Abstract
The ability to develop cultural adaptations to local environments is critical to the biological success of humans. Although overall population size and connectedness are thought to play an important role in increasing the rate of cumulative cultural evolution, the independent effect of dispersal rules on rates of cultural evolution has not been examined. Here, a computational model is used to explore the effect of dispersal on the rate of cultural evolution in traits transmitted patrilineally (from father to son), matrilineally (mother to daughter) and bilineally (through both sexes). Two dispersal conditions are modelled: patrilocality (where females disperse and males stay) and bilocality (where either sex may disperse). The results suggest that when only females disperse, the capacity for cumulative cultural evolution in traits shared only among males is severely constrained. This occurs even though overall rates of dispersal and the number of cultural models available to males and females are identical in both dispersal conditions. The constraints on the evolution of patrilineally inherited traits could be considered to represent a process of ‘cultural inbreeding', analogous to genetic inbreeding.
Keywords: cultural evolution, dispersal, hunter–gatherers, social organization, human evolution
1. Background
Even before the emergence of agriculture, humans had colonized almost all parts of the Old and New World [1]. Critical to this extraordinary biological success is our ability to establish cultural adaptations to local environments, an ability underpinned by our propensity for social learning, language and advanced social cognition [2]. Learning socially, we are able not only to inherit skills and technologies but also to innovate upon them, leading to a ‘ratcheting' process of cumulative cultural evolution that allows the emergence of cultural traits and technologies that could never be generated through individual learning alone [3].
Several modelling, empirical and experimental studies have demonstrated the role of population size and connectedness in driving cultural evolution [4–6] and increased population size and density has been advanced as a potential explanation for increases in cultural complexity in the archaeological record [4]. Empirical evidence, however, does not consistently support the population size hypothesis [7,8] and if population size were the sole driver of cultural evolution, we might ask how hunter–gatherers, living in small bands of around 20 adults [9,10], are able to maintain effective cultural and technological adaptations.
The answer may lie in distinctive aspects of hunter–gatherer social organization. The multi-family social structure within bands and the high mobility of households between bands may both facilitate local cultural exchange [11,12]. Some hunter–gatherer populations also have cultural institutions that facilitate long-distance social relationships, such as the hxaro exchange system of the !Kung San [13]. One aspect of social organization that requires greater attention with respect to cultural evolution is dispersal. Human societies show great variation in patterns of dispersal and residence, varying between female dispersal (patrilocality) and male dispersal (matrilocality). Most small-scale hunter–gatherer societies fall between these extremes, having flexible ‘multi-local' systems of residence in which either men or women may leave their natal group to marry (bilocality) and where households frequently move between camps [9,10,14]. Although the residence systems of hunter–gatherers may represent an extreme case of flexibility, social institutions that facilitate interactions between groups are a common feature of human social organization more generally and are in contrast to the more bounded, territorial and female-dispersal system typical of chimpanzees and, to some extent, bonobos [15,16].
Here, a computational model is used to explore the effect of bilocal versus patrilocal dispersal on the rate of culutral evolution in traits that are transmitted matrilineally (through females only), patrilienally (through males only) and bilineally (through both sexes). The distinction between these kinds of inheritance is particualrly salient for hunter–gatherer societies, where the sexual division of labour in foraging means that many technologies and skills are likely to be transmitted only among one sex [17].
2. Material and methods
The model considers a population consisting of G groups of N individuals. In every iteration of the model, a new generation of N/2 agents are born. These agents are randomly assigned a father and mother from the existing males and females in their group. In all versions of the model, half of the new generation of agents disperse to another group, and half remain in their natal group. In the ‘patrilocal' condition, only females disperse from their natal group—males always remain. In the ‘bilocal' condition, an equal number of males and females disperse. Although single-sex dispersal is here framed as patrilocality, the same results would be generated for matrilocality (where males disperse and females remain).
Cultural transmission in the model is based on the process of transmission used by Powell et al. [4], building on Henrich [5]. All individuals in the initial population have a skill value (z-score) that represents their proficiency in some cultural or technological domain. Individuals acquire their z-score in a two-step process. First, they inherit skills ‘vertically' from their parents. This inheritance is assumed to be an imperfect process that can result in the learner having a lower skill level than their parent but which can also result in innovation, allowing offspring to have a greater skill level than their parent. After dispersal has occurred, agents can learn ‘horizontally' from members of their group. Agents will acquire the z-score of any group member who has a higher score than their own with a probability proportional to the difference between the two scores. Since individuals are, therefore, able to recognize and preferentially learn from more successful agents, this process of horizontal transmission can be considered rational and directed—individuals actively seek to increase their skill level.
The rate of evolution is explored for three kinds of inheritance: bilineal, patrilineal and matrilineal. Patrilineal traits are transmitted vertically from father to son and then horizontally among male group members. Matrilineal traits are transmitted vertically from mother to daughter and then horizontally among female group members. Bilineal traits can be transmitted from both parents to both male and female offspring and are then horizontally transmitted among all group members. Unless otherwise stated, the population consists of 20 groups of 24 individuals, approximating group and population sizes for ethnographically observed hunter–gatherer populations [9]. Further description of the simulations is provided in the electronic supplementary material.
3. Results
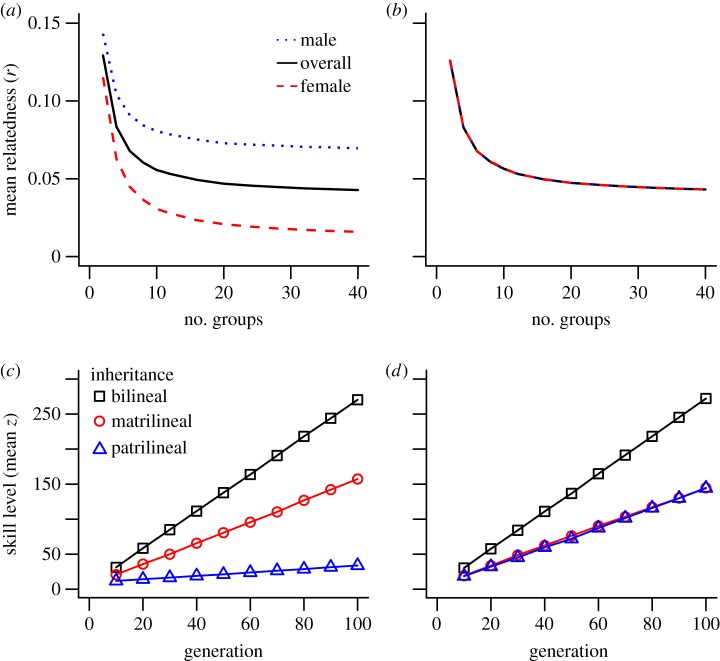
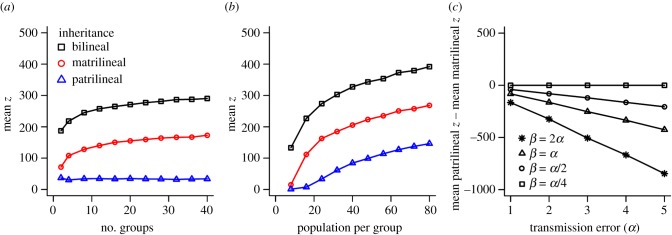
The patrilocal condition resulted in substantial differences in the relatedness of men and women to their group, with men being more than three times as closely related to group members across much of the range of G (figure 1a,b). Compared with bilocal dispersal, patrilocality (where females disperse and males remain) also significantly limited the mean z of patrilineally inherited traits (e.g. at α = 3 and β = 1.5, mean patrilineal z in the patrilocal condition z = 34.70, s.d. = 3.94; mean patrilineal z in the bilocal condition = 143.97, s.d. = 12.32 after 100 generations, figure 1c,d). This difference is observed across a large number of generations (electronic supplementary material, figure S1) and across the range for group numbers, group size and values of α and β (figure 2; electronic supplementary material, S2).
Figure 1.
Effect of dispersal on group relatedness and rate of cultural evolution. Mean coefficient of relatedness (r) to the group for males (dotted blue), females (dashed red) and overall (solid black) under (a) patrilocal dispersal and (b) bilocal dispersal after 100 generations of the model. Accumulation of cultural skills transmitted bilineally (squares), matrilineally (circles), patrilineally (triangles) under (c) patrilocal dispersal and (d) bilocal dispersal. All points are the mean of 200 simulations with α = 3 and β = 1.5. Maximum standard deviation of any point is ±0.017 in (a,b) and ±11.97 in (c,d). (Online version in colour.)
Figure 2.
Dynamics of cultural transmission across parameter space given patrilocality. (a) Mean skills levels (z) after 100 generations across varying number of groups of 24 individuals. (b) Mean skill levels (z) after 100 generations across 20 groups of varying size. (c) The difference between mean patrilineal and matrilineal skill levels (z) after 100 generations across a range of values of α and β, with lines linking the values of β relative to α. In all panels, points are the average of 30 simulations. Maximum standard deviation of any point is ±17.85 in (a), ±15.28 in (b) and ±89.05 in (c). (Online version in colour.)
The reduction in the mean z of patrilineally inherited traits caused by patrilocal residence occurs without causing a substantial reduction in the mean z scores for matrilineal traits (mean matrilineal z in the patrilineal condition = 152.62, s.d. = 13.13; mean matrilineal z in the bilocal condition = 146.33, s.d. = 12.32) and without any change in mean z for bilineal traits (mean bilineal z in the patrilineal condition = 269.85, s.d. = 12.98; mean bilineal z in the bilocal condition = 269.01, s.d. = 11.10, figure 1c,d). Critically, the differences in the evolution of patrilineally inherited traits between the patrilocal and bilocal conditions emerge despite the fact that in all conditions of the model men and women are learning from the same number of cultural models and that the overall dispersal rates are identical (in both the bilocal and patrilocal conditions, 50% of individuals leave their natal group and 50% remain).
4. Discussion
The results suggest that single-sex dispersal may constrain the rate of cultural evolution in traits transmitted only among the non-dispersing sex. These results have several implications. Firstly, the flexible ‘bilocal’ residence typical of small-scale hunter–gatherers may promote cultural exchange, especially when combined with high mobility between groups. Secondly, sex-biased residence in humans may result in sex differences in the diversity of cultural traits, although these differences may be mitigated by other mechanisms that promote exchange between groups (e.g. affiliation to a wider tribal or linguistic community). Finally, beyond humans, the bounded and female-dispersal system typical of chimpanzees may create sex differences in cultural traits. Although further empirical evidence would be needed to associate sex differences with dispersal, such sex differences do appear to exist, with female chimpanzees using tools more frequently than males across sites (e.g. females are more likely to hunt with tools [18] and use natural hammers to crack nuts in the wild [19]).
Of course, flexible systems of residence may have benefits beyond facilitating cultural evolution. Where households can switch between living with the family of the husband and the family of the wife, they may have access to a much larger number of communities, allowing them to avoid local ecological depletion, to take advantage of seasonally available resources and to more easily ‘walk-away' from tyrannical or uncooperative group members [20,21]. At a population level, a fluid social organization may also promote genetic diversity. Indeed, a hunter–gatherer style system of social organization has even been invoked to explain the lower levels of inbreeding found among anatomically modern humans as compared with Neanderthals in the European Upper Palaeolithic [22]. The results presented here suggest that more flexible systems of residence may promote not only genetic but also cultural diversity, allowing individuals to avoid a process of cultural inbreeding analogous to that of genetic inbreeding.
Supplementary Material
Supplementary Material
Data accessibility
Model code is available as the electronic supplementary material.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Gamble C. 2014. Settling the Earth: the archaeology of deep human history. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Richerson PJ, Boyd R. 2005. Not by genes alone: how culture transformed human evolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Boyd R, Richerson PJ, Henrich J. 2011. The cultural niche: why social learning is essential for human adaptation. Proc. Natl Acad. Sci. USA 108, 10 918–10 925. ( 10.1073/pnas.1100290108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell A, Shennan S, Thomas MG. 2009. Late Pleistocene demography and the appearance of modern human behavior. Science 324, 1298–1301. ( 10.1126/science.1170165) [DOI] [PubMed] [Google Scholar]
- 5.Henrich J. 2004. Demography and cultural evolution: how adaptive cultural processes can produce maladaptive losses: the Tasmanian case. Am. Antiq. 69, 197–214. ( 10.2307/4128416) [DOI] [Google Scholar]
- 6.Derex M, Beugin M-P, Godelle B, Raymond M. 2013. Experimental evidence for the influence of group size on cultural complexity. Nature 503, 389–391. ( 10.1038/nature12774) [DOI] [PubMed] [Google Scholar]
- 7.Vaesen K, Collard M, Cosgrove R, Roebroeks W. 2016. Population size does not explain past changes in cultural complexity. Proc. Natl Acad. Sci. USA 113, E2241–E2247. ( 10.1073/pnas.1520288113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collard M, Vaesen K, Cosgrove R, Roebroeks W. 2016. The empirical case against the ‘demographic turn’ in Palaeolithic archaeology. Phil. Trans. R. Soc. Lond. B 371, 20150242 ( 10.1098/rstb.2015.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill KR, et al. 2011. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science 331, 1286–1289. ( 10.1126/science.1199071) [DOI] [PubMed] [Google Scholar]
- 10.Dyble M, Salali G, Chaudhary N, Page A, Smith D, Thompson J, Vinicius L, Mace R, Migliano A. 2015. Sex equality can explain the unique social structure of hunter-gatherer bands. Science 348, 796–798. ( 10.1126/science.aaa5139) [DOI] [PubMed] [Google Scholar]
- 11.Migliano A, et al. 2017. Characterization of hunter–gatherer networks and implications for cumulative culture. Nat. Hum. Behav. 1, 43 ( 10.1038/s41562-016-0043) [DOI] [Google Scholar]
- 12.Hill KRK, Wood BBM, Baggio J, Hurtado AM, Boyd RRT. 2014. Hunter-gatherer inter-band interaction rates: implications for cumulative culture. PLoS ONE 9, e102806 ( 10.1371/journal.pone.0102806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiessner P. 2002. Hunting, healing, and hxaro exchange: a long-term perspective on !Kung (Ju/’hoansi) large-game hunting. Evol. Hum. Behav. 23, 407–436. ( 10.1016/S1090-5138(02)00096-X) [DOI] [Google Scholar]
- 14.Kramer KLK, Greaves RDR. 2011. Postmarital residence and bilateral kin associations among hunter-gatherers: Pumé foragers living in the best of both worlds. Hum. Nat. 22, 41–63. ( 10.1007/s12110-011-9115-7) [DOI] [PubMed] [Google Scholar]
- 15.Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior. Cambridge, MA: Harvard University Press. [Google Scholar]
- 16.Gerloff U, Hartung B, Fruth B, Hohmann G, Tautz D. 1999. Intracommunity relationships, dispersal pattern and paternity success in a wild living community of bonobos (Pan paniscus) determined from DNA analysis of faecal samples. Proc. R. Soc. Lond. B 266, 1189–1195. ( 10.1098/rspb.1999.0762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly R. 2013. The lifeways of hunter-gatherers: the foraging spectrum. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Pruetz JD, Bertolani P, Ontl KB, Lindshield S, Shelley M, Wessling EG. 2015. New evidence on the tool-assisted hunting exhibited by chimpanzees (Pan troglodytes verus) in a savannah habitat at Fongoli, Sénégal. R. Soc. Open Sci. 2, 140507 ( 10.1098/rsos.140507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boesch C, Boesch H. 1981. Sex differences in the use of natural hammers by wild chimpanzees: a preliminary report. J. Hum. Evol. 10, 585–593. ( 10.1016/S0047-2484(81)80049-8) [DOI] [Google Scholar]
- 20.Venkataraman VV, Kraft TS, Dominy NJ, Endicott KM. 2017. Hunter-gatherer residential mobility and the marginal value of rainforest patches. Proc. Natl Acad. Sci. USA 114, 3097–3102. ( 10.1073/pnas.1617542114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis HM, Vinicius L, Strods J, Mace R, Migliano AB. 2014. High mobility explains demand sharing and enforced cooperation in egalitarian hunter–gatherers. Nat. Commun. 5, 5789 ( 10.1038/ncomms6789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sikora M, et al. 2017. Ancient genomes show social and reproductive behavior of early Upper Paleolithic foragers. Science 358, 659–662. ( 10.1126/science.aao1807) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Model code is available as the electronic supplementary material.