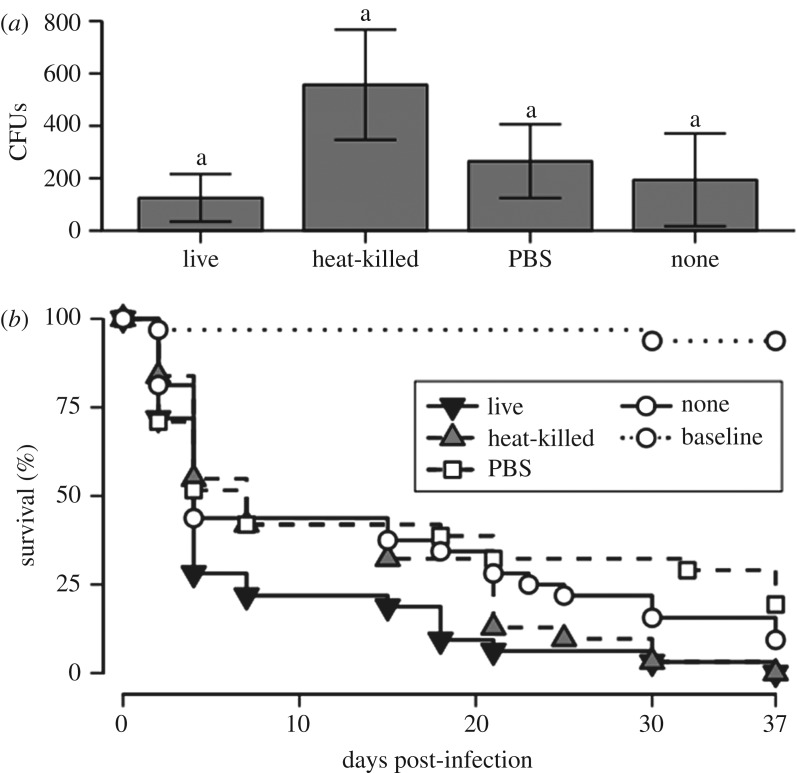
Despite no overall effect of cohabitant treatment on haemolymph antibacterial activity following live bacterial injection of focal beetles (F3,71 = 1.41, p = 0.246; figure 1a), there appeared to be some impairment of bacterial clearance by heavier beetles when cohabitants had been treated with heat-killed bacteria (electronic supplementary material, S5.3). There was an overall effect of cohabitation treatment on focal beetle survival after live bacterial injection (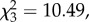 p = 0.02; figure 1b; electronic supplementary material, S5.4 and S5.5), driven by lower survival in those cohabiting with live-bacteria-challenged individuals compared with those whose cohabitants were wounded (z =−3.12, p = 0.01; figure 1b; electronic supplementary material, S5.6).
p = 0.02; figure 1b; electronic supplementary material, S5.4 and S5.5), driven by lower survival in those cohabiting with live-bacteria-challenged individuals compared with those whose cohabitants were wounded (z =−3.12, p = 0.01; figure 1b; electronic supplementary material, S5.6).
Figure 1.
Results from experiment 1: (a) bacterial clearance (mean ± s.e. Staphylococcus aureus colony forming units (CFUs) recovered from haemolymph 8 h post-inoculation; lettering denotes Tukey's HSDs (honest significant differences); and (b) survival by focal beetles.
(b). Experiment 2
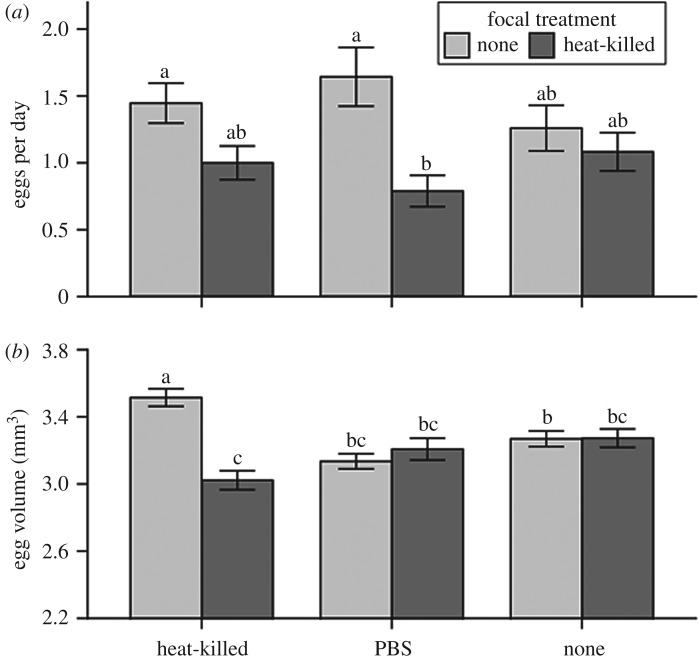
There was no overall effect of cohabitant treatment on egg-laying rate of focal beetles (F2,218 = 0.07, p = 0.936), but focal treatment was important (F1,218 = 14.13, p < 0.001; electronic supplementary material, S5.7), an effect primarily driven by a difference in egg-laying rate in those cohabiting with a wounded beetle (figure 2a; electronic supplementary material, S5.8). By contrast, there was an interaction between cohabitant treatment and focal treatment on egg volume (F2,416 = 11.43, p < 0.001; figure 2b; electronic supplementary material, S5.9), driven mainly by a difference in egg volume in focal beetles cohabiting with heat-killed-bacteria-challenged beetles (figure 2b; electronic supplementary material, S5.10). There were no differences in survival between any of the treatment groups (electronic supplementary material, S5.12 and S5.13).
