Abstract
Organisms use a variety of environmental cues to orient their movements in three-dimensional space. Here, we show that the upward movement of young Chinook salmon (Oncorhynchus tshawytscha) emerging from gravel nests is influenced by the geomagnetic field. Fish in the ambient geomagnetic field travelled farther upwards through substrate than did fish tested in a field with the vertical component inverted. This suggests that the magnetic field is one of several factors that influences emergence from the gravel, possibly by serving as an orientation cue that helps fish determine which way is up. Moreover, our work indicates that the Oncorhynchus species are sensitive to the magnetic field throughout their life cycles, and that it guides their movements across a range of spatial scales and habitats.
Keywords: magnetoreception, orientation, compass, Oncorhynchus tshawytscha
1. Introduction
The geomagnetic field has been a ubiquitous feature of Earth since life evolved [1] and organisms ranging from bacteria to mammals use it to orient [2]. Animals can use the geomagnetic field as a ‘compass’ to set and maintain a heading, as a ‘map’ to assess location, or both [2,3]. In addition to facilitating orientation in the horizontal plane, the geomagnetic field has also been implicated in some vertical movements. For example, certain anaerobic bacteria swim along magnetic field lines to move downward into mud where oxygen is lower [4], and nematode worms (Caenorhabditis elegans) exploit magnetic information to guide upwards and downwards movements [5]. Similar tendencies might be adaptive for many species that undertake vertical movements in environments where sources of directional information are limited. Here, we examine the influence of the magnetic field on vertical movements of young Chinook salmon (Oncorhynchus tshawytscha) emerging from gravel nests.
Adult female salmonid fishes bury their eggs after fertilization within gravel beds in streams and lake habitats [6–9]. After hatching, the embryos (termed alevins) initially move downward in the gravel and develop there [6,7]. When their residual yolk stores become depleted, the young fish migrate upwards, emerge from the gravel and subsequently live above the substrate [10–12]. The process of emergence from the gravel appears to be influenced by many factors. As yolk is reduced and the digestive tract develops, there is a period when the fish can emerge if environmental conditions (e.g. low oxygen) stress them, so some young salmon emerge with yolk showing [8]. Pre-emergent salmon move towards areas with higher dissolved oxygen; they also orient to water flow and avoid light [6–8,10–12]. While in the gravel, the alevins may move little, or quite a lot [6,8,11]. Prior to emergence, negative phototaxis helps them remain in the gravel. As the time to emerge approaches, they become more tolerant of light, though they tend to emerge from gravel at night [7,8]. In the absence of light and water currents, fish still move upwards and out of the substrate, but the basis of this orientation has not been determined [7,11,12].
One possibility is that fish moving vertically out of gravel nests derive some directional information from Earth's magnetic field. In principle, swimming against field lines in the northern hemisphere would lead fish out of the gravel and into the water column, whereas swimming with field lines would lead them deeper into the substrate (figure 1). To investigate this hypothesis, fish were placed at the bottom of tubes filled with glass marbles and exposed to one of three conditions: (i) the ambient magnetic field (field lines directed downwards), to provide a baseline of salmon movement, (ii) an ‘intensified’ magnetic field (field lines directed downwards), to serve as a control for a sudden change in electromagnetic conditions, and (iii) an ‘inverted vertical’ field (field lines directed upwards) to test whether reversing the vertical component of the magnetic field alters the upward movement of fish (figure 1). If fish use the direction of magnetic field lines to orient their vertical movement, we predicted that those in the inverted vertical field would not swim as far up the tube as those in the ambient field, but there would be no difference between the ambient and intensified fields.
