Abstract
The cost of pregnancy is hard to study in marine mammals, particularly in species that undergo pregnancy while diving continuously at sea such as elephant seals (genus Mirounga). We analysed the diving behaviour of confirmed pregnant and non-pregnant northern elephant seals (M. angustirostris, n = 172) and showed that after an initial continuous increase in dive duration, dives of pregnant females become shorter after week 17. The reasons for this reduction in dive duration remain unknown, but we hypothesize that increased fetal demand for oxygen could be the cause. Our findings reveal an opportunity to explore the use of biologging data to investigate pregnancy status of free-ranging marine mammals and factors that could affect pregnancy success.
Keywords: marine mammals, pregnancy, diving behaviour, elephant seal
1. Introduction
The cost of reproduction is central to understanding the trade-offs between current and future reproductive success [1,2]. Research on mammals has primarily examined the energetics of gestation and lactation [3,4], but females incur additional costs associated with morphological changes and an increased physiological burden [5]. In marine mammals, these costs are particularly hard to account for, because pregnancy occurs while at sea. One of the few studies on this subject links morphological changes in pregnant bottlenose dolphins (Tursiops truncatus) to an increase in energetic costs as they experience higher drag forces [6]. Whether pregnancy affects the aerobic capacity of diving mammals is still unknown, yet their foraging ability depends on their capacity to remain underwater, and is ultimately determined by their body oxygen storage [7]. Pregnancy could affect dive performance if the oxygen demand of a growing fetus constrains the time a mother can spend underwater foraging.
Gestation in mammals is typically associated with higher blood volume [8,9], which implies larger total body O2 stores. Pregnancy is also linked with an increase in the basal metabolic rate (i.e. higher rate of O2 consumption) [10,11]. Furthermore, although the presence of specialized fetal haemoglobin has not been confirmed in pinnipeds, their fetuses do have a haemoglobin molecule with higher O2 affinity than adults [12], which may protect the fetus from hypoxia. Therefore, pregnancy likely impacts the O2 available to the mother.
Adult female elephant seals (genus Mirounga) haul out twice a year: to breed (approx. four weeks) and moult (approx. six weeks); they spend the rest of their time diving continuously at sea [13,14] (figure 1). These dives can be close to 2 h in duration, and reach depths of over 2000 m [13,14]. Deep-diving species typically maximize their O2 storage by having greater blood volume and higher concentrations of haemoglobin and myoglobin than shallow-diving species [7]. Blood volume also increases as animals spend more time at sea, representing a possible conditioning effect from repeated breath-holds and hypoxia [15,16].
Figure 1.
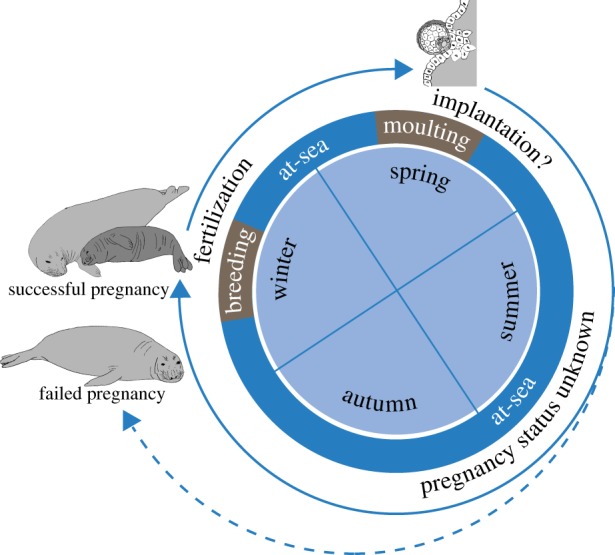
Annual cycle of adult female northern elephant seals. Breeding (approx. four weeks) and moulting haul-out (approx. six weeks) are indicated in brown. Individuals spend the rest of their annual cycle diving continuously at sea (blue). Timing of egg is uncertain. Pregnancy can only be confirmed when the female hauls out. (Online version in colour.)
Because elephant seals undergo pregnancy at-sea during their 8+ months post-moult trip (figure 1), our knowledge about this essential life-history phase is extremely limited. Fortunately, recent technological advances have enabled a wide range of physiological and behavioural measurements of free-ranging animals, including their diving behaviour [17]. We analysed diving data from confirmed pregnant and non-pregnant northern elephant seals (Mirounga angustirostris) to analyse whether dive duration, here understood as a measure of their diving/foraging performance, changes over the course of gestation.
2. Material and methods
Throughout this manuscript, we will use the term ‘pregnant’ to refer to animals that successfully returned to land and gave birth to a pup. ‘Non-pregnant’ refers to animals that returned to land but did not pup.
