Abstract
Organic semiconductors (OSCs) are promising materials for cost-effective production of electronic devices because they can be processed from solution employing high-throughput techniques. However, small-molecule OSCs are prone to structural modifications because of the presence of weak van der Waals intermolecular interactions. Hence, controlling the crystallization in these materials is pivotal to achieve high device reproducibility. In this perspective article, we focus on controlling polymorphism and morphology in small-molecule organic semiconducting thin films deposited by solution-shearing techniques compatible with roll-to-roll systems. Special attention is paid to the influence that the different experimental deposition parameters can have on thin films. Further, the main characterization techniques for thin-film structures are reviewed, highlighting the in situ characterization tools that can provide crucial insights into the crystallization mechanisms.
1. Introduction
Organic semiconductors (OSCs) have emerged as promising materials for cost-effective production of new flexible electronic devices because they can be processed from solution and at temperatures compatible with polymeric substrates. Traditionally, thin films of small-molecule semiconductors were deposited employing vacuum-based processes and investigated as active materials in organic field-effect transistors (OFETs). Nonetheless, the advances in the design of new small molecules with higher solubility have allowed depositing them with solution-based methods.1 Hence, OFETs fabricated utilizing different solution deposition techniques have been reported in the last few years exhibiting impressive field-effect mobility values.2−11 To raise industrial interest, though, it is crucial that such deposition techniques are simple, cheap, and compatible with scalable and high-throughput processes such as roll-to-roll. In this direction, solution-shearing techniques are highly appealing (Figure 1).12,13
Figure 1.
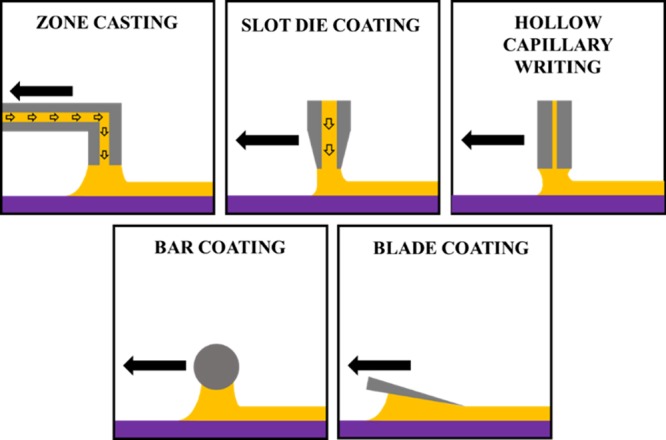
Schematic illustration of some of the most common solution-shearing deposition techniques that are mentioned in this paper.
Another critical issue in the field of organic electronics is achieving high device-to-device reproducibility. The charge transport in OSCs is ruled by anisotropic intermolecular interactions, and hence controlling thin-film morphology and molecular packing is essential.14 Because organic molecules are held together by weak nondirectional van der Waals forces, they are expected to exhibit many alternative packing arrangements, with minor differences in structure and energy, leading to polymorphism. Different polymorphs can have electronic performances that differ by orders of magnitude.15−17 Access to each polymorph depends on both kinetic and thermodynamic parameters and stems from the weak molecule–molecule and molecule–solvent interactions that are established, which depend on parameters such as the crystallization rate or temperature applied.18 Thin-film morphology also plays an important role as it determines the number of grain boundaries and its anisotropy.
In this perspective article, we focus on controlling crystallization and thin-film morphology in small-molecule organic semiconducting thin films prepared by solution-shearing techniques. It will be shown that the modification of the deposition parameters has a strong influence on the thin-film formation, which in turn affects the electrical characteristics of the device. The progress in the understanding of this complex scenario will result in the fabrication of more highly performing devices with higher reproducibility, key for the future of organic electronics.
2. Solution-Shearing Techniques
In general terms, the solution-shearing method consists of dragging a solution meniscus formed in between a substrate and a top element, such as a bar, blade, or nozzle, by the movement of the substrate or the top element. Typically, both the dragging speed and the substrate temperature can be controlled. In Figure 1, some of the most common specific techniques used in the literature are schematically represented. In the zone-casting technique, the solution is supplied continuously by a nozzle at a casting rate in the range of micrometers per second obtaining highly oriented crystalline films.19−21 The slot-die-coating technique, widely used in the paint industry, allows the continuous deposition of a high volume of solution through a slot in a determined crystal growth direction.22 The deposition by hollow capillary writing is accomplished by allowing the solution microdroplet at the end of a hollow pen to contact the surface and then laterally translating the pen, typically at a rate of 0.1–4.0 cm·min–1.23 Recently, the well-known wire-bar-coating technique, where a wired bar in contact with a substrate is used to spread a solution, has been adapted for the deposition of OSCs. For instance, the bar coating makes use of a smooth cylindrical bar placed a few hundred microns above a heated substrate.24 Another derived technique is the blade coating, which consists of shearing a solution placed between a heated substrate and a blade, typically with a tilt angle of 8°.5,6,25
3. Thin-Film Preparation
The basic parameters that can be tuned in a solution-shearing experiment are the substrate temperature and the shearing speed, that is, the rate at which the substrate or the top bar/blade/nozzle is dragged (Figure 2). Such parameters have a strong influence on the thermodynamics and kinetics of the OSC crystallization. Undoubtedly, parameters such as the device materials used, surface treatments, and solution formulation also determine the film morphology and crystallinity. Further, the application of external stimuli such as an electric field during the deposition process can cause some alterations. In the following section, we overview the impact that all of these experimental parameters can have on the thin-film structure and morphology. Post-treatments13 such as solvent or temperature annealing have not been considered here because they are, in principle, less appealing for fast device manufacturing.
Figure 2.
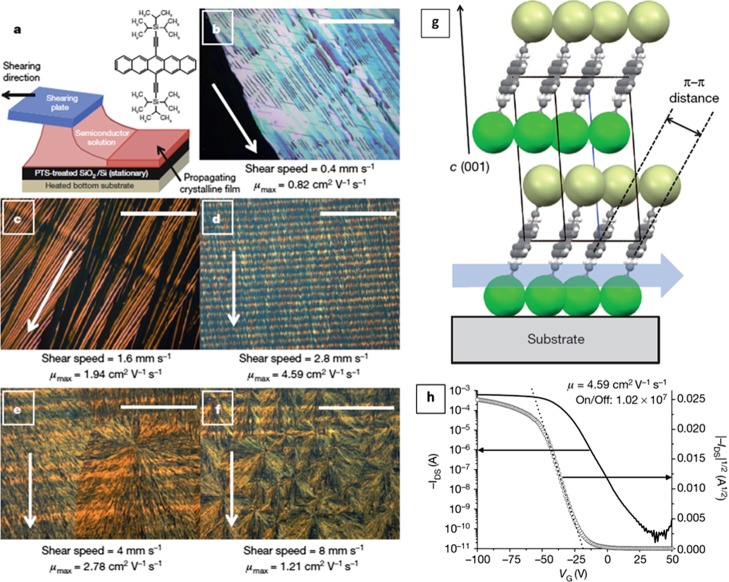
(a) Scheme of the blade-coating technique and (b–f) cross-polarized optical microscopy images of TIPS-Pen thin films formed with different shearing speeds ranging from 0.4 to 8 mm·s–1. Scale bars are all 200 μm, and white arrows represent the shearing direction. (g) Molecular packing structure of TIPS-Pen thin films obtained at a shearing speed of 8 mm·s–1. Spheres represent the TIPS groups: yellow and green correspond to the front and back of the pentacene moiety. (h) Transfer curve of the device prepared at a shearing speed of 2.8 mm·s–1 with a high field-effect mobility (4.6 cm2 V–1 s–1). Reprinted with permission from ref (8) (2011 Macmillan Publishers Limited).
3.1. Coating Shearing Speed
The coating shearing speed has a strong influence on the crystallization and nucleation processes. The most obvious effect is related to the thin-film texture. In several works, it has been demonstrated that at low speeds in the convective regime, oriented nearly single-crystalline films can be produced because the crystallization takes place at the meniscus contact line as the solvent is evaporated.8,21,22,26−32 However, at higher speeds, the crystallization occurs thanks to nucleation and coalescence from a supersaturated solution. This tendency can be clearly observed in Figure 2b–f.8 In this work, the OSC 6,13-bis(triisopropylsilylethinyl)pentacene) (TIPS-Pen) is deposited via blade shearing at speeds ranging from 0.4 to 8 mm·s–1, leading to thin-film morphologies that vary from comet-shaped long crystals to isotropic spherulitic crystalline domains. Such single-crystal-like films were also previously achieved using the zone-casting technique, where a solution of an OSC was supplied through a stationary flat nozzle onto a substrate moving very slowly at 0.02–0.03 mm·s–1.21,30,33 The electrical anisotropy was investigated in a zone-casted thin film of a tetrathiafulvalene (TTF) derivative giving higher field-effect mobilities (μ) along the casting direction with an anisotropy ratio (μ∥/μ⊥) of up to 102.34
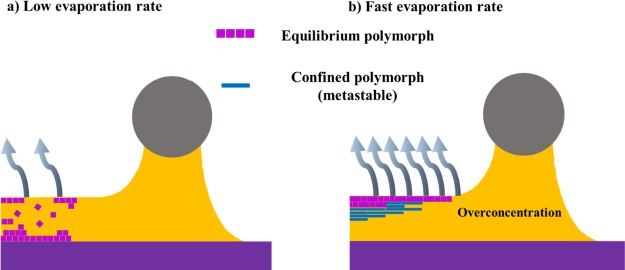
In addition to tuning the grain structure, the solution-shearing speed can also have an impact on the OSC lattice strain.18 Giri et al. reported that increasing the shearing speed in the deposition of TIPS-Pen films at elevated temperatures induces a decrease in the π–π stacking distance from 3.33 to 3.08 Å (Figure 2g).8 Accordingly, the charge carrier mobility increased from 0.8 cm2 V–1 s–1 for unstrained films deposited at 0.4 mm·s–1 to a high mobility of 4.6 cm2 V–1 s–1 for a strained film prepared at 2.8 mm·s–1 (Figure 2h), which was attributed to an improved electronic coupling between molecules. This was rationalized in terms of how the crystallization took place. In a deposition experiment where the solvent evaporates slowly, such as in drop-casting, the crystallization occurs near the substrate–liquid interface and the crystal growth is fed by molecules from the bulk of the solution (Figure 3a). On the contrary, under fast drying conditions, typically, the crystallization initiates at the air–liquid interface and proceeds toward the substrate, spatially confining the crystallization (Figure 3b).26 Generally, during solution shearing in the convective regime, the liquid film thickness decreases as the solution-shearing speed increases. In thinner films, there is a steeper gradient of temperature between the surface that is exposed to the ambient and the heated substrate. Further, in thinner films, the evaporation rate is also faster and thus the solution flows toward the growing crystalline film, without reaching the thermodynamic equilibrium. Hence, the authors hypothesized that all of these factors promoted the kinetic trapping of metastable polymorphs above a specific solution rate.
Figure 3.
Scheme of the crystallization process that takes place in (a) a low evaporation rate and (b) a fast evaporation rate deposition experiment. Adapted from ref (26) (2014 Macmillan Publishers Limited).
In a similar experiment, Headrick’s group investigated in situ the crystallization of TIPS-Pen films deposited by the hollow capillary writing technique using a heated substrate.27,29 They proposed that the large variation in mobility found as a function of the writing speed in the convective regime was primary because of the elimination of strain-induced defects, whereas lattice strain effects played a smaller role. The authors argue that because the films solidify before reaching the thermal equilibrium with the substrate, strain is imparted in the films because of the large thermal expansion coefficient of the solid film compared with the substrate. Such a strain can be relieved only by cracking or buckling. Because these effects are dependent on the film thickness, at higher writing speeds, when thinner films are achieved, buckling and cracking can be eliminated.
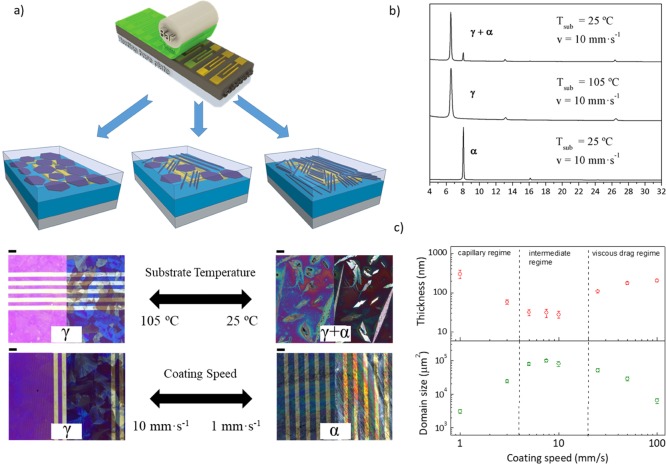
We recently demonstrated that the modification in the coating speed during the deposition of the organic semiconductor dibenzo-TTF (DB-TTF) blended with polystyrene (PS) by bar-assisted meniscus-shearing method (BAMS)24,35 at 105 °C can lead to the formation of either the kinetic or the thermodynamic polymorph (Figure 4a).31 Indeed, X-ray data revealed that the thin-film structure obtained at 1 mm·s–1 corresponded to the more thermodynamically stable α-polymorph, whereas the kinetic γ-phase was formed at higher coating speeds (Figure 4b).36 Morphologically, three different regions were distinguished depending on the coating speed. The first region was at the lowest coating speed (1 mm·s–1) where the thickness and roughness were higher and the domains were smaller because of the high number of nucleation points giving long but very thin crystallites. The coating speeds ranging from 3 to 10 mm·s–1 gave rise to very smooth and thin films characterized by large crystallites. Finally, in the region with coating speeds above 10 mm·s–1, the films were thicker and the crystallite size domain was smaller. Plotting film thickness versus coating speed gave a U-shaped curve in agreement with the three different regimes (Figure 4c). The low coating speed region corresponds to the capillary or convective regime of the deposition, and it is governed by the evaporation rate. The viscous drag regime was found at the highest coating speeds (i.e., above 10 mm·s–1), which is related to the Landau–Levich model.37,38 An intermediate regime, described by both intermixed regimes was identified,39 which gave the thinner films with larger crystalline domains. The best device performance was found in this intermediate regime, achieving a maximum mobility of around 0.25 cm2 V–1 s–1 at 7.5 mm·s–1 (γ-phase). The mobility of the devices prepared at 1 mm·s–1 that corresponded to the α-phase of DB-TTF was of the order of 0.05 cm2 V–1 s–1, that is, five times less than that of the γ-polymorph.
Figure 4.
(a) Conceptual illustration of how crystal growth and polymorph formation can be programmed during the solution-shearing of DB-TTF/PS by varying the substrate temperature or the coating speed, and representative optical microscopy images of some of the films prepared (left and right parts of the images correspond to with and without polarizer). Scale bar: 100 μm for all images. (b) X-ray diffractograms of DB-TTF/PS 1:2 thin films deposited by BAMS at different conditions. (c) Film thickness and domain size as functions of the coating speed in DB-TTF/PS 1:2 films deposited by BAMS at 105 °C. Figures are adapted with permission from ref (31) (2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim) and ref (24) (2015 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim).
3.2. Substrate Temperature
Substrate temperature is one of the most used parameters to control the deposition of active layers in OSCs using thermal evaporation or the drop-casting technique.40−45 In solution-shearing deposition processes, the temperature is selected based on the boiling point of the solvent used to control the crystallization rate. However, there are not many examples reporting a systematic study of the influence of the stage temperature on the thin-film crystallization.31,46 Galindo et al. reported that by adopting a constant solution-shearing speed (10 mm·s–1) and varying the substrate temperature in the range of 25–120 °C, the DB-TTF polymorph purity could be controlled.31 The authors found a crystal-phase mixture of DB-TTF (i.e., α + γ) at a low substrate temperature, but above 100 °C, a temperature close to the solvent boiling point (i.e., PhCl), only γ-phase was found (Figure 4a,b). Hence, when the substrate temperature is high, the thermodynamic equilibrium is not reached during the deposition process and only the kinetic phase is formed. On the contrary, below 100 °C, the films are still somewhat wet after spreading the solution on the substrate, and then, the thermodynamic α-phase also coexists with the γ-phase. Importantly, it was observed that the phase mixture and the grain boundaries between domains of different phases had a clear negative impact on the device performance.
It is known that TIPS-Pen exhibits two polymorphic forms. Form I is formed at a low temperature, but it shows a bulk phase transition at 124 °C, giving form II.47 By modifying the substrate temperature when depositing TIPS-Pen by hollow capillary writing, it is also possible to control the TIPS-Pen polymorph formed.27,48 Form I is formed when the OSC is deposited at room temperature or at 50 °C, whereas form II is formed at 135 °C. Importantly, it was demonstrated that form II can be stabilized at room temperature if the film thickness is below the critical thickness for cracking, and form I does not show a phase transition when heated to 135 °C.27
3.3. Ink Formulation
Considering the ink formulation, the choice of the OSC clearly determines the device performance.35 However, other parameters of the ink formulation also have a key role. The concentration of a given material has a direct impact on the film thickness (i.e., lower concentration, thinner films). Hence, the same effects that have been previously observed by modifying the shearing speed can also be realized by controlling the concentration of the solution.26,28,29
In general, the solvent is selected to ensure a good enough solubility of OSCs. Commonly, high boiling point solvents are chosen (e.g., chlorobenzene, toluene, and tetralin) to control better the evaporation rate, which is also defined by the substrate temperature applied.46 Lower evaporation rates will more efficiently permit the migration and reorganization of the molecules during the crystallization. Also, the solvent can have an impact on the nucleation and crystallization depending on the OSC solubility, molecule solvation, and molecule–solvent interactions. In the preparation of organic single crystals, it has been widely known for a long time that different polymorphs can be obtained depending on the nature of the solvent.49 In solution shearing, this has also been proved in the blade shearing of TIPS-Pen.26 The authors found that solvents with a larger molar volume led to the metastable polymorph, whereas solvents with a smaller molar volume negated almost completely the effects of the spatial confinement. An interesting approach is the use of a mixture of two solvents. Playing with a mixture of anisole and toluene, thin films of TIPS-Pen were prepared by slot-die coating.22 This OSC has a good solubility in toluene but not in anisole. Further, toluene has a lower boiling point. The modification of the ratio between solvents could be exploited to control the crystallization to achieve an optimized device performance.
The use of a mixture of solvents has also been very recently used to compensate for the Marangoni flow caused by a temperature-dependent surface tension gradient near the meniscus line.50 This has a negative impact on the thin-film deposition and its electrical properties. Interestingly, the authors demonstrated that by preparing solutions of the OSC with two good solvents exhibiting different volatilities and surface tensions, it is possible to control the surface tension gradient at the meniscus to assist the mass transport of the OSC molecules toward the contact line. In this way, ultrathin films with better film coverage and showing an improved device performance could be achieved at higher shearing speeds.
A very powerful strategy employed in the last few years to fabricate crystalline OSC thin films of small molecules is the use of polymeric blends, usually mixing the OSC with an insulating polymer that acts as a matrix.2,7,9,51,52 This facilitates the processability of the OSC because the polymer acts as a binder and helps to overcome issues related to dewetting, which hampers the film uniformity. Further, the use of blends also promotes the OSC crystallization that takes place, induced by a vertical phase separation of the two materials. Solution shearing of blends of OSCs has also been reported recently, demonstrating that it represents a very successful route to achieve high OFET performance.24,25,35 In these blend-based inks, more parameters can be tuned that can have an important influence on the resulting films. In addition to the type of polymer employed, the molecular weight (Mw) of a polymer might have a remarkable effect on the thin film and hence the electrical device characteristics. The Mw determines the viscosity, solubility, and miscibility and might cause different stratification processes. Niazi et al.25 observed that blade-sheared films of blends of 2,8-difluoro-5,11-bis(triethylsilylethynyl)anthradithiophene (diF-TES-ADT) and PS revealed higher mobilities when increasing the Mw of PS, which was mainly accounted for the higher viscosity of the solution that gave rise to crack-free and smoother crystalline domains (Figure 5a). In the same work, the authors also reported that the use of solvent mixtures (i.e., a polar and nonpolar solvent) in which the two components of the blend show different degrees of solubility can be modified to optimize the thin-film crystal domains.25 Moreover, it has been shown on the blends of DB-TTF and PS that the ratio OSC/PS can be critical not only to the film morphology but also to the type of polymorph formed.31 Thin films deposited from blends with more than 50 wt % of DB-TTF content showed oriented crystals along the casting direction, but they corresponded to a mixture of crystals from the α and γ polymorphs. On the other hand, thin films deposited from 1:1 blends or blends containing less than 50 wt % of DB-TTF were more homogeneous with an isotropic plate-like crystalline domain and belonged to the γ-polymorph. Finally, it should also be highlighted that blending the OSC with insulating polymers also offer other advantages such as the reduction of charge traps at the dielectric/OSC interface53,54 and an enhanced device stability.24,55
Figure 5.
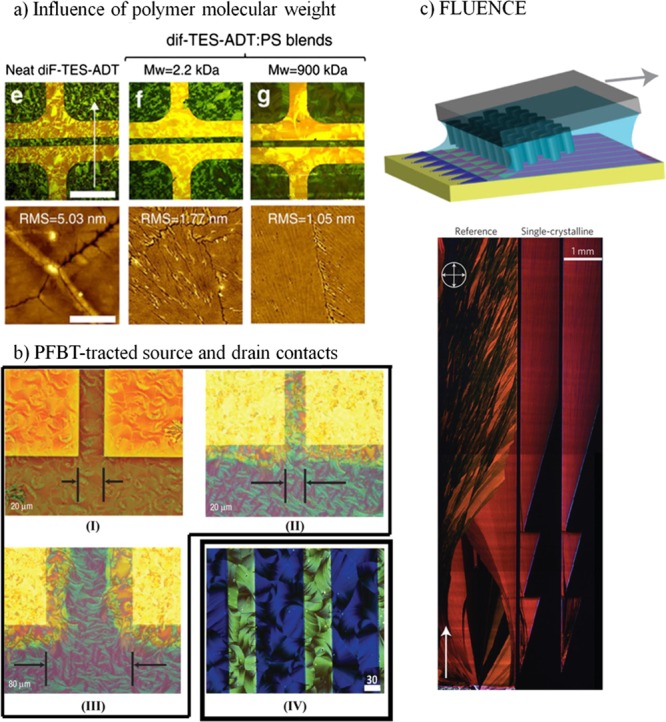
(a) Polarized optical micrographs and atomic force microscopy (AFM) images of (e) neat diF-TES-ADT, (f) low Mw blend (PS 2.2 kDa), and (g) high Mw blend (PS 900 kDa). The white arrow shows the direction of blade coating. Scale bars: 250 μm [in polarized optical microscopy (POM) images] and 4 μm (in AFM images). The diF-TES-ADT thin films were deposited by blade coating. (b) POM images of diF-TES-ADT thin films deposited by spin-coating (I, II, and III) and BAMS (IV). The source and drain contacts in (II, III, and IV) were treated with a PFBT SAM, whereas the ones in (I) were not functionalized. (c) Scheme of the FLUENCE methodology (top) and POM images of a TIPS-Pen film coated with (bottom right) and without FLUENCE (bottom left). Adapted with permission from ref (25) (2015 Macmillan Publishers Limited), ref (35) (2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim), ref (63) (2008 Macmillan Publishers Limited), and ref (6) (2013 Macmillan Publishers Limited).
3.4. Device Configuration
During the deposition of OSCs, a common challenge is to overcome surface dewetting issues that result in poor film homogeneity or low substrate coverage. This effect is determined by the surface energy. One of the most commonly used strategies to control the wettability of silicon dioxide (the most employed dielectric) is treating it with ozone or argon plasma, which results in an increase of the surface hydrophilicity that is often required to ensure solution wettability. Miskiewicz et al. reported the influence of the SiO2 surface energy on the performance of TTF-based transistors deposited by zone-casting.56 The authors concluded that a smaller mismatch in surface energy may result in better molecular packing and consequently more efficient charge transport. However, higher hydrophilic surfaces are generally detrimental to the electrical performance of the device because they generate charge traps. Hence, another approach is to modify the SiO2 substrate with molecular self-assembled monolayers (SAMs) that can tune the surface energy depending on the molecular functional group.57 In solution-shearing experiments, the meniscus formation and wetting/dewetting aspects are determined by the surface energy of the substrate and the blade/bar. Therefore, the growth of SAMs is often applied both on the substrate and on the oxide-based blades.58 Alternatively, changing the material of the bar has also been demonstrated to be a promising route to modify the solution meniscus and hence the resulting film homogeneity and coverage. Indeed, it was previously shown that the deposition by BAMS of a blend of the p-type semiconductor meso-diphenyl tetrathia[22]annulene[2,1,2,1] with PS was successfully achieved only when the metallic bar was replaced with a highly hydrophobic polytetrafluoroethylene bar.53 Problems related to dewetting are also encountered when employing hydrophobic polymeric substrates. When Parylene C and poly(vinylidene fluoride) were used as dielectrics in devices prepared by solution shearing of the OSCs, surface treatments with ozone or argon plasma were required.59,60
In bottom-contact devices, gold source and drain electrodes can also be modified by SAMs. This has commonly been done to modify the work function of the electrode to improve the charge injection, which in turn reduces the contact resistance, enhancing the device electrical characteristics.61 However, modification of the electrodes can also affect the morphology of the OSC layer adjacent to the SAM-covered electrodes. The first nanometers of the OSC layers are highly affected by the surface affinity and the chemical composition of the SAMs, which consecutively affects the upper film. The chemical composition mainly affects the interaction and induces extra nuclei, whereas the surface affinity affects the drying/crystallization time and thickness of the film.61 Previously, it was reported that the use of gold electrodes modified with a pentafluorobenzenethiol (PFBT) SAM dramatically improved the crystallization of TIPS-Pen and diF-TES-ADT in spin-coated films.62−65 It was observed that, because of the contact-induced crystallization, crystals grew from the contacts and extended 10 μm into the transistor channel (Figure 5b). However, in thin films of TIPS-Pen and diF-TES-ADT fabricated by blade shearing at 1.5 mm·s–1, PFBT SAMs disrupted the formation of spherulitic crystalline structures.62 Thus, the authors concluded that strongly interacting contacts were not suitable for achieving larger crystalline domains typically obtained by solution-shearing techniques. In stark contrast, when the same materials were deposited by BAMS at 10 mm·s–1, it was found that PFBT SAMs lead to more homogeneous and interconnected crystallites, which in turn resulted in higher-mobility OFET devices.35 Remarkably, such crystalline domains extended along the whole channel (in the range of 25–100 μm) (Figure 5b). The discrepancy between these results can be attributed to differences in the coating speed and in the solution confinement between the two techniques.
Crystal growth defects are very often observed in rapid solution-coating processes, such as in solution shearing, where voids or dendritic growth are found to be detrimental to the charge carrier transport. This is caused by mass transport limitations. A very interesting approach, namely, FLUENCE (i.e., fluid-enhanced crystal engineering), was recently reported aiming at gaining control on the fluid flow to enhance the crystallization and nucleation during the deposition process.6 FLUENCE consists of introducing specifically designed three-dimensional micropillars on the blade to induce recirculation of the ink (Figure 5c). Solution shearing mainly drives mass transport in the coating direction. The use of micropillars permits a rapid flow expansion of the solution, improving the lateral mass transport, which is perpendicular to the shearing direction, causing a more homogeneous crystal growth. Furthermore, the introduction of micropillars in the blade was combined with the addition of patterns on the substrate with solvent wetting and nonwetting regions using SAMs. The design was carried out considering that nucleation takes place preferentially at highly convex points along the contact line. Hence, the manipulation of the curvature of the contact line allows for the control of the crystal nucleation. Using FLUENCE, the fast coating of TIPS-Pen was demonstrated to give centimeter-long highly aligned single-crystalline films with a metastable crystal structure that led to an unprecedented average mobility of 8 cm2 V–1 s–1 (Figure 5c).
As previously mentioned, depending on the experimental conditions, anisotropic films can be prepared. In these cases, therefore, the coating direction, that is, the direction of the solution shearing, with respect to the channel length is pivotal. An exhaustive study of the influence of the shearing direction was performed by Lee et al. in a solution-sheared naphthalene diimide derivative film.32 The devices prepared with the channel length direction perpendicular to the shearing direction (i.e., angle of 90°) showed a maximum mobility of 0.41 cm2 V–1 s–1, whereas devices with parallel orientation (0°) exhibited almost one-third of the mobility. The worst mobility was found in the devices prepared at 45°. This mobility anisotropy was accounted to be dominated by the grain boundaries and cracks. In another report, a blend of TIPS-Pen and PS was bar-coated parallel and orthogonal to the channel length on a Parylene C dielectric with gold source/drain electrodes.60 It was observed that the crystal domain dimensions were much smaller on the devices prepared with the solution-shearing direction parallel to the channel length, which was ascribed to the step between the gold contacts and the Parylene C (ranging around 60–80 nm) and also to the different wettabilities of these materials. Surprisingly, the change in domain sizes did not make an impact on the mobility of the two types of devices; however, it caused important differences in their response to mechanical deformations.
3.5. External Stimuli: Electric Field
The application of an external stimulus during deposition can offer a promising tool to control the packing structure of a given OSC.66 This route has hardly been explored in solution-shearing processes, although external perturbations during deposition could potentially have a strong impact. Recently, Molina-Lopez et al. reported that the application of alternating electric fields can be exploited to modify the molecular packing of solution-sheared OSCs, specifically TIPS-Pen and 2,7-dioctyl[1]benzothieno[3,2-b][1]benzothiophene (C8-BTBT).67 The setup employed is sketched in Figure 6. A sharp blade moving at a steady velocity was used to cast the OSC solution, whereas a difference of electrical potential was maintained between the blade and the substrate. In this system, voltage and frequency could be modulated. Strong dielectropheretic (DEP) forces (FDEP) and torques (TDEP), which can cause translational and rotational motion of the molecules, are expected near the blade edge where nucleation and crystallization takes place. The experiments were limited to low coating velocities and low temperatures to give the molecules enough time to move in the solution. Interestingly, the authors observed a gradual transition between polymorphs as the DEP force increased, and metastable polymorphs, some of them unprecedented, were trapped. As a consequence, a twofold enhancement in the OFET mobilities was found for TIPS-Pen along the transport direction, and a threefold decrease was observed in the C8-BTBT films accompanied by an increase in anisotropy.
Figure 6.
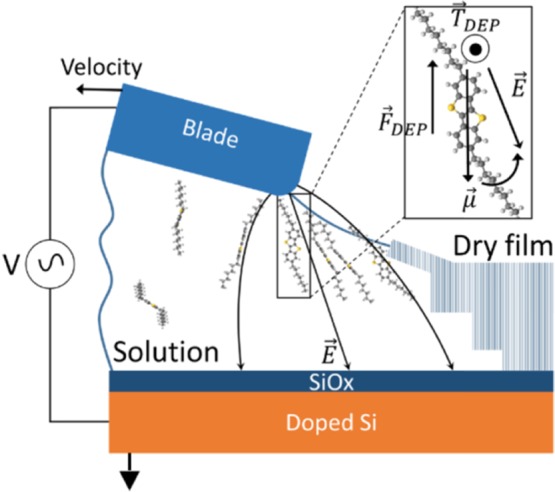
Setup employed for the application of dielectrophoresis to solution-sheared C8-BTBTand TIPS-Pen. Reprinted with permission from ref (67) (2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim).
4. Thin-Film Characterization
To progress toward the understanding of the influence of morphology and crystal structure on the charge transport properties, it is of paramount importance to advance the development of characterization techniques. The study of the thin-film morphology and the identification of polymorphs can be realized employing a variety of techniques. POM is a very accessible tool that gives information about the morphology of the film as well as its crystallinity. Such information can be complemented by other microscopy techniques such as AFM, which can bring out additional information regarding the film thickness or the terrace heights. To determine the crystal structure, X-ray techniques are obviously the most powerful ones.68 They provide information related to the crystal packing and also the orientation of the crystals with respect to the surface. Polarized absorption and Raman spectroscopies can also be used to find out about the in-plane orientation of the molecules in thin films.27,34,69 Lattice-phonon confocal Raman spectroscopy has also been proved to be a highly prevailing tool to identify OSC polymorphs in thin films because it is very sensitive to intermolecular interactions.36,70−72
For the characterization of OSCs, thin films are of great interest to gain information about the molecular ordering of the molecular layer close to the substrate. It is known that such a buried interfacial layer, which might have a different packing than the top layers,73−75 is mainly responsible for the charge transport properties in OFETs.76 Near-edge X-ray absorption fine-structure spectroscopy combined with thin-film delamination77,78 as well as vibrational sum frequency generation spectroscopy79,80 has been utilized to investigate such an interfacial layer. More recently, the combination of Raman spectroscopy and surface-enhanced Raman scattering has also been shown to afford useful information related to the interfacial packing of OSCs.28
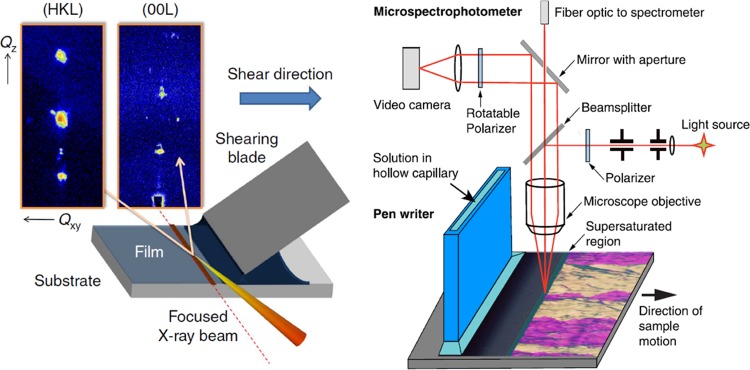
To be able to predict the polymorph formed in some given experimental conditions or to optimize the deposition parameters for a certain material, it is crucial to understand the crystallization mechanisms that take place.81 For this purpose, it is hence necessary to develop in situ characterization tools. This is extremely challenging in solution-shearing processes because OSC deposition and crystallization occur very rapidly. The introduction of very powerful laboratory X-ray sources, mainly a synchrotron radiation source, permits faster measurements due to the higher intensity and more sensitive diffraction analysis because the wavelength can be adjusted at Armstrong distance.82 In the last few years, in situ characterization of the nucleation and crystallization taking place in solution-sheared thin films have been reported using microbeam grazing incidence wide-angle X-ray scattering (Figure 7 (left)).26,83,84 Acquisition of the reciprocal space region probed by a fast two-dimensional (2D) X-ray detector gives information regarding the evolution of the crystallization process and the formation of transient structures. These studies are often combined with in situ high-speed POM measurements that are useful to observe the morphology and crystallinity changes.26,83 Further, in situ optical spectroscopy in the reflection mode has also been used to investigate the growth mechanism and thermal stability of solution-sheared TIPS-Pen.27 Spectra were obtained in the supersaturated region at the meniscus and in the solid film and evaluated using the setup shown in Figure 7 (right). The response is highly sensitive on the molecular packing and thermal expansion effects. Here, the authors concluded that it was likely that the crystallization process proceeds by the incorporation of molecular aggregates instead of individual molecules. The progress in in situ characterization techniques along with suitable theoretical models is definitely of high importance to progress in this field and achieve a controlled material crystallization.
Figure 7.
Left: Schematic representation of in situ X-ray diffraction used to study the crystallization and nucleation process during deposition by blade coating. Right: Schematic of in situ optical reflectance measurements and microscope system used to follow the crystallization and nucleation processes during deposition by hollow capillary writing. Figures are adapted with permission from ref (27) (2016 Macmillan Publishers Limited) and ref (26) (2014 Macmillan Publishers Limited).
5. Outlook
In the area of organic electronics, it is imperative to find routes for processing OSCs at high speed while ensuring high device-to-device reproducibility. OSCs are highly prone to polymorphism because of the weak intermolecular van der Waals forces. Thus, the access to different polymorphs depends on the competition between thermodynamics and kinetics. Further, because of the anisotropic character of the intermolecular interactions, the electron coupling in the different directions might vary significantly. Therefore, gaining control of the thin-film morphology (i.e., domain alignment and gain boundaries) and the polymorphism is essential. Previously, we discussed some of the parameters that can be exploited as a tool box to modulate the thin-film crystallinity and morphology, such as substrate temperature, speed, ink formulation, and so forth. We tried to address all these factors separately with the aim of understanding their specific influence. However, all of these parameters are strongly interrelated, making this panorama very complex. For instance, depending on the boiling point of the solvent chosen, different temperatures will be applied, or achieving an immediate evaporation of the solvent will depend on both temperature and speed of the bar/blade. The combination of all these variables determine the driving crystallization regime. It also seems that adding external stimuli during solution shearing, quite an unexplored approach, could also bring new tools to control the resulting thin films.66 Further, technological advances aimed at achieving a patterned OSC film with a controlled morphology and structure will probably be a focus of research in the near future.85 Undoubtedly, to progress in the control of thin-film structure, it is also key to develop in situ characterization techniques. Another critical issue that has been quite overlooked is the study of the time stability of the different polymorphic forms because they are often metastable, that is, the thermodynamic form is not the one formed. Further, it should be noticed that even a low degree of polymorphs mixture, which is sometimes difficult to detect, can have a very negative impact on the device performance. Only when the thin-film polymorphism and morphology can be controlled at will, it will be possible to fabricate highly reproducible, durable, high-performing organic-based devices.
Acknowledgments
This work was funded by the European Research Council (ERC) StG 2012-306826 e-GAMES project. The authors also thank the Networking Research Center on Bioengineering, Biomaterials, and Nanomedicine (CIBER-BBN), the Generalitat de Catalunya (2014-SGR-17), and the Spanish Ministry of Economy and Competitiveness through the project FANCY CTQ2016-80030-R and through the “Severo Ochoa” Programme for Centers of Excellence in R&D (SEV-2015-0496). A.T. is enrolled in the Materials Science Program of UAB and acknowledges FPU fellowship from the Ministry.
The authors declare no competing financial interest.
References
- Mas-Torrent M.; Rovira C. Novel Small Molecules for Organic Field-Effect Transistors: Towards Processability and High Performance. Chem. Soc. Rev. 2008, 37, 827–838. 10.1039/b614393h. [DOI] [PubMed] [Google Scholar]
- Smith J.; Zhang W.; Sougrat R.; Zhao K.; Li R.; Cha D.; Amassian A.; Heeney M.; McCulloch I.; Anthopoulos T. D. Solution-Processed Small Molecule-Polymer Blend Organic Thin-Film Transistors with Hole Mobility Greater than 5 cm2/Vs. Adv. Mater. 2012, 24, 2441–2446. 10.1002/adma.201200088. [DOI] [PubMed] [Google Scholar]
- Stingelin-Stutzmann N.; Smits E.; Wondergem H.; Tanase C.; Blom P.; Smith P.; de Leeuw D. Organic Thin-Film Electronics from Vitreous Solution-Processed Rubrene Hypereutectics. Nat. Mater. 2005, 4, 601–606. 10.1038/nmat1426. [DOI] [PubMed] [Google Scholar]
- Matsidik R.; Komber H.; Luzio A.; Caironi M.; Sommer M. Defect-Free Naphthalene Diimide Bithiophene Copolymers with Controlled Molar Mass and High Performance via Direct Arylation Polycondensation. J. Am. Chem. Soc. 2015, 137, 6705–6711. 10.1021/jacs.5b03355. [DOI] [PubMed] [Google Scholar]
- Becerril H. A.; Roberts M. E.; Liu Z.; Locklin J.; Bao Z. High-Performance Organic Thin-Film Transistors through Solution-Sheared Deposition of Small-Molecule Organic Semiconductors. Adv. Mater. 2008, 20, 2588–2594. 10.1002/adma.200703120. [DOI] [Google Scholar]
- Diao Y.; Tee B. C.-K.; Giri G.; Xu J.; Kim D. H.; Becerril H. A.; Stoltenberg R. M.; Lee T. H.; Xue G.; Mannsfeld S. C. B.; Bao Z. Solution Coating of Large-Area Organic Semiconductor Thin Films with Aligned Single-Crystalline Domains. Nat. Mater. 2013, 12, 665–671. 10.1038/nmat3650. [DOI] [PubMed] [Google Scholar]
- Paterson A. F.; Treat N. D.; Zhang W.; Fei Z.; Wyatt-Moon G.; Faber H.; Vourlias G.; Patsalas P. A.; Solomeshch O.; Tessler N.; Heeney M.; Anthopoulos T. D. Small Molecule/Polymer Blend Organic Transistors with Hole Mobility Exceeding 13 cm2 V-1 s-1. Adv. Mater. 2016, 28, 7791–7798. 10.1002/adma.201601075. [DOI] [PubMed] [Google Scholar]
- Giri G.; Verploegen E.; Mannsfeld S. C. B.; Atahan-Evrenk S.; Kim D. H.; Lee S. Y.; Becerril H. A.; Aspuru-Guzik A.; Toney M. F.; Bao Z. Tuning Charge Transport in Solution-Sheared Organic Semiconductors Using Lattice Strain. Nature 2011, 480, 504–508. 10.1038/nature10683. [DOI] [PubMed] [Google Scholar]
- Ford M. J.; Wang M.; Patel S. N.; Phan H.; Segalman R. A.; Nguyen T.-Q.; Bazan G. C. High Mobility Organic Field-Effect Transistors from Majority Insulator Blends. Chem. Mater. 2016, 28, 1256–1260. 10.1021/acs.chemmater.5b04774. [DOI] [Google Scholar]
- Tseng H.-R.; Phan H.; Luo C.; Wang M.; Perez L. A.; Patel S. N.; Ying L.; Kramer E. J.; Nguyen T.-Q.; Bazan G. C.; Heeger A. J. High-Mobility Field-Effect Transistors Fabricated with Macroscopic Aligned Semiconducting Polymers. Adv. Mater. 2014, 26, 2993–2998. 10.1002/adma.201305084. [DOI] [PubMed] [Google Scholar]
- Back J. Y.; Yu H.; Song I.; Kang I.; Ahn H.; Shin T. J.; Kwon S.-K.; Oh J. H.; Kim Y.-H. Investigation of Structure-Property Relationships in Diketopyrrolopyrrole-Based Polymer Semiconductors via Side-Chain Engineering. Chem. Mater. 2015, 27, 1732–1739. 10.1021/cm504545e. [DOI] [Google Scholar]
- Xu Y.; Liu C.; Khim D.; Noh Y.-Y. Development of High-Performance Printed Organic Field-Effect Transistors and Integrated Circuits. Phys. Chem. Chem. Phys. 2015, 17, 26553–26574. 10.1039/c4cp02413c. [DOI] [PubMed] [Google Scholar]
- Diao Y.; Shaw L.; Bao Z.; Mannsfeld S. C. B. Morphology Control Strategies for Solution- Processed Organic Semiconductor Thin Films. Energy Environ. Sci. 2014, 7, 2145–2159. 10.1039/c4ee00688g. [DOI] [Google Scholar]
- Pfattner R.; Bromley S. T.; Rovira C.; Mas-Torrent M. Tuning Crystal Ordering, Electronic Structure, and Morphology in Organic Semiconductors: Tetrathiafulvalenes as a Model Case. Adv. Funct. Mater. 2016, 26, 2256–2275. 10.1002/adfm.201502446. [DOI] [Google Scholar]
- Coropceanu V.; Cornil J.; da Silva Filho D. A.; Olivier Y.; Silbey R.; Brédas J.-L. Charge Transport in Organic Semiconductors. Chem. Rev. 2007, 107, 926–952. 10.1021/cr050140x. [DOI] [PubMed] [Google Scholar]
- Mas-Torrent M.; Rovira C. Role of Molecular Order and Solid-State Structure in Organic Field-Effect Transistors. Chem. Rev. 2011, 111, 4833–4856. 10.1021/cr100142w. [DOI] [PubMed] [Google Scholar]
- Chung H.; Diao Y. Polymorphism as an Emerging Design Strategy for High Performance Organic Electronics. J. Mater. Chem. C 2016, 4, 3915–3933. 10.1039/c5tc04390e. [DOI] [Google Scholar]
- Hiszpanski A. M.; Baur R. M.; Kim B.; Tremblay N. J.; Nuckolls C.; Woll A. R.; Loo Y.-L. Tuning Polymorphism and Orientation in Organic Semiconductor Thin Films via Post-Deposition Processing. J. Am. Chem. Soc. 2014, 136, 15749–15756. 10.1021/ja5091035. [DOI] [PubMed] [Google Scholar]
- Tracz A.; Jeszka J. K.; Watson M. D.; Pisula W.; Müllen K.; Pakula T. Uniaxial Alignment of the Columnar Super-Structure of a Hexa (Alkyl) Hexa-Peri-Hexabenzocoronene on Untreated Glass by Simple Solution Processing. J. Am. Chem. Soc. 2003, 125, 1682–1683. 10.1021/ja028945z. [DOI] [PubMed] [Google Scholar]
- Pisula W.; Menon A.; Stepputat M.; Lieberwirth I.; Kolb U.; Tracz A.; Sirringhaus H.; Pakula T.; Müllen K. A Zone-Casting Technique for Device Fabrication of Field-Effect Transistors Based on Discotic Hexa-Peri-Hexabenzocoronene. Adv. Mater. 2005, 17, 684–688. 10.1002/adma.200401171. [DOI] [Google Scholar]
- Mas-Torrent M.; Masirek S.; Hadley P.; Crivillers N.; Oxtoby N. S.; Reuter P.; Veciana J.; Rovira C.; Tracz A. Organic Field-Effect Transistors (OFETs) of Highly Oriented Films of Dithiophene-Tetrathiafulvalene Prepared by Zone Casting. Org. Electron. 2008, 9, 143–148. 10.1016/j.orgel.2007.09.007. [DOI] [Google Scholar]
- Chang J.; Chi C.; Zhang J.; Wu J. Controlled Growth of Large-Area High-Performance Small-Molecule Organic Single-Crystalline Transistors by Slot-Die Coating Using A Mixed Solvent System. Adv. Mater. 2013, 25, 6442–6447. 10.1002/adma.201301267. [DOI] [PubMed] [Google Scholar]
- Headrick R. L.; Wo S.; Sansoz F.; Anthony J. E. Anisotropic Mobility in Large Grain Size Solution Processed Organic Semiconductor Thin Films. Appl. Phys. Lett. 2008, 92, 063302. 10.1063/1.2839394. [DOI] [Google Scholar]
- Del Pozo F. G.; Fabiano S.; Pfattner R.; Georgakopoulos S.; Galindo S.; Liu X.; Braun S.; Fahlman M.; Veciana J.; Rovira C.; Crispin X.; Berggren M.; Mas-Torrent M. Single Crystal-like Performance in Solution-Coated Thin-Film Organic Field-Effect Transistors. Adv. Funct. Mater. 2016, 26, 2379–2386. 10.1002/adfm.201502274. [DOI] [Google Scholar]
- Niazi M. R.; Li R.; Li E. Q.; Kirmani A. R.; Abdelsamie M.; Wang Q.; Pan W.; Payne M. M.; Anthony J. E.; Smilgies D.-M.; Thoroddsen S. T.; Giannelis E. P.; Amassian A. Solution-Printed Organic Semiconductor Blends Exhibiting Transport Properties on Par with Single Crystals. Nat. Commun. 2015, 6, 8598. 10.1038/ncomms9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri G.; Li R.; Smilgies D.-M.; Li E. Q.; Diao Y.; Lenn K. M.; Chiu M.; Lin D. W.; Allen R.; Reinspach J.; Mannsfeld S. C. B.; Thoroddsen S. T.; Clancy P.; Bao Z.; Amassian A. One-Dimensional Self-Confinement Promotes Polymorph Selection in Large-Area Organic Semiconductor Thin Films. Nat. Commun. 2014, 5, 3573. 10.1038/ncomms4573. [DOI] [PubMed] [Google Scholar]
- Li Y.; Wan J.; Smilgies D.-M.; Bouffard N.; Sun R.; Headrick R. L. Nucleation and Strