Abstract
Activation of the immune system is associated with an increase in the breakdown of various peripheral tissues, including bone. Despite the widely appreciated role of inflammatory mediators in promoting bone resorption, the functional value behind this process is not completely understood. Recent advances in the field of immunometabolism have highlighted the metabolic reprogramming that takes place in activated immune cells. It is now believed that the breakdown of peripheral tissue provides metabolic substrates to fuel metabolic anabolism in activated immune cells. We argue that phosphate, liberated by bone resorption, plays an indispensable role in sustaining immune cell metabolism. The liberated phosphate is then incorporated into macromolecules such as nucleotides and phospholipids, and is also used for the phosphorylation of metabolites (e.g. glycolytic intermediates). In addition, magnesium, also liberated during the breakdown of bone, is an essential cofactor required by various metabolic enzymes which are upregulated in activated immune cells. Finally, calcium activates various additional molecules involved in immune cell migration. Taken together, these factors suggest a key role for bone resorption during infection.
Keywords: bone resorption, calcium, immunometabolism, magnesium, phosphate
1. Introduction
Bone is a dynamic organ which is repeatedly subjected to processes that promote resorption, followed by the deposition of matrix proteins and bone minerals. Normally, these processes are balanced, resulting in no net loss or gain in bone mineral density. However, during an inflammatory insult, a disproportional shift towards bone resorption takes place, resulting in a net loss of bone tissue. Indeed, inflammatory mediators have a well-established role in inducing bone resorption [1–3] and low-grade chronic inflammation is known to result in a decrease in bone quality [4,5]. Mechanistically, nuclear factor kappa B (NF-κB), a classic inflammatory transcription factor, plays a central role in bone resorption: NF-κB activation has been shown to suppress osteoblast function [6], while in osteoclasts, activation of the NF-κB transcription cascade through receptor activator of NF-κB (RANK) plays a critical role in promoting osteoclast differentiation and activation [7]. Here, inflammatory mediators promote bone resorption via a number of processes. As an example, inflammatory mediators such as tumour necrosis factor (TNF) not only render osteoclast precursors far more sensitive toward RANK ligand (RANKL) [8], but along with IL-1β and IL-6 also enhance the expression of RANKL on osteoblasts [9]. Thus by both increasing the sensitivity of RANK towards RANKL, as well as increasing the expression of RANKL enhance bone resorption by promoting the maturation of osteoclasts.
Although it is now well established that inflammatory mediators promote bone resorption, the exact reason why an inflammatory insult induces bone resorption remains unclear. Two lines of evidence suggest that bone resorption represents a dedicated immunological response, and is not merely an ‘accidental’ by-product of inflammation. Firstly, the fact that inflammatory mediators promote bone resorption through various mechanisms, strongly suggest that bone resorption represents a dedicated immunological response. Secondly, bone resorption induced by inflammatory insults are observed in various species, signifying that bone resorption may be an evolutionary conserved response, which would then suggests an adaptive function for bone resorption during infection or severe trauma.
Here we review a number of mechanisms by which bone resorption may enhance immune function during infection. In particular, we highlight recent developments in the field of immunometabolism which suggest that bone resorption may support the metabolic needs of activated immune cells. It is argued that bone resorption plays an evolutionary conserved role in liberating phosphate, which in turn sustains biosynthetic activities of immune cells, and promotes cell proliferation. In addition, magnesium liberated from bone, is a key cofactor for various enzymes involved in reactions that catalyse the addition of phosphate groups, for example the phosphorylation of glycolytic intermediates, as well as nucleotide polymerization. Finally, liberated calcium may play a central immunological role in facilitating immune cell trafficking during infection (e.g. activation of adhesion molecules) and in upregulating autophagy.
2. Immunometabolism
The current re-emerging interest in immune cell metabolism (immunometabolism) has highlighted the extent to which metabolic reprogramming in these cells influences immune function [10,11]. Various immune cells, once activated, engage in glycolysis despite the presence of oxygen—a phenomenon first described in cancer cells and referred to as aerobic glycolysis or the ‘Warburg effect’ [10]. It is now understood that aerobic glycolysis represents a strategy to rapidly produce ATP [12] as well as to provide carbon units for biosynthetic pathways [10,11]. However, the rapid expansion of immunological tissue imposes a challenge, particularly in light of sickness-associated anorexia (SAA), as it necessitates a redistribution of biomass. In this regard, we have recently argued that hyperglycaemia observed in critically ill patients is precisely directed to address this problem [13]. Peripheral catabolism of muscle provides amino acids for gluconeogenesis and for protein synthesis in expanding immune cells, which also helps explain why the levels of various amino acids may decrease during sepsis [14]. In addition, inflammatory mediators induce a state of insulin resistance, ensuring the availability of glucose to immune cells [13]. Thus, catabolism in peripheral tissue drives anabolism in immune cells.
3. Bone resorption supports immunological anabolism
Immune cell activation is thus associated with an increased demand for metabolic substrates such as glucose and amino acids. However, another indispensable requirement of rapidly dividing cells is phosphate. Phosphate groups, acquired via the hydrolysis of ATP, are added to a number of glycolytic intermediates that will ultimately be assimilated into structural molecules such as nucleotides and phospholipids. Indeed, the very first committing step in glycolysis involves the phosphorylation of glucose. Consequently, rapid cell division, as well as an increase in biosynthetic activity would impose a greater demand for exogenous phosphate.
Here, bone resorption could play an integral role in supplying phosphate during immune activation. Even effector immune cells would require large amounts of phosphate. This is well exemplified in plasma cells, where thousands of immunoglobulins are produced per cell per second. Sustaining such high levels of protein synthesis would necessitate a substantial pool of nucleotides e.g. for ribosomal and messenger RNA, as well as the generation of phospholipids to expand the endoplasmic reticulum to ultimately accommodate the processing and maturation of protein products. Similarly, lipid synthesis to sustain expanding cell membranes of phagocytic or antigen presenting cells would also ultimately depend on the availability of phosphate.
A number of observations highlight the importance of phosphate in maintaining rapid cell metabolism. Employing a biological stoichiometric analysis of the elements carbon, nitrogen, and phosphorus, Elser and co-workers demonstrated that some, although not all cancers exhibited an increase in phosphorus content [15]. The authors speculate that in phosphate-rich cancers, tumour growth is sustained by rapid growth and high levels of cell death, whereas cancers not exhibiting elevated phosphate content might be indicative of tumours with lower cell turnover. The dependency of rapidly dividing cells on phosphate is also demonstrated by hypophosphataemia that develops in patients with certain fast-growing haematological cancers [16,17]. Hypophosphataemia may also manifest during the rapid expansion of non-malignant tissue. For example, a decrease in serum phosphate levels following haematopoietic reconstitution correlates with an increase in leucocyte count [18,19]. It is also evident that a shortfall in phosphate may adversely affect immune function. For instance, earlier studies conducted on dogs demonstrated that hypophosphataemia following parenteral feeding results in decreased phagocytosis and bactericidal activity of granulocytes [20,21]. These observations suggest that the role of phosphate in sustaining rapid cell proliferation is not merely theoretical, but may exert clinically relevant effects. Finally, the dependency of activated immune cells on phosphate is also implicated by the observation that inflammatory mediators such as IL-1β and IL-6 promote the upregulation of calcium-sensing receptor (CaSR), which in turn lowers the threshold for parathyroid hormone (PTH) release [22]. Since PTH induces the release of phosphate, suppression of PTH levels would enhance phosphate retention. These observations suggest that inflammatory mediators may influence hormonal control of bone resorption in order to supply phosphate to activated immune cells.
The metabolism of activated immune cells may also be indirectly supported by bone resorption. Bone is a major storage site for magnesium [23], an important cofactor in various enzymes involved in glycolysis, as well as in nucleic acid biochemistry, for example polymerase enzymes and enzymes that phosphorylate and dephosphorylate proteins [24–26]. Thus, bone resorption not only supplies phosphate groups critical for cell metabolism, but also provides magnesium, a key enzymatic cofactor for various enzymes that catalyse reactions involving the addition of phosphates (e.g. glycolysis and nucleotide polymerization).
4. Calcium
Calcium has a host of important physiological functions. In this regard, Straub and colleagues [27] have pointed out that an inflammatory insult resulting in bone resorption is likely to also induce SAA, suggesting that enhanced bone resorption may function to supply the calcium during fasting. In addition to a ‘housekeeping’ function, calcium released by bone resorption may also promote cell survival and immune function.
Emerging evidence suggests that calcium release by bone resorption may also promote cell survival by upregulating autophagy. Although a cellular influx of calcium can induce apoptosis, a marginal increase in calcium may activate AMPK via calmodulin-dependent protein kinase kinase-β [28]. In turn, AMPK induces catabolic activities and coordinates various cell stress response systems, including autophagy [29]. Similarly, recent findings have also highlighted the role of calcium signalling in regulating autophagy by induction of lysosomal biogenesis [30]. Elevated calcium enhances the activity of calcineurin which in turn dephosphorylates the transcription factor EB, leading to its translocation into the nucleus where it controls various genes involved in the biogenesis of lysosomal vesicles [30].
Sustaining elevated levels of autophagic activity are believed to be important in promoting host survival during an infection as autophagy is involved in both cell survival and host defence [31]. Autophagy plays a role in mitochondrial quality control [32], a process likely to play an important role in severe inflammatory insults such as sepsis where mitochondrial dysfunction has been implicated as a contributing factor in the manifestation of organ dysfunction [33]. Furthermore, autophagy may enhance cell survival by removing aggregated proteins resulting from an increase in misfolded proteins due to a febrile response, or oxidative stress [34] and it may also modulate inflammation by degradation of inflammasomes [35]. Finally, autophagy plays a multifaceted role in host defence as induction of autophagy by serum and amino acid starvation enhances the degradation of Mycobacterium tuberculosis by macrophages [36]. In fact, autophagy also plays a pivotal role in cell-autonomous defence mechanisms where the same catabolic machinery of autophagy is repurposed to degrade pathogens [37]. This would suggest that an influx of calcium into peripheral tissue may not only promote cell survival, but also augments host defence during infection.
In addition to supporting housekeeping functions in immune cells, calcium may also play a more direct role in augmenting immune function. As an example, a transient increase in intracellular calcium levels in endothelium cells facilitate the loosening of gap junction integrity between cells and allows for the transmigration of immune cells [38]. Also of note, various adhesion molecules, e.g. cadherin proteins, which are used by leucocytes to attach to cells during migration, are calcium-dependent [39]. These observations suggest that calcium released during bone resorption may facilitate immune cell trafficking. Calcium also acts as a danger signal inducing the release of inflammatory cytokines, as well as acting as a chemotactic agent [40,41], highlighting the importance of calcium in modulating immune responses. However, serum calcium levels are supressed in various inflammatory contexts [42], suggesting that these calcium-mediated effects are more relevant within a paracrine context.
Finally, the movement of calcium liberated by bone resorption into cells of the vascular system may also have an adaptive function. It was found that an influx of calcium may alter contractile function in smooth muscle [43], thus modulating vascular tone. Calcium also plays an important role in facilitating coagulation [44]. These observations suggest that calcium release may represent a pre-emptive response to potential blood loss following severe trauma.
5. Growth factors
Bone resorption would not only liberate various ionic molecules, but could also liberate various growth factors deposited by osteoblasts [45]. Many of these growth factors such as vascular endothelial growth factor, fibroblast growth factor and insulin-like growth factors are mitogenic and known to promote tissue repair [45]. These factors can also exert additional effects. For instance, TGF-β also induces autophagy [46]. Interestingly, TGF-β is usually associated with a strong immunosuppressive effect, playing an important role in the resolution of inflammation [47]. In fact, TGF-β has also been shown to promote the alternative activation of macrophages [48], thus playing a key role in polarizing the immune response towards a tolerogenic phenotype associated with wound healing. This suggests that bone resorption could act as a counter measure of excessive inflammation. However, these factors are usually associated with a paracrine function relating to bone remodelling. It remains to be established whether bone resorption can support a systemic effect via the release of these growth factors.
6. Bone resorption: supporting the immune system
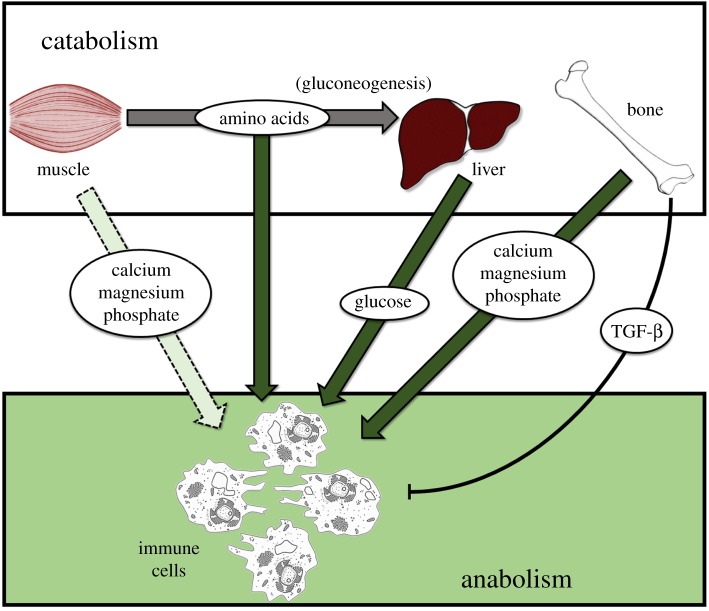
Taken together, these observations highlight how bone resorption in response to an inflammatory stimulus represents a dedicated, evolutionary conserved response aimed at supporting immune function (figure 1). Muscle breakdown would mobilize amino acids for gluconeogenesis in the liver as well as protein synthesis in immune cells. Of note, the breakdown of muscle also contributes to the release of phosphate, calcium and magnesium, but to a much lower extent than bone.
Figure 1.
Catabolism in peripheral tissue drives anabolism in immune cells. Bone represents the largest source of calcium, magnesium and phosphate. However, muscle breakdown, induced by inflammatory mediators, also supplies these ions. (Online version in colour.)
Interestingly, these bone-derived ions are often decreased during severe inflammation. Although magnesium deficiency is usually very rare in healthy individuals, low serum magnesium levels are common in sepsis [49]. Similarly, hypophosphataemia is often observed in the context of severe inflammatory conditions such as sepsis [50] as well as in surgical intensive care patients [51] while hypocalcaemia has likewise long been known to manifest in most critically ill patients [42]. A decrease in serum levels of these ions may result from an influx into cells, or may be a consequence of increased urinary excretion.
An earlier study report that intravenous infusion of phosphate results in urinary loss of phosphate [52]. Similarly, in the context of supplementing patients with magnesium during sepsis, the tissue absorption of magnesium is slow, with almost half of all magnesium being excreted in the urine [49]. Thus, supplementation with phosphate and magnesium may result in increased urinary output of these electrolytes and suggest that urinary loss of ions may contribute to decreased serum levels of magnesium and phosphate. In contrast, calcium loss seem to be more constrained: Lind and colleagues [53] reported that, despite the high incidence of hypocalcaemia among ICU patients, such a decrease in serum calcium was not the result of suppressed bone resorption or increased urinary loss [53]. This would suggest that hypercalciuria is not the only mechanism by which calcium is lost. This implies that the liberated calcium may be rapidly cleared by influx into cells, or possibly through associating with adhesion molecules.
It is unlikely that bone resorption results in the liberation of magnesium, calcium and phosphate at the exact stoichiometric relations in which they are required by the body. Rather, an increase in urinary loss of phosphate may result from too much phosphate being liberated in an effort to acquire sufficient amounts of calcium. Also, a variety of clinical interventions as well as co-morbidities may also alter excretion of electrolytes. As an example, fluid resuscitation could also alter electrolyte balance, while insulin administration to treat hyperglycaemia also induce an influx of phosphate into cells, resulting in a drop in serum phosphate levels [54]. Also of interest, it was reported that the urinary loss of ions during sepsis was lower than that of surgical patients [53], suggesting that either the intensity, or the type of inflammatory insult potentially impacts urinary loss of ions. Clarifying the fate of liberated ions could indicate the significance of bone resorption and highlight the role of particular ions in supporting host survival during various inflammatory insults.
7. Conclusion
Bone is a dynamic organ with a widely appreciated structural role. In addition to facilitating locomotion, protecting organs, and facilitating sensory function (e.g. ossicles in the ear), bone also acts as a storage organ. Induction of bone resorption by inflammatory mediators would ensure a robust immune response by providing calcium (with a broad range of cellular functions) as well as the necessary phosphate to maintain elevated glycolytic activity in activated immune cells. It is also interesting to note that magnesium, a cofactor to various enzymes which interact with high-energy phosphate groups (e.g. enzymes acting in glycolysis and nucleotide polymerases) are also liberated by bone resorption. However, though plausible, these claims require further investigation. Of particular interest is the decrease in certain electrolytes despite bone resorption. We have outlined various processes that would use bone-derived electrolytes. However, other factors may also contribute to the decrease in electrolytes, including a refractory increase in bone mineral deposition following an inflammatory-mediated bone resorption.
Acknowledgements
The authors thank Jasper and Jacki Niesing for insightful discussions on this work.
Data accessibility
This article has no additional data.
Authors' contributions
G.v.N., M.M. and A.-M.E. drafted the manuscript. All authors read and approved the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
The authors acknowledge funding support from the Cancer Association of South Africa (CANSA), National Research Foundation (NRF) and the Medical Research Council of South Africa (SAMRC).
References
- 1.Steeve KT, Marc P, Sandrine T, Dominique H, Yannick F. 2004. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology . Cytokine Growth Factor Rev. 15, 49–60. ( 10.1016/j.cytogfr.2003.10.005) [DOI] [PubMed] [Google Scholar]
- 2.Redlich K, Smolen JS. 2012. Inflammatory bone loss: pathogenesis and therapeutic intervention . Nat. Rev. Drug Discov. 11, 234–250. ( 10.1038/nrd3669) [DOI] [PubMed] [Google Scholar]
- 3.Hardy R, Cooper MS. 2009. Bone loss in inflammatory disorders . J. Endocrinol. 201, 309–320. ( 10.1677/JOE-08-0568) [DOI] [PubMed] [Google Scholar]
- 4.Wang X, et al. 2016. Fracture risk and bone mineral density levels in patients with systemic lupus erythematosus: a systematic review and meta-analysis . Osteoporosis Int. 27, 1413–1423. ( 10.1007/s00198-015-3449-7) [DOI] [PubMed] [Google Scholar]
- 5.Schett G, et al. 2006. High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study . Arch. Intern. Med. 166, 2495–2501. ( 10.1001/archinte.166.22.2495) [DOI] [PubMed] [Google Scholar]
- 6.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang C. 2009. Inhibition of osteoblastic bone formation by nuclear factor-κB . Nat. Med. 15, 682–689. ( 10.1038/nm.1954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada T, Nakashima T, Hiroshi N, Penninger JM. 2006. RANKL–RANK signaling in osteoclastogenesis and bone disease . Trends Mol. Med. 12, 17–25. ( 10.1016/j.molmed.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 8.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. 2000. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand . J. Clin. Invest. 106, 1481–1488. ( 10.1172/JCI11176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori T, et al. 2011. IL-1β and TNFα-initiated IL-6–STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis . Int. Immunol. 23, 701–712. ( 10.1093/intimm/dxr077) [DOI] [PubMed] [Google Scholar]
- 10.O'Neill LA, Kishton RJ, Rathmell J. 2016. A guide to immunometabolism for immunologists . Nat. Rev. Immunol. 16, 553–565. ( 10.1038/nri.2016.70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly B, O'Neill LA. 2015. Metabolic reprogramming in macrophages and dendritic cells in innate immunity . Cell Res. 25, 771–784. ( 10.1038/cr.2015.68) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeiffer T, Schuster S, Bonhoeffer S. 2001. Cooperation and competition in the evolution of ATP-producing pathways . Science 292, 504–507. ( 10.1126/science.1058079) [DOI] [PubMed] [Google Scholar]
- 13.van Niekerk G, Davis T, Engelbrecht A. 2017. Hyperglycaemia in critically ill patients: the immune system's sweet tooth . Crit. Care. 21, 202 ( 10.1186/s13054-017-1775-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su L, Li H, Xie A. et al 2015. Dynamic changes in amino acid concentration profiles in patients with sepsis . PLoS ONE 10, e0121933 ( 10.1371/journal.pone.0121933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elser J, Kyle M, Smith M, Nagy J. 2006. Biological stoichiometry in human cancer. PLoS ONE 2, e1028 ( 10.1371/journal.pone.0001028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milionis HJ, Bourantas CL, Siamopoulos KC, Elisaf MS. 1999. Acid–base and electrolyte abnormalities in patients with acute leukemia . Am. J. Hematol. 62, 201–207. () [DOI] [PubMed] [Google Scholar]
- 17.Soyoral Y, Aslan M, Ebinc S, Dirik Y, Demir C. 2014. Life-threatening hypophosphatemia and/or phosphate depletion in a patient with acute lymphoblastic leukemia: a rare case report . Am. J. Emerg. Med. 32, 1437.e3–1437.e5. ( 10.1016/j.ajem.2014.04.011) [DOI] [PubMed] [Google Scholar]
- 18.Steiner M, Steiner B, Wilhelm S, Freund M, Schuff-Werner P. 2000. Case report severe hypophosphatemia during hematopoietic reconstitution after allogeneic peripheral blood stem cell transplantation . Bone Marrow Transplant. 25, 1015–1016. ( 10.1038/sj.bmt.1702407) [DOI] [PubMed] [Google Scholar]
- 19.Raanani P, Levi I, Holzman F, Grotto I, Brok-Simoni F, Avigdor A, Davidson J, Shpilberg O, Ben-Bassat I. 2001. Engraftment-associated hypophosphatemia-the role of cytokine release and steep leukocyte rise post stem cell transplantation . Bone Marrow Transplant. 27, 311 ( 10.1038/sj.bmt.1702761) [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen A, Segel E, Hessov I, Borregaard N. 1988. Reduced function of neutrophils during routine postoperative glucose infusion . Acta Chir. Scand. 154, 429–433. [PubMed] [Google Scholar]
- 21.Craddock P, Yawata Y, VanSanten L, Gilberstadt S, Silvis S, Jacob H. 1974. Acquired phagocyte dysfunction: a complication of the hypophosphatemia of parenteral hyperalimentation . N. Engl. J. Med. 290, 1403–1407. ( 10.1056/NEJM197406202902504) [DOI] [PubMed] [Google Scholar]
- 22.Porter C, Sousse LE, Irick R, Schryver E, Klein GL. 2017. Interactions of phosphate metabolism with serious injury, including burns. JBMR Plus 1, 59–65. ( 10.1002/jbm4.10011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahnen-Dechent W, Ketteler M. 2012. Magnesium basics . Clin. Kidney J. 5, i3–i14. ( 10.1093/ndtplus/sfr163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garfinkel L, Garfinkel D. 1985. Magnesium regulation of the glycolytic pathway and the enzymes involved . Magnesium 4, 60–72. [PubMed] [Google Scholar]
- 25.Ko YH, Hong S, Pedersen PL. 1999. Chemical mechanism of ATP synthase. Magnesium plays a pivotal role in formation of the transition state where ATP is synthesized from ADP and inorganic phosphate. J. Biol. Chem. 274, 28 853–28 856. ( 10.1074/jbc.274.41.28853) [DOI] [PubMed] [Google Scholar]
- 26.Cowan J. 2002. Structural and catalytic chemistry of magnesium-dependent enzymes . Biometals 15, 225–235. ( 10.1023/A:1016022730880) [DOI] [PubMed] [Google Scholar]
- 27.Straub RH, Cutolo M, Pacifici R. 2015. Evolutionary medicine and bone loss in chronic inflammatory diseases—a theory of inflammation-related osteopenia. Semin. Arthritis. Rheum. 45, 220–228. ( 10.1016/j.semarthrit.2015.04.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. 2005. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase . Cell Metab. 2, 9–19. ( 10.1016/j.cmet.2005.05.009) [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Song P, Zou MH. 2012. AMP-activated protein kinase, stress responses and cardiovascular diseases . Clin. Sci. (Lond). 122, 555–573. ( 10.1042/CS20110625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina DL, et al. 2015. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB . Nat. Cell Biol. 17, 288–299. ( 10.1038/ncb3114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Niekerk G, Loos B, Nell T, Engelbrecht A. 2016. Autophagy—a free meal in sickness-associated anorexia . Autophagy 12, 727–734. ( 10.1080/15548627.2016.1147672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashrafi G, Schwarz T. 2013. The pathways of mitophagy for quality control and clearance of mitochondria . Cell Death Diff. 20, 31–42. ( 10.1038/cdd.2012.81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. 2002. Association between mitochondrial dysfunction and severity and outcome of septic shock . Lancet 360, 219–223. ( 10.1016/S0140-6736(02)09459-X) [DOI] [PubMed] [Google Scholar]
- 34.Lamark T, Johansen T. 2012. Aggrephagy: selective disposal of protein aggregates by macroautophagy. Int. J. Cell Biol. 2012, 736905 ( 10.1155/2012/736905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi C, Shenderov K, Huang N, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. 2012. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction . Nat. Immunol. 13, 255–263. ( 10.1038/ni.2215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. 2004. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages . Cell 119, 753–766. ( 10.1016/j.cell.2004.11.038) [DOI] [PubMed] [Google Scholar]
- 37.Randow F, MacMicking JD, James LC. 2013. Cellular self-defense: how cell-autonomous immunity protects against pathogens . Science 340, 701–706. ( 10.1126/science.1233028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller WA. 2016. Transendothelial migration: unifying principles from the endothelial perspective . Immunol. Rev. 273, 61–75. ( 10.1111/imr.12443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlos TM, Harlan JM. 1994. Leukocyte-endothelial adhesion molecules . Blood 84, 2068–2101. [PubMed] [Google Scholar]
- 40.Hendy GN, Canaff L. 2016. Calcium-sensing receptor, proinflammatory cytokines and calcium homeostasis. Semin. Cell Dev. Biol. 49, 37–43. ( 10.1016/j.semcdb.2015.11.006) [DOI] [PubMed] [Google Scholar]
- 41.Klein GL, Castro SM, Garofalo RP. 2016. The calcium-sensing receptor as a mediator of inflammation. Semin. Cell Dev. Biol. 49, 52–56. ( 10.1016/j.semcdb.2015.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zivin JR, Gooley T, Zager RA, Ryan MJ. 2001. Hypocalcemia: a pervasive metabolic abnormality in the critically ill. Am. J. Kidney Dis. 37, 689–698. ( 10.1016/S0272-6386(01)80116-5) [DOI] [PubMed] [Google Scholar]
- 43.Adelstein RS, Sellers JR. 1987. Effects of calcium on vascular smooth muscle contraction . Am. J. Cardiol. 59, B4–B10. ( 10.1016/0002-9149(87)90076-2) [DOI] [PubMed] [Google Scholar]
- 44.Mann K, Whelihan M, Butenas S, Orfeo T. 2007. Citrate anticoagulation and the dynamics of thrombin generation . J. Thromb. Haemost. 5, 2055–2061. ( 10.1111/j.1538-7836.2007.02710.x) [DOI] [PubMed] [Google Scholar]
- 45.Devescovi V, Leonardi E, Ciapetti G, Cenni E. 2008. Growth factors in bone repair . Chir. Organi. Mov. 92, 161–168. ( 10.1007/s12306-008-0064-1) [DOI] [PubMed] [Google Scholar]
- 46.Kiyono K, Suzuki HI, Matsuyama H, Morishita Y, Komuro A, Kano MR, Sugimoto K, Miyazono K. 2009. Autophagy is activated by TGF-beta and potentiates TGF-beta-mediated growth inhibition in human hepatocellular carcinoma cells . Cancer Res. 69, 8844–8852. ( 10.1158/0008-5472.CAN-08-4401) [DOI] [PubMed] [Google Scholar]
- 47.Li MO, Wan YY, Sanjabi S, Robertson AL, Flavell RA. 2006. Transforming growth factor-β regulation of immune responses . Annu. Rev. Immunol. 24, 99–146. ( 10.1146/annurev.immunol.24.021605.090737) [DOI] [PubMed] [Google Scholar]
- 48.Gong D, Shi W, Yi S, Chen H, Groffen J, Heisterkamp N. 2012. TGFβ signaling plays a critical role in promoting alternative macrophage activation . BMC Immunol. 13, 31 ( 10.1186/1471-2172-13-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velissaris D, Karamouzos V, Pierrakos C, Aretha D, Karanikolas M. 2015. Hypomagnesemia in critically ill sepsis patients . J. Clin. Med. Res. 7, 911–918. ( 10.14740/jocmr2351w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barak V, Schwartz A, Kalickman I, Nisman B, Gurman G, Shoenfeld Y. 1998. Prevalence of hypophosphatemia in sepsis and infection: the role of cytokines . Am. J. Med. 104, 40–47. ( 10.1016/S0002-9343(97)00275-1) [DOI] [PubMed] [Google Scholar]
- 51.Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultz MJ. 2010. Treatment of hypophosphatemia in the intensive care unit: a review . Crit. Care. 14, R147 ( 10.1186/cc9215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charron T, Bernard F, Skrobik Y, Simoneau N, Gagnon N, Leblanc M. 2003. Intravenous phosphate in the intensive care unit: more aggressive repletion regimens for moderate and severe hypophosphatemia . Intensive Care Med. 29, 1273–1278. ( 10.1007/s00134-003-1872-2) [DOI] [PubMed] [Google Scholar]
- 53.Lind L, et al. 2000. Hypocalcemia and parathyroid hormone secretion in critically ill patients . Crit. Care Med. 28, 93–99. ( 10.1097/00003246-200001000-00015) [DOI] [PubMed] [Google Scholar]
- 54.Liamis G, Milionis H, Elisaf M. 2010. Medication-induced hypophosphatemia: a review . QJM 103, 449–459. ( 10.1093/qjmed/hcq039) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.