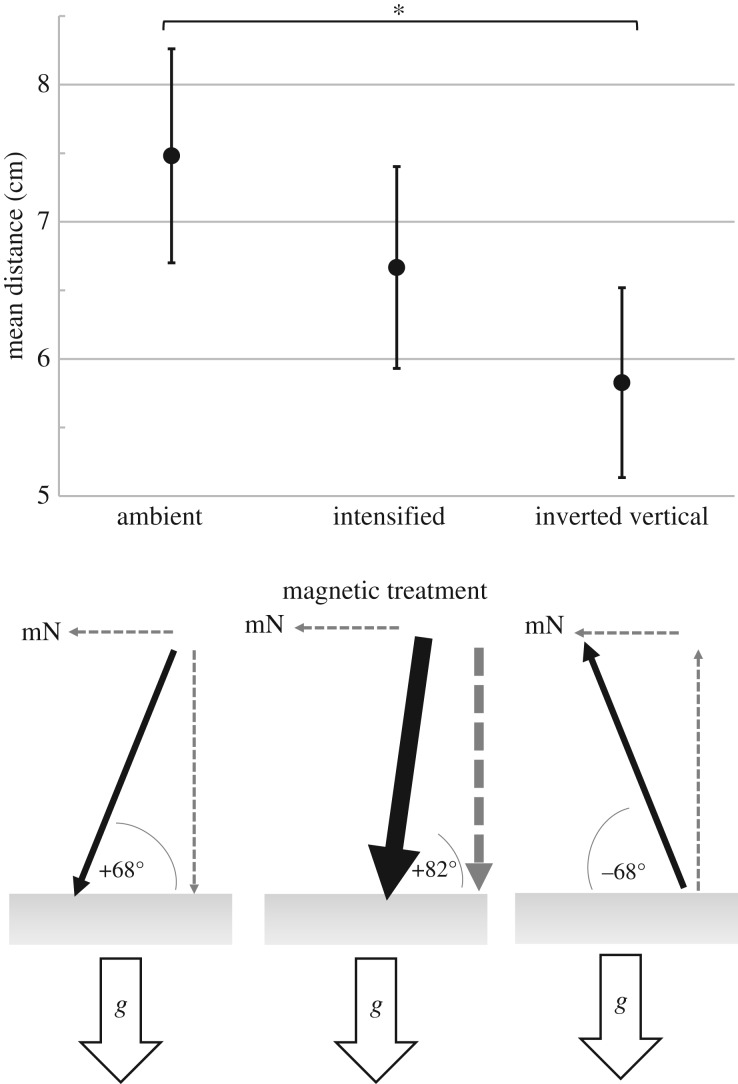
Figure 1.
Mean height of fish movement (y-axis) under different magnetic conditions. Error bars indicate the 95% confidence interval of the mean. Significant differences between treatments are indicated by an asterisk (Mann–Whitney U-test, U = 29871, p = 0.0036). Beneath the graph is a schematic of the magnetic conditions experienced by fish. The geomagnetic vector is shown by a black arrow; the horizontal and vertical components of the field are grey dashed arrows (arrow thickness shows relative intensity). Magnetic north is designated by ‘mN’. The gravity vector is shown as a white arrow, ‘g’.
2. Material and methods
Experiments were performed from 29 March through 1 April 2016 at the Oregon Hatchery Research Center (44.40° N, 123.75° W) using Chinook salmon from Oregon, USA. The fish were incubated in fibreglass hatching trays. After absorption of the yolk sac, they were moved into a shallow trough (table 1). Testing occurred after full yolk absorption, prior to first exogenous feeding.
Table 1.
Magnetic conditions experienced by young Chinook salmon. Field values represent measurements made using a tri-axial magnetometer (Applied Physics, 520A) within rearing areas and experimental treatments. Values indicate the mean (and range) of fields that the group of fish experienced, not necessarily each individual.
| location | inclination angle (°) | total field intensity (μT) | electrical current (Amp) |
|---|---|---|---|
| hatching trays | +65° (+63 to +67°) | 50.6 (49.3–52.1) | NA |
| rearing trough | +65° (+64 to +66°) | 49.7 (49.4–50.0) | NA |
| ambient experimental treatment | +68° (+64 to +71°) | 49.9 (49.4–50.5) | 0 |
| intensified experimental treatment | +82° (+82 to +83°) | 138.8 (135.4–141.3) | +1.98 |
| inverted vertical experimental treatment | −68° (−66 to −71°) | 49.8 (49.3–50.1) | −1.98 |
The testing apparatus consisted of a clear aquarium (51.4 cm × 26.7 cm × 31.8 cm) containing 27 wells (height = 3.8 cm, diameter = 3.8 cm) constructed from clear, plastic fluorescent light bulb covers glued to the surface of mesh matting. Tubes of the same material were cut to 22.9 cm in length and fit into the wells vertically. Clear glass marbles (1.9 cm in diameter) were used to simulate gravel. Each tube and well were filled with marbles to a height of 20.3 cm. The aquarium was placed within a coil system consisting of four square coils (each 100 cm on a side) constructed in accordance with Merritt et al. [13] and arranged so that the magnetic field produced by the coil could be directed up or down. Wires from the coil were attached to a DC power supply so that current through the wires could be varied, allowing control of the vertical component of the magnetic field. The entire apparatus was draped in black plastic to minimize light exposure. For each trial, tanks were filled with water from nearby Fall Creek until each well was half-full. While in the ambient magnetic field, one fish was placed in each well and a marble-filled tube was fit over the well. After fish were in place, tanks were filled with water up to the 22.9 cm marks on the tubes (electronic supplementary material, figures S1–S3).