Figure 2.
Results from experiment 2: effect of cohabitant treatment on mean ± s.e. (a) Egg-laying rate and (b) egg volume from focal beetles either before (none) or after (heat-killed) challenge. Lettering denotes Tukey's HSDs.
4. Discussion
We found no evidence in this study for SI in T. molitor, as focal beetles were no better protected against a live bacterial challenge following exposure to a conspecific challenged with the same pathogen. However, differences in immune and fitness traits between focal beetles housed with bacterially challenged and healthy conspecifics suggest that T. molitor do respond to immune-related cues in the social environment, but not in a way we predicted. Longevity of focal beetles after live bacterial challenge was reduced following social exposure to the same live bacteria, which, considering that haemolymph antibacterial activity in these beetles was not impaired, suggests a trade-off between resistance and longevity. However, longevity of focal beetles was maintained when their cohabitant was challenged with heat-killed bacteria, suggesting this group of beetles were better able to tolerate a live bacterial challenge [11]. Social exposure to an immune-challenged conspecific also appeared to alter reproductive investment by inducing production of larger eggs, an effect that was reversed when the egg-producer was itself immune-challenged, suggesting further trade-offs between tolerance and fitness in this species.
Although physical interactions between hosts can result in parasite transmission [15], most bacterial infections occur via oral uptake [1]. Focal beetles cohabiting with a live-bacteria-challenged conspecific may have contacted S. aureus by ingesting food contaminated with bacteria from excreta; bacteria in the haemolymph have been shown to colonize the insect gut [22]. Immune activation has been shown to incur survival costs, and challenge with a live pathogen is likely to inflict costs through pathological damage [23]. Impaired survival may therefore be explained by considering post-cohabitation challenge as a secondary infection with additive or synergistic negative effects for an already-immunocompromised host; infective bacteria have been recovered from T. molitor haemolymph up to 21 days after initial infection [12], meaning that secondary challenge could have resulted in multiple infection. Alternatively, survival costs could be a consequence of illness-induced anorexia during cohabitation [24], as nutritional stress from fasting can have comparable effects to infection on fitness traits in tenebrionids ([25]; electronic supplementary material, S1).
In contrast to those exposed to live-bacteria-challenged conspecifics, focal beetles cohabiting with heat-killed-bacteria-challenged individuals appeared to exhibit some impairment of haemolymph antibacterial activity (an effect that was weight-dependent; electronic supplementary material, S5.1), yet their survival was not impaired in the same way. These beetles may have contacted non-viable bacterial fragments via the excreta of cohabitants, such as the bacterial cell wall components lipopolysaccharide and peptidoglycan which can be highly immunogenic in T. molitor [13,26], or responded to modifications of the cohabitant cuticular hydrocarbon profile which are known to occur during immune stimulation in T. molitor [9]. Whatever the mechanism, heavier beetles appeared to better tolerate infection by preserving survival without clearing the pathogen as effectively [11], suggesting that larger individuals can better afford to invest in tolerance strategies [26].
Host tolerance allows an organism to protect itself from pathogens by reducing negative impacts on fitness [11], and females are predicted to invest in survival strategies which preserve the capacity for continued reproduction [11,27]. In line with this prediction, we found that egg-laying rate was not negatively impacted by cohabitation with heat-killed-bacteria-challenged individuals. Indeed, egg-laying rate was only impeded when focal beetles cohabited with wounded beetles and were themselves challenged with bacteria. However, fitness investment was augmented by exposure to bacteria-challenged conspecifics as it led to production of larger eggs in unchallenged focal beetles, but relatively smaller eggs when the focal beetle was bacterially challenged itself. Egg size does not always predict egg quality [28], but immune-challenged female T. molitor are known to transfer immune factors to their eggs that improve offspring quality, which appears to influence egg size [29]. Our findings on how immune traits might trade-off with fitness investments therefore fit with predictions relating to trans-generational immune priming in this species; however, this is the first evidence of social cues influencing fitness traits.