We collected diving data from 172 northern elephant seals captured at Año Nuevo State Park, California, USA, between 2004 and 2016 (table 1). Animals were sedated and handled following standard protocols [13], and instrumented with MK9 Time Depth Recorders (TDR, Wildlife Computers, WA, USA), programmed to collect diving data every 8 s. Animals were also outfitted with satellite tags to obtain location at sea and determine when animals came ashore [13].
Table 1.
Mass-at-departure, recovery, trip duration and diving metrics of adult female northern elephant seals, Mirounga angustirostris, according to pregnancy status.
| n | mass at departure (kg) | mass at recovery (kg) | trip duration (d) | dive depth (m) | dive duration (s) | |
|---|---|---|---|---|---|---|
| pregnant | 155 | 308.8 ± 36.0 | 478.2 ± 49.2 | 227.7 ± 35.7 | 475 ± 142 | 1427 ± 535 |
| non-pregnant | 17 | 299.5 ± 37.5 | 507.4 ± 46.7 | 204.5 ± 61.8 | 466 ± 150 | 1462 ± 714 |
Non-pregnant elephant seals (n = 17) returned either much earlier or later than pregnant females, and we confirmed whether individuals were nursing a pup upon instrument recovery. Diving data were corrected and analysed using the package diveMove [18], in R [19]. We used generalized additive mixed models (GAMMs) to explore the relationship between dive duration (DDur) and day of the trip (DoT), with pregnancy (fPreg) as categorical variable, individual identity as random effect and a gamma distribution (other diving metrics were also explored but are not included here; see the electronic supplementary material). GAMMs were fitted using the package mgcv [20], in R. Data are presented as mean ± s.d., unless stated otherwise.
3. Results and discussion
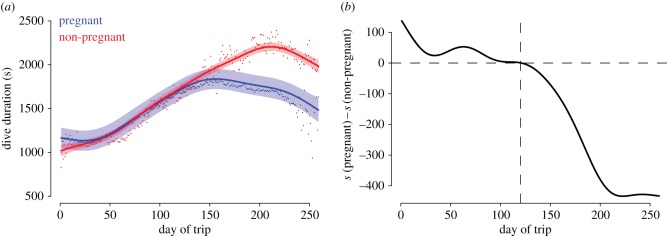
We found no difference in the mass at departure (ANOVA, F = 0.78, p = 0.38) or arrival (F = 1.705, p = 0.19) between pregnant and non-pregnant seals (table 1). Previous studies using linear mixed models have found no effects of pregnancy status on the dive duration of elephant seals in the weeks before or after their haul-outs [15]. Using the same approach, we found no differences in dive duration (t = 0.64, p = 0.52), or diving depths (t = 0.45, p = 0.65) between pregnant and non-pregnant seals. However, when we used GAMMs, we found that dive duration does change across the trip, and it does so in a nonlinear manner. Our model explained 67.8% of the deviance and revealed a significant relationship between DDur and DoT (F = 631.4, p ≪ 0.01), and a significant interaction between DoT : fPreg, resulting in significantly different smoothers for pregnant and non-pregnant animals (F = 67.2, p ≪ 0.01, figure 2).
Figure 2.
Predicted variation in dive duration along the gestational foraging trip of northern elephant seals. The shaded areas represent the 95% confidence intervals and dots represent daily geometric means. (a) Predicted dive duration for pregnant females (blue, n = 155, ndives = 1 909 136) and non-pregnant females (red, n = 17, ndives = 207 716). (b) Differences in trends between pregnant and non-pregnant females (pregnant – non-pregnant).
Prior to day 120 (week 17), dive duration for both groups increased and pregnant animals dived for approximately 100 s longer than non-pregnant animals. After day 120, dive durations of pregnant seals decreased, while those of non-pregnant seals continued to increase; by day 204, non-pregnant females dived 400 s longer (figure 2). Previous research has shown that dive duration increases with trip duration, which has been related to a concurrent increase in mass and blood volume [15,21]. While this increase in dive duration had previously been linked to pregnancy [21], another study suggested that pregnancy status was not associated with the increased dive duration along a foraging trip [15]. Our results demonstrate that a nonlinear approach is required to fully characterize how dive duration changes across the period of gestation, and that pregnancy status does affect how this critical foraging parameter changes with time.
Although the impacts of gestation on O2 stores in freely diving animals are still unknown, we expect that pregnancy will affect the diving ability. We suggest that the initial increase in blood volume and mass of the mother during the early stages of the pregnancy increases body O2 stores. However, this trend reverses in the second half of the gestation, probably owing to increased fetal O2 demand. Humans have a gestation length similar to elephant seals and experience a period of significant development of connections in their brain [22] (estimated to consume 46–80% of the total fetal oxygen supply in developing humans [23]) and exponential increase in overall growth around weeks 23–27 of gestation [22,24], coinciding with the rapid decline in dive duration that we see in our data after day 150 (figure 2).
Although it is well documented that pinnipeds undergo embryonic diapause [25], it is still undetermined when the fertilized egg implants. As elephant seals gestate during their post-moult trip, pregnancy can only be confirmed when animals return to land, and it is uncertain whether females that did not have a pup were pregnant and aborted, or were never pregnant. Current biologging technology, however, allows us to obtain daily dive duration from wild-ranging marine animals via satellite [26], information that has the potential to be useful to discriminate pregnancy status of adult females.
We demonstrated that pregnancy potentially poses a cost for adult female elephant seals as it affects their diving capacity (duration), limiting the time that they can spend foraging underwater during the last weeks of their pregnancy. The reasons for this reduction in dive duration remain unknown, but we hypothesize that an increased fetal O2 demand could be the cause, as oxygen-demanding tissues (i.e. brain) develop and the fetus experiences exponential growth during the second half of pregnancy. If this pattern holds for other marine mammals, it could be possible to assess reproductive state in free-ranging individuals from other populations and species, as well as explore the behavioural and environmental factors that contribute to the success of a pregnancy.
Supplementary Material
Acknowledgements
We thank the many people that have worked in the field as part of the Costa Lab, particularly C. Kuhn, P. Robinson, S. Simmons, J. Hassrick, M. Fowler and S. Peterson. We also thank Año Nuevo State Park (San Mateo, California) for their support.
Ethics
Animal procedures were authorized under the National Marine Fisheries Services permits nos 786-1463, 87-143, 14636 and 19108. All protocols were approved by the IACUC, University of California Santa Cruz.
Data accessibility
Data available from Dryad (http://dx.doi.org/10.5061/dryad.k58fr) [27].
Authors' contributions
L.A.H. conceived the study. L.A.H., R.R.H. and M.S.T. analysed the data and wrote the manuscript. D.P.C. obtained funding and led data collection. All authors read and approved the final manuscript and agree to be held accountable for the content therein.
Competing interests
The authors declare no competing interests.
Funding
Support was provided by the US Office of Naval Research (ONR) through grant nos N00014-00-1-0880, N00014-03-1-0651, N00014-08-1-1195 and N00014-10-1-0356, the National Ocean Partnership Program N00014-02-1-1012, the E & P Sound and Marine Life Joint Industry Project of the IAGOP no. JIP 22 07-23, the Alfred P. Sloan and David and Lucile Packard Foundation and the Gordon and Betty Moore Foundation.
References
- 1.Williams GC. 1966. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690. ( 10.1086/282461) [DOI] [Google Scholar]
- 2.Stearns SC. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268. ( 10.2307/2389364) [DOI] [Google Scholar]
- 3.Miller KE, Bales KL, Ramos JH, Dietz JM. 2006. Energy intake, energy expenditure, and reproductive costs of female wild golden lion tamarins (Leontopithecus rosalia). Am. J. Primatol. 68, 1037–1053. ( 10.1002/ajp.20306) [DOI] [PubMed] [Google Scholar]
- 4.Oftedal OT. 1997. Lactation in whales and dolphins: evidence of divergence between baleen- and toothed-species. J. Mammary Gland Biol. Neoplasia 2, 205–230. ( 10.1023/a:1026328203526) [DOI] [PubMed] [Google Scholar]
- 5.Speakman JR. 2008. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B 363, 375–398. ( 10.1098/rstb.2007.2145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noren SR, Redfern JV, Edwards EF. 2011. Pregnancy is a drag: hydrodynamics, kinematics and performance in pre- and post-parturition bottlenose dolphins (Tursiops truncatus). J. Exp. Biol. 214, 4151–4159. ( 10.1242/jeb.059121) [DOI] [PubMed] [Google Scholar]
- 7.Kooyman G, Ponganis P. 1998. The physiological basis of diving to depth: birds and mammals. Annu. Rev. Physiol. 60, 19–32. ( 10.1146/annurev.physiol.60.1.19) [DOI] [PubMed] [Google Scholar]
- 8.Hytten F. 1986. Blood volume changes in normal pregnancy. Obstet. Gynecol. Surv. 41, 426–428. ( 10.1097/00006254-198607000-00001) [DOI] [Google Scholar]