Fish were then exposed to one of three treatments: (i) the ambient field, (ii) an intensified field or (iii) a field with the vertical component inverted (table 1). After 30 min, an observer entered the enclosure and recorded the vertical position of each fish, using marks on the outside of the tubes (2.5 cm spacing) to score the position (electronic supplementary material, figure S3). Each fish was tested once. The tanks were drained and refilled for subsequent trials. Tests were conducted between 04:30 and 12:00 h, alternating the three treatments to control for diurnal effects on motivation to swim upwards. Non-parametric tests were used to determine if there was a significant difference (p < 0.05) in the height fish moved among and between treatments.
3. Results
The mean height fish moved upwards in the ambient field was 7.5 cm (95% confidence interval ± 0.78 cm, n = 267). Those in the intensified field moved up 6.7 cm (95% confidence interval ± 0.74 cm, n = 267). Fish in the inverted vertical field moved up 5.8 cm (95% confidence interval ± 0.69 cm, n = 263). A difference in upwards movement existed among the three treatments (Kruskal–Wallis test H = 8.58, p = 0.014, d.f. = 2). In pairwise tests, a difference in movement was detected between the ambient and inverted vertical fields (Mann–Whitney U-test, U = 29871, p = 0.0036, d.f. = 1). No difference in movement was detected between the ambient and intensified fields (Mann–Whitney U-test, U = 33022, p = 0.1615, d.f. = 1) (figure 1).
4. Discussion
The results demonstrate that Chinook salmon are sensitive to the magnetic field during their emergence from gravel. Fish in a magnetic field with an inverted vertical component did not move as far upwards as fish tested in the ambient field. By contrast, exposure to an intensified downward magnetic field had no detectable effect on this behaviour (figure 1). Thus, inverting the vertical component of the field affected the upward movement of alevins, consistent with the hypothesis that salmon use the direction of field lines to orient vertically.
Nonetheless, fish moved upwards in all treatments, including the ‘inverted vertical’ field. Several factors may explain this occurrence. Like orientation in the horizontal plane [2,3], magnetic information is likely one of several complementary or redundant sensory inputs used to direct vertical movement. In this experiment, other potential cues for guiding upward movement remained available (e.g. the direction of the gravity vector and, perhaps, gradients in odours associated with the surface). Second, by placing fish at the bottom of the plastic tubes, downward movement could not occur, and even random activity (e.g. equal amounts of downward and upward orientation) presumably resulted in net upward movement. Third, it is possible that rather than using the magnetic field as an orientation cue, fish were startled by a sudden change in the magnetic field, causing them to move less—and this effect was less pronounced in a stronger magnetic intensity than when the vertical component of the field was inverted.
Irrespective of these considerations, our work indicates that the Oncorhynchus species uses Earth's magnetic field throughout their life cycles, across a wide range of spatial scales, and for a variety of navigational tasks [14–18]. Interestingly, the magnetic field is one of the few common features shared across the diverse and expansive environments that salmon and trout occupy (gravel beds, streams, lakes, rivers, estuaries, coastal waters and the open sea) and may, therefore, be particularly useful as a spatial reference system. Finally, our study contributes to the growing literature that suggests vertical movements of burrowing, flying and swimming organisms are influenced by magnetic cues [4,5,19,20]. Further exploration of organisms' use of the magnetic field to orient in three-dimensional space is warranted.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Alex Powell for fabricating testing arenas.
Ethics
Experiments were conducted in accordance with Oregon State University Animal Care Use Protocol no. 4394.
Data accessibility
Data are available in the electronic supplementary material.
Authors' contributions
Conception by T.P.Q. and D.L.G.N.; experimental design by N.F.P., M.M.S., A.M.P., T.P.Q., K.J.L., J.S.S., J.P.O., R.B.C. and D.L.G.N.; construction of experimental set-up by N.F.P., M.M.S., A.M.P., J.P.O., R.B.C., D.L.G.N.; data collection by M.M.S. and A.M.P.; interpretation of results by N.F.P., M.M.S., K.J.L., T.P.Q., A.M.P., J.S.S. and D.L.G.N.; initial draft by N.F.P.; editing by N.F.P., M.M.S., A.M.P., K.J.L., T.P.Q., J.S.S., J.P.O. and D.L.G.N. All authors agree to be held accountable for the content and approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Oregon Sea Grant, the Fisheries and Wildlife Department of Oregon State University, the Oregon Hatchery Research Center and the Oregon Department of Fish and Wildlife contributed funding.
References
- 1.Taraduno JA, et al. 2010. Geodynamo, solar wind, and magnetopause 3.4 to 3.45 billion years ago. Science 327, 1238–1240. ( 10.1126/science.1183445) [DOI] [PubMed] [Google Scholar]
- 2.Wiltschko R, Wiltschko W. 1995. Magnetic orientation in animals. Berlin, Germany: Springer. [Google Scholar]
- 3.Lohmann KJ, Lohmann CMF, Putman NF. 2007. Magnetic maps in animals: nature's GPS. J. Exp. Biol. 210, 3697–3705. ( 10.1242/jeb.001313) [DOI] [PubMed] [Google Scholar]
- 4.Blakemore RP. 1982. Magnetotactic bacteria. Annu. Rev. Microbiol. 36, 217–238. ( 10.1146/annurev.mi.36.100182.001245) [DOI] [PubMed] [Google Scholar]
- 5.Vidal-Gadea A, et al. 2015. Magnetosensitive neurons mediate geomagnetic orientation in Caenorhabditis elegans. Elife 4, e07493 ( 10.7554/eLife.07493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dill LM, Northcote TG. 1970. Effects of gravel size, egg depth, and egg density on intragravel movement and emergence of coho salmon (Oncorhynchus kisutch) alevins. J. Fish. Res. Board Canada 27, 1191–1199. ( 10.1139/f70-141) [DOI] [Google Scholar]
- 7.Carey WE, Noakes DLG. 1981. Development of photobehavioural responses in young rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 19, 285–296. ( 10.1111/j.1095-8649.1981.tb05832.x) [DOI] [Google Scholar]
- 8.Mason JC. 1976. Some features of coho salmon, Oncorhynchus kisutch, fry emerging from simulated redds and concurrent changes in photobehavior. Fish. Bull. 74, 167–175. [Google Scholar]
- 9.Curry RA, Noakes DLG. 1995. Groundwater and the selection of spawning sites by brook trout (Salvelinus fontinalis). Can. J. Fish. Aquat. Sci. 52, 1733–1740. ( 10.1139/f95-765) [DOI] [Google Scholar]
- 10.Nunan CP, Noakes DLG. 1985. Response of rainbow trout (Salmo gairdneri) embryos to current flow in simulated substrates. Can. J. Zool. 63, 1813–1815. ( 10.1139/z85-270) [DOI] [Google Scholar]
- 11.Bams RA. 1969. Adaptations of sockeye salmon associated with incubation in stream gravels. In Symposium on salmon and trout in streams 1969 (ed. Northcote TG.), pp. 71–87. Vancouver, Canada: H. R. MacMillan Lectures in Fisheries, University of British Columbia. [Google Scholar]
- 12.Nunan CP, Noakes DLG. 1987. Effects of light on movement of rainbow trout embryos within, and their emergence from, artificial redds. Am. Fish. Soc. Symp. 2, 151–156. [Google Scholar]
- 13.Merritt R, Purcell C, Stroink G. 1983. Uniform magnetic field produced by three, four, and five square coils. Rev. Sci. Inst. 54, 879–882. ( 10.1063/1.1137480) [DOI] [Google Scholar]
- 14.Quinn TP. 1980. Evidence for celestial and magnetic compass orientation in lake migrating sockeye salmon fry. J. Comp. Physiol. 137, 243–248. ( 10.1007/BF00657119) [DOI] [Google Scholar]
- 15.Quinn TP, Brannon EL. 1982. The use of celestial and magnetic cues by orienting sockeye salmon smolts. J. Comp. Physiol. 147, 547–552. ( 10.1007/BF00612020) [DOI] [Google Scholar]
- 16.Putman NF, Scanlan MM, Billman EJ, O'Neil J, Couture R, Quinn TP, Lohmann KJ, Noakes DLG. 2014. Inherited magnetic map guides ocean navigation in juvenile Pacific salmon. Curr. Biol. 24, 446–450. ( 10.1016/j.cub.2014.01.017) [DOI] [PubMed] [Google Scholar]
- 17.Putman NF, Lohmann KJ, Putman EM, Klimley AP, Quinn TP, Noakes DLG. 2013. Evidence for geomagnetic imprinting as a homing mechanism in Pacific salmon. Curr. Biol. 23, 312–316. ( 10.1016/j.cub.2012.12.041) [DOI] [PubMed] [Google Scholar]
- 18.Chew GL, Brown GE. 1987. Orientation of rainbow trout (Salmo gairdneri) in normal and null magnetic fields. Can. J. Zool. 67, 641–643. ( 10.1139/z89-092) [DOI] [Google Scholar]
- 19.Fedele G, Green EW, Rosato E, Kyriacou CP. 2014. An electromagnetic field disrupts negative geotaxis in Drosophila via a CRY-dependent pathway. Nat. Commun. 5, 4391 ( 10.1038/ncomms5391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae JE, Bang S, Min S, Lee SH, Kwon SH, Lee Y, Lee YH, Chung J, Chae KS. 2016. Positive geotactic behaviors induced by geomagnetic field in Drosophila. Mol. Brain 9, 55 ( 10.1186/s13041-016-0235-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the electronic supplementary material.