Socially induced immune augmentation likely occurs via two routes [15]. Firstly, passive SI protects naive hosts through the proxy action of immune factors transferred from infected nestmates, mainly via mutual feeding or coprophagy in eusocial insects [2,15]. Secreted antimicrobials also form a plausible mechanism in non-social insects [7]; tenebrionids secrete quinones which have broad antimicrobial action [14] and may trade-off with internal immune defences [30]. Their upregulation during parasitism [31] (but not during wounding [4]) suggests a secondary role in group-level defence [7,32]. Secondly, active SI involves investment in immunity induced by transmission of low-level infection or detection of pathogen-associated signals [15], such as modification of cuticular hydrocarbon profiles in immune-challenged individuals [9]. Here, reduced survival in focal beetles cohabiting with live-bacteria-challenged individuals suggests that transmission of infection is not involved in SI in this species but that other cues from immune-challenged individuals can influence both immune investment and reproductive strategy, a phenomenon that requires further investigation.
Supplementary Material
Acknowledgements
We thank Rebecca Wilson, Tobit Dehnen, Daniel McDowell and Josh Hooker for technical assistance; Will Hentley and Joel Pick for statistical advice; Jurriaan Ton and anonymous reviewers for advice on manuscript preparation.
Data accessibility
Data used in this manuscript and associated code are archived in the Dryad data repository: http://dx.doi.org/10.5061/dryad.s1636 [33].
Authors' contributions
The study was designed by all authors; laboratory work carried out by J.D.G. and S.E.F.E.; data analysis by J.D.G.; writing of the manuscript by J.D.G. and S.E.F.E. All authors gave final approval and agree to be held accountable for the content.
Competing interests
We declare we have no competing interests.
Funding
J.D.G. was supported by a University of Sheffield Faculty of Science Scholarship.
References
- 1.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Cremer S, Armitage SAO, Schmid-Hempel P. 2007. Social immunity. Curr. Biol. 17, R693–R702. ( 10.1016/j.cub.2007.06.008) [DOI] [PubMed] [Google Scholar]
- 3.Masri L, Cremer S. 2014. Individual and social immunisation in insects. Trends Immunol. 35, 471–482. ( 10.1016/j.it.2014.08.005) [DOI] [PubMed] [Google Scholar]
- 4.Peuãÿ R, Eggert H, Armitage SAO, Kurtz J. 2015. Downregulation of the evolutionary capacitor Hsp90 is mediated by social cues. Proc. R. Soc. B 282, 20152041 ( 10.1098/rspb.2015.2041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton WD. 1964. The genetical evolution of social behaviour. II. J. Theor. Biol. 7, 17–52. ( 10.1016/0022-5193(64)90039-6) [DOI] [PubMed] [Google Scholar]
- 6.Elliot SL, Hart AG. 2010. Density-dependent prophylactic immunity reconsidered in the light of host group living and social behavior. Ecology 91, 65–72. ( 10.1890/09-0424.1) [DOI] [PubMed] [Google Scholar]
- 7.Otti O, Tragust S, Feldhaar H. 2014. Unifying external and internal immune defences. Trends Ecol. Evol. 29, 625–634. ( 10.1016/j.tree.2014.09.002) [DOI] [PubMed] [Google Scholar]
- 8.Sadd B, Holman L, Armitage H, Lock F, Marland R, Siva-Jothy MT. 2006. Modulation of sexual signalling by immune challenged male mealworm beetles (Tenebrio molitor L.): evidence for terminal investment and dishonesty. J. Evol. Biol. 19, 321–325. ( 10.1111/j.1420-9101.2005.01062.x) [DOI] [PubMed] [Google Scholar]
- 9.Nielsen ML, Holman L. 2011. Terminal investment in multiple sexual signals: immune-challenged males produce more attractive pheromones. Funct. Ecol. 26, 20–28. ( 10.1111/j.1365-2435.2011.01914.x) [DOI] [Google Scholar]
- 10.Worden BD, Parker PG, Pappas PW. 2000. Parasites reduce attractiveness and reproductive success in male grain beetles. Anim. Behav. 59, 543–550. ( 10.1006/anbe.1999.1368) [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335, 936–941. ( 10.1126/science.1214935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haine ER, Moret Y, Siva-Jothy MT, Rolff J. 2008. Antimicrobial defense and persistent infection in insects. Science 322, 1257–1259. ( 10.1126/science.1165265) [DOI] [PubMed] [Google Scholar]
- 13.Jacobs CGC, Gallagher JD, Evison SEF, Heckel DG, Vilcinskas A, Vogel H. 2016. Endogenous egg immune defenses in the yellow mealworm beetle (Tenebrio molitor). Dev. Comp. Immunol. 70, 1–8. ( 10.1016/j.dci.2016.12.007) [DOI] [PubMed] [Google Scholar]
- 14.Yezerski A, Cussatt G, Glick D, Evancho M. 2005. The effects of the presence of stored product pests on the microfauna of a flour community. J. Appl. Microbiol. 98, 507–515. ( 10.1111/j.1365-2672.2004.02479.x) [DOI] [PubMed] [Google Scholar]
- 15.Konrad M, et al. 2012. Social transfer of pathogenic fungus promotes active immunisation in ant colonies. PLoS Biol. 10, e1001300 ( 10.1371/journal.pbio.1001300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geissmann Q. 2013. OpenCFU, a new free and open-source software to count cell colonies and other circular objects. PLoS ONE 8, e54072 ( 10.1371/journal.pone.0054072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org. [Google Scholar]
- 18.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 3rd edn New York, NY: Springer. [Google Scholar]
- 19.Pinheiro J, et al. 2017. nlme: linear and nonlinear mixed effects models. Version 3.1-131. See https://CRAN.R-project.org/package=nlme .