- 9.Prince H. 1982. Blood volume in the pregnant rabbit. Q. J. Exp. Physiol. 67, 87–95. ( 10.1113/expphysiol.1982.sp002628) [DOI] [PubMed] [Google Scholar]
- 10.Lavigne D, Innes S, Worthy G, Kovacs K, Schmitz O, Hickie J. 1986. Metabolic rates of seals and whales. Can. J. Zool. 64, 279–284. ( 10.1139/z86-047) [DOI] [Google Scholar]
- 11.Thompson SD, Nicoll ME. 1986. Basal metabolic rate and energetics of reproduction in therian mammals. Nature 321, 690–693. ( 10.1038/321690a0) [DOI] [PubMed] [Google Scholar]
- 12.Qvist J, Weber RE, Zapol WM. 1981. Oxygen equilibrium properties of blood and hemoglobin of fetal and adult Weddell seals. J. Appl. Physiol. 50, 999–1005. ( 10.1152/jappl.1981.50.5.999) [DOI] [PubMed] [Google Scholar]
- 13.Robinson PW, et al. 2012. Foraging behavior and success of a mesopelagic predator in the northeast Pacific Ocean: insights from a data-rich species, the northern elephant seal. PLoS ONE 7, e36728 ( 10.1371/journal.pone.0036728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hindell MA, et al. 2016. Circumpolar habitat use in the southern elephant seal: implications for foraging success and population trajectories. Ecosphere 7, e01213 ( 10.1002/ecs2.1213) [DOI] [Google Scholar]
- 15.Hassrick J, Crocker DE, Teutschel N, McDonald B, Robinson P, Simmons S, Costa D. 2010. Condition and mass impact oxygen stores and dive duration in adult female northern elephant seals. J. Exp. Biol. 213, 585–592. ( 10.1242/jeb.037168) [DOI] [PubMed] [Google Scholar]
- 16.Bennett KA, McConnell B, Fedak MA. 2001. Diurnal and seasonal variations in the duration and depth of the longest dives in southern elephant seals (Mirounga leonina): possible physiological and behavioural constraints. J. Exp. Biol. 204, 649–662. [DOI] [PubMed] [Google Scholar]
- 17.Wilmers CC, Nickel B, Bryce CM, Smith JA, Wheat RE, Yovovich V. 2015. The golden age of bio-logging: how animal-borne sensors are advancing the frontiers of ecology. Ecology 96, 1741–1753. ( 10.1890/14-1401.1) [DOI] [PubMed] [Google Scholar]
- 18.Luque SP. 2007. diveMove: diving behaviour analysis in R. R News 7, 8–14. [Google Scholar]
- 19.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- 20.Wood SN. 2004. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 99, 673–686. ( 10.1198/016214504000000980) [DOI] [Google Scholar]
- 21.Le Boeuf BJ. 1994. Variation in the diving pattern of northern elephant seals with age, mass, sex, and reproductive condition. In Elephant seals: population ecology, behavior, and physiology (eds Le Boeuf BJ, Laws RM), pp. 237–252. Berkeley, CA: University of California Press. [Google Scholar]
- 22.Jakab A, Schwartz E, Kasprian G, Gruber GM, Prayer D, Schöpf V, Langs G. 2014. Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Front. Hum. Neurosci. 8, 852 ( 10.3389/fnhum.2014.00852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonds DR, Mwape B, Kumar S, Gabbe SG. 1984. Human fetal weight and placental weight growth curves. Neonatology 45, 261–274. ( 10.1159/000242016) [DOI] [PubMed] [Google Scholar]
- 24.Passingham RE. 1985. Rates of brain development in mammals including man. Brain Behav. Evol. 26, 167–175. ( 10.1159/000118773) [DOI] [PubMed] [Google Scholar]
- 25.Boyd IL. 1991. Environmental and physiological factors controlling the reproductive-cycles of pinnipeds. Can. J. Zool. 69, 1135–1148. ( 10.1139/Z91-162) [DOI] [Google Scholar]
- 26.Fedak MA, Lovell P, Grant SM. 2001. Two approaches to compressing and interpreting time-depth information as collected by time-depth recorders and satellite-linked data recorders. Mar. Mammal Sci. 17, 94–110. ( 10.1111/j.1748-7692.2001.tb00982.x) [DOI] [Google Scholar]
- 27.Hückstädt LA, Holser RR, Tift MS, Costa DP. 2018. Data from: The extra burden of motherhood: reduced dive duration associated with pregnancy status in a deep-diving mammal, the northern elephant seal Dryad Data Repository. ( 10.5061/dryad.k58fr) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hückstädt LA, Holser RR, Tift MS, Costa DP. 2018. Data from: The extra burden of motherhood: reduced dive duration associated with pregnancy status in a deep-diving mammal, the northern elephant seal Dryad Data Repository. ( 10.5061/dryad.k58fr) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from Dryad (http://dx.doi.org/10.5061/dryad.k58fr) [27].