Abstract
Research on animal personality explains the coexistence of distinct behavioural phenotypes within a species and demonstrates limits to individual plasticity. However, the mechanisms guiding the lifelong development of personality should receive more attention, because many elements of personality are emergent properties of interactions between the environment and an individual's genetic background. In these interactions, mechanisms (e.g. genetic regulatory networks, epigenetic processes and neuroendocrine regulation) influencing personality may be modified. An approach integrating proximate mechanisms with a view of lifelong personality development will crucially improve understanding stability, plasticity and inter-individual variability of personalities and clarify the effects of selection on the phenomenon.
Keywords: ontogeny, evo-devo, plasticity, regulatory systems, sensitive windows
1. Introduction
Empirical and theoretical research on animal personalities, i.e. individuals showing consistently different behaviours across contexts and time, has taken up the challenge to explain the coexistence of different behavioural phenotypes within one population and demonstrated limits to individual plasticity [1]. Nevertheless, apparently stable (i.e. repeatable [2]) personality traits and correlations among these traits were shown to change over ontogeny in response to shifting environmental conditions [3,4]. Such plasticity is often particularly high during periods called ‘sensitive windows’ [5]. This raises the question, how the relative stability of individually different personalities can be reconciled with ontogenetic changes in response to experience.
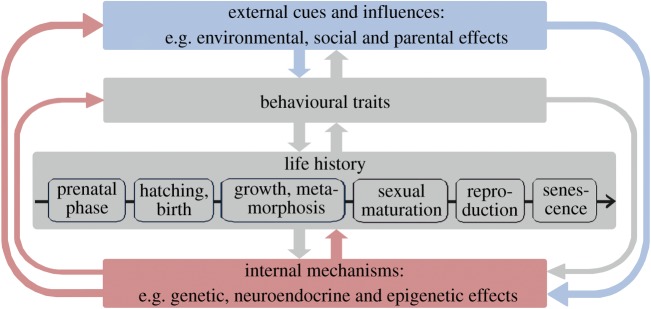
Molecular studies revealed phylogenetically old mechanisms providing plasticity in the development of complex morphological and physiological traits [6,7]. Such mechanisms are available and influential in a wide taxonomic range of species. Behavioural characters appear to be even more plastic than morphological characters. Thus, principles of evolutionary developmental biology (evo-devo), i.e. use of the same genetic machinery to regulate the production of different phenotypic traits at different stages in ontogeny, may fruitfully be integrated into personality research [8,9]. We urge researchers to investigate the early development of personality traits and trait changes across the lifetime from the perspective of ontogenetic mechanisms. We highlight the potential for changes and discuss some of the available evidence suggesting that epigenetic and neuroendocrine mechanisms in interaction with the environment may enable lifelong plasticity (figure 1). Influenced by unique, sometimes chance events, such mechanisms lead to the development of relatively stable personality traits that may differ among individuals even with the same genetic background [10,11]. Developmental history needs to be considered to fully understand emerging personality traits and modifications of underlying mechanisms. Ultimately, these mechanisms and their plasticity rather than the traits may respond to selection.
Figure 1.
During development, internal and external cues interact with the response and regulation of internal mechanisms (red) that determine the state and the development of individuals throughout life. In turn, the individual's state and behaviour (grey) influence its environment and how external cues (blue) are sensed and evaluated.
The development of sex shows that stable individual differences can ontogenetically arise in interaction with the environment even with an identical genetic background. In reptiles and fish, sex determination is modulated from strict genetic to environmental, thermal determination [9,11]. In Drosophila, selection for sex ratio can lead to changes in the sex-determining mechanism, while the sex ratio remains unaltered [12] demonstrating that underlying mechanisms rather than traits may change under selection. This suggests that investigating personality within the framework of developmental mechanisms will provide novel insights in its origin, stability, inter-individual variability and potential lifetime modulation.
2. The relationship between personality traits and underlying mechanisms
Theoretical models demonstrated that mechanisms underlying alternative behavioural traits in evolutionary games influence the course of evolution and the equilibrium frequencies of strategies, for example, in the extent of cooperation in the iterated Prisoner's dilemma a game that shows why two ‘rational’ individuals might not cooperate even if that would benefit them. Unexpectedly, the extent of cooperation (i.e. behavioural phenotype frequency) that evolves as well as the dynamics of evolution vary substantially depending on the assumed mechanism [13].
Empirically, evo-devo has provided many examples where the same mechanism produces distinct outcomes depending on environmental conditions [6,9]. Similarly, different, temporally stable personality structures may emerge on the same genetic background from identical mechanisms when environments and the resulting states of the organisms differ [6,9]. A bottom-up approach investigating mechanisms that influence stability and plasticity of multiple behavioural traits across an individual's life history is therefore expected to prove fruitful.
Personality traits may change, because mechanisms induce behavioural shifts during ontogeny in response to the environment [3]. For example, much of post-embryonic development is coordinated by hormones produced and controlled by the nervous system. This regulatory network may be reprogrammed through changes in gene expression mediated by neuroendocrine feedback loops that induce alterations in hormone signalling in response to environmental and social cues [14–16]. Neuroendocrine mechanisms permit high plasticity in the development of behavioural phenotypes [16,17], as explained further below. Candidate mechanisms acting individually or in combination are the development of the hypothalamic–pituitary–adrenal (HPA) and hypothalamic–pituitary–gonadalaxis, neural self-organization processes and epigenetic mechanisms [16–21].
When the state of individuals changes rapidly, e.g. during dispersal, around maturation [15] or metamorphosis [4,22], modifications are particularly likely. During such sensitive windows, certain traits may stay consistent while others show major modifications [15,23]. Thus, ontogeny offers substantial opportunities to modify traits [14,18,23] according to relevant updating information, i.e. environmental [5] or internal state-related cues. Indeed, morphological, behavioural and physiological traits can change remarkably fast as shown in a lek breeding fish. After winning or losing a fight the individual's state shifts, followed within minutes by shifts in gene activation and behaviour based on a potent neuroendocrine mechanism [14]. Given the potential for changes of personality traits throughout life it is quite puzzling that personality displays substantial within-individual stability.
3. Mechanisms inducing a change in state
Mechanisms that change personality during ontogeny may be regulated by gene action. They also interact with each other and the environment, feeding back on the environment (e.g. niche choice) and on gene regulation (figure 1). The following examples highlight mechanism diversity and suggest that their properties and plasticity might evolve rapidly [24] in response to selection that targets personality traits.
(a). Epigenetic modification
Epigenetic variation influencing phenotypic traits is co-shaped by genes and environments, adding further complexity to gene × environment interactions. Differences in epigenetic programming in response to maternal behaviour may emerge early in life and remain stable in adults, leading to long-term consequences on personality traits and fitness [19]. Among other mechanisms, DNA methylation and histone acetylation reversibly mediate diversification of genome function in response to ‘experience’ [11]. Trauma, ageing, social interactions or maternal effects acting through modifications of the offspring's HPA axis [16,20] constitute such experiences that may (adaptively) modify personality traits. Such modifications may even be transmitted through the germ line to the next generation [20,21]. Thus, epigenetic variation may provide raw material for phenotypic selection when genetic variation proves limited [11,25].
(b). Neuroendocrine cascades
Early social influences can substantially alter the response to stressors in later life. Such developmental plasticity of the brain depends on social influences beginning during intra-uterine life. Effects may be mediated through elevated maternal plasma glucocorticoid levels influencing glucocorticoid receptor density in the embryonic amygdala or the timing and level of testosterone surges in male fetuses. After birth, the mother–offspring relationship further influences neuroendocrine interactions that may modify DNA methylation and gene expression [15,26]. These influences play out against a variable genetic background that is sensitive to environmental influences and may lead to different behavioural phenotypes. Major re-programming is also possible during maturation, when frequency and intensity of social interactions can modulate testosterone secretion and, in turn, the cortisol response controlling aggressive responses to competitors [15]. Such endocrine effects across ontogeny may adaptively shape differences in adult personality [16,18].
(c). Personality expression across metamorphosis
Insects and amphibians pass through a reorganization of morphology and physiology during metamorphosis. Surprisingly, few studies examined the repeatability of personality traits across metamorphosis [4,22], revealing species-, trait- and sex-specific effects and providing mixed evidence for intra-individual consistency. For an impact of juvenile experience on adult behaviour, the persistence of information through metamorphosis, e.g. via neurons generated in the larva, is a prerequisite [27]. Again, hormonal regulation may be involved, because metamorphosis is mediated by juvenile (insects) or thyroid hormones (amphibians), which play important roles at all life stages. Furthermore, impacts of distinct epigenetic processes in juveniles versus adults may be decisive components in shaping behavioural repeatability over ontogeny and across generations [21].
4. What is evolving: traits or underlying mechanisms?
The above examples highlight that what is called the evolution of personality may be better understood in terms of the evolution of the regulation of underlying mechanisms (gene expression and otherwise) that lead to personality trait development. Indeed, hundreds of genes can differ in expression in distinct environments [11,28]. For many traits, basic neuronal and neuroendocrine circuits are common to most vertebrates [17] and invertebrates [29]. They can be similarly recruited across species for developmental regulation in interaction with both extrinsic and intrinsic events. Investigating endocrine and other molecular mechanisms with wide-ranging effects should explain stability and plasticity of resulting different personalities in a general framework.
5. Conclusion
Mechanistically, the observed heritability of correlated behavioural traits may restrict the regulation of developmental feedback loops that lead to trait expression in the adult. If so, we need to place more emphasis on studying the evolution of all kinds of mechanisms underlying this heritability. Knowing the evo-devo mechanisms behind stability and plasticity will improve our understanding of personality more than studying the evolution of traits in isolation or the personality of adults as the endpoint of development. As also suggested by theory [13] the latter may be misleading, because it treats traits as the evolving units, which may be inadequate, if traits are emergent properties of underlying mechanisms. The physiological mechanisms are under selection to produce reaction norms that allow flexible adaptation to highly dynamic environments [16,30]. Therefore, we suggest focusing particularly on developmental mechanisms and periods of plasticity that enable adjustment to different types of ecological challenges. Environmental variability or shifts may, through selection on the phenotype, lead to evolution by modifying the original mechanisms' sensitivity to internal and external cues, establishing an optimal balance of traits and continuing to refine the mechanisms’ adaptive plasticity.
Acknowledgements
We thank N. Sachser, three reviewers and the editor for constructive criticism.
Data accessibility
This article has no additional data.
Authors' contributions
F.T. conceived the idea and wrote the first draft, all authors contributed ideas and cooperated in the write-up.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the Deutsche Forschungsgemeinschaft (FOR 1232).
References
- 1.Dingemanse NJ, Wolf M. 2013. Between-individual differences in behavioural plasticity within populations: causes and consequences. Anim. Behav. 85, 1031–1039. ( 10.1016/j.anbehav.2012.12.032) [DOI] [Google Scholar]
- 2.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. [DOI] [PubMed] [Google Scholar]
- 3.Stamps J, Groothuis TGG. 2010. The development of animal personality: relevance, concepts and perspectives. Biol. Rev. Camb. Phil. Soc. 85, 301–325. ( 10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- 4.Müller T, Müller C. 2015. Behavioural phenotypes over the lifetime of a holometabolous insect. Front. Zool. 12(Suppl. 1), S8 ( 10.1186/1742-9994-12-S1-S8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawcett TW, Frankenhuis WE. 2015. Adaptive explanations for sensitive windows in development. Front. Zool. 12(Suppl. 1), S3 ( 10.1186/1742-9994-12-S1-S3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nijhout HF. 2015. A developmental–physiological perspective on the development and evolution of phenotypic plasticity. In Conceptual change in biology (ed. Love AC.), pp. 147–173. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 7.Wagner A. 2008. Robustness and evolvability: a paradox resolved. Proc. R. Soc. B 275, 91–100. ( 10.1098/rspb.2007.1137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duckworth RA. 2015. Neuroendocrine mechanisms underlying behavioral stability: implications for the evolutionary origin of personality. Ann. NY Acad. Sci. 1360, 54–74. ( 10.1111/nyas.12797) [DOI] [PubMed] [Google Scholar]
- 9.Crews D, Weisberg SA, Sarkar S. 2015. Hazards inherent in interdisciplinary behavioral research. Front. Zool. 12(Suppl. 1), S21 ( 10.1186/1742-9994-12-S1-S21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bierbach D, Laskowski KL, Wolf M. 2017. Behavioural individuality in clonal fish arises despite near-identical rearing conditions. Nat. Comm. 8, 15361 ( 10.1038/ncomms15361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J, Barrett RDH. 2017. Epigenetics in natural animal populations. J. Evol. Biol. 30, 1612–1632. ( 10.1111/jeb.13130) [DOI] [PubMed] [Google Scholar]
- 12.Kozielska M, Pen I, Beukeboom LW, Weissing FJ. 2006. Sex ratio selection and multi-factorial sex determination in the housefly: a dynamic model. J. Evol. Biol. 19, 879–888. ( 10.1111/j.1420-9101.2005.01040.x) [DOI] [PubMed] [Google Scholar]
- 13.van den Berg P, Weissing FJ. 2016. The importance of mechanisms for the evolution of cooperation. Proc. R. Soc. B 282, 20151382 ( 10.1098/rspb.2015.1382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernald RD. 2015. Social behaviour: can it change the brain? Anim. Behav. 103, 259–265. ( 10.1016/j.anbehav.2015.01.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachser N, Kaiser S, Hennessy MB. 2013. Behavioural profiles are shaped by social experience: when, how and why. Phil. Trans. R. Soc. B 368, 20120344 ( 10.1098/rstb.2012.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seebacher F, Krause J (eds). 2017. Physiological determinants of social behaviour in animals. Phil. Trans. R. Soc. B 372, 20160231 ( 10.1098/rstb.2016.0231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann HA, et al. 2014. An evolutionary framework for studying mechanisms of social behavior. Trends Ecol. Evol. 29, 581–589. ( 10.1016/j.tree.2014.07.008) [DOI] [PubMed] [Google Scholar]
- 18.Schoech SJ, Rensel MA, Heiss RS. 2011. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr. Zool. 57, 514–530. ( 10.1093/czoolo/57.4.514) [DOI] [Google Scholar]
- 19.Zhang X, Ho S-M. 2011. Epigenetics meets endocrinology. J. Mol. Endocrinol. 46, R11–R32. ( 10.1677/JME-10-0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohacek J, Gapp K, Saab BJ, Mansuy IM.. 2013. Transgenerational epigenetic effects on brain functions. Biol. Psychiatry 73, 313–320. ( 10.1016/j.biopsych.2012.08.019) [DOI] [PubMed] [Google Scholar]
- 21.Hales NR, Schield DR, Andrew AL, Card DC, Walsh MR, Castoe TA. 2017. Contrasting gene expression programs correspond with predator-induced phenotypic plasticity within and across generations in Daphnia. Mol. Ecol. 26, 5003–5015. ( 10.1111/mec.14213) [DOI] [PubMed] [Google Scholar]
- 22.Wilson ADM, Krause J. 2012. Personality and metamorphosis: is behavioral variation consistent across ontogenetic niche shifts? Behav. Ecol. 23, 1316–1323. ( 10.1093/beheco/ars123) [DOI] [Google Scholar]
- 23.Bell AM, Sih A. 2007. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834. ( 10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- 24.Hairston NG Jr, Ellner SP, Geber MA, Yoshida T, Fox JA.. 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127. ( 10.1111/j.1461-0248.2005.00812x) [DOI] [Google Scholar]
- 25.Kronholm I, Collins S. 2016. Epigenetic mutations can both help and hinder adaptive evolution. Mol. Ecol. 25, 1856–1868. ( 10.1111/mec.13296) [DOI] [PubMed] [Google Scholar]
- 26.Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, Meaney MJ. 2008. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J. Neuroendocrinol. 20, 795–801. ( 10.1111/j.1365-2826.2008.01725.x) [DOI] [PubMed] [Google Scholar]
- 27.Blackiston DJ, Casey ES, Weiss MR. 2008. Retention of memory through metamorphosis: can a moth remember what it learned as a caterpillar? PLoS ONE 3, e1736 ( 10.1371/journal.pone.0001736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snell-Rood EC, van Dyken JD, Cruickshank T, Wade MJ, Moczek AP. 2010. Toward a population genetic framework of developmental evolution: the costs, limits, and consequences of phenotypic plasticity. Bioessays 32, 71–81. ( 10.1002/bies.200900132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartenstein V. 2007. The neuroendocrine system of invertebrates: a developmental and evolutionary perspective. J. Endocrinol. 190, 555–570. ( 10.1677/joe.1.06964) [DOI] [PubMed] [Google Scholar]
- 30.Levis NA, Pfennig DW. 2016. Evaluating ‘plasticity-first’ evolution in nature: key criteria and empirical approaches. Trends Ecol. Evol. 31, 563–574. ( 10.1016/j.tree.2016.03.012) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.