- 20.Therneau T.2015. A package for survival analysis in S. Version 2.38. See https://CRAN.R-project.org/package=survival .
- 21.Calvo D, Molina JM. 2005. Fecundity–body size relationship and other reproductive aspects of Streblote panda (Lepidoptera: Lasiocampidae). Ann. Entomol. Soc. Am. 98, 191–196. ( 10.1603/0013-8746(2005)098[0191:FSRAOR]2.0.CO;2) [DOI] [Google Scholar]
- 22.Silva CP, et al. 2002. Bacterial infection of a model insect: Photrhabdus luminescens and Manduca sexta. Cell. Microbiol. 4, 329–339. ( 10.1046/j.1462-5822.2002.00194.x) [DOI] [PubMed] [Google Scholar]
- 23.Armitage SAO, Thompson JJW, Rolff J, Siva-Jothy MT. 2003. Examining costs of induced and constitutive immune investment in Tenebrio molitor. J. Evol. Biol. 16, 1038–1044. ( 10.1046/j.1420-9101.2003.00551.x) [DOI] [PubMed] [Google Scholar]
- 24.Ayres JS, Schneider DS. 2009. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 7, e1000150 ( 10.1371/journal.pbio.1000150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shostak AW. 2009. Tapeworm (Hymenolepis diminuta) infection in flour beetles (Tribolium confusum): does it cause a trade-off between host fecundity and egg size? Can. J. Zool. 87, 1087–1095. ( 10.1139/Z09-102) [DOI] [Google Scholar]
- 26.Moret Y, Siva-Jothy MT. 2003. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. B 270, 2475–2480. ( 10.1098/rspb.2003.2511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreau J, Martinaud G, Troussard J-P, Zanchi C, Moret Y. 2012. Trans-generational immune priming is constrained by the maternal immune response in an insect. Oikos 121, 1715–1922. ( 10.1111/j.1600-0706.2011.19933.x) [DOI] [Google Scholar]
- 28.Mcintyre GS, Gooding RH. 2000. Effects of maternal age on larval competitiveness in house flies. Heredity 85, 480–489. ( 10.1046/j.1365-2540.2000.00787.x) [DOI] [PubMed] [Google Scholar]
- 29.Dhinaut J, Chogne M, Moret Y. 2017. Trans-generational immune priming in the mealworm beetle protects eggs through pathogen-dependent mechanisms imposing no immediate fitness cost for the offspring. Dev. Comp. Immunol. 79, 105–112. ( 10.1016/j.dci.2017.10.017) [DOI] [PubMed] [Google Scholar]
- 30.Joop G, et al. 2014. Experimental evolution of external immune defences in the red flour beetle. J. Exp. Biol. 27, 1562–1571. [DOI] [PubMed] [Google Scholar]
- 31.Yan GY, Phillips TW. 1996. Influence of tapeworm infection on the production of aggregation pheromone and defensive compounds in Tribolium castaneum. J. Parasitol. 82, 1037–1039. ( 10.2307/3284221) [DOI] [PubMed] [Google Scholar]
- 32.Gokhale CS, Traulsen A, Joop G. 2017. Social dilemma in the external immune system of the red flour beetle? It is a matter of time. Evol. Ecol. 7, 6758–6765. ( 10.1002/ece3.3198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher JD, Siva-Jothy MT, Evison SEF. 2018. Data from: Social cues trigger differential immune investment strategies in a non-social insect, Tenebrio molitor Dryad Digital Repository. ( 10.5061/dryad.s1636) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gallagher JD, Siva-Jothy MT, Evison SEF. 2018. Data from: Social cues trigger differential immune investment strategies in a non-social insect, Tenebrio molitor Dryad Digital Repository. ( 10.5061/dryad.s1636) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data used in this manuscript and associated code are archived in the Dryad data repository: http://dx.doi.org/10.5061/dryad.s1636 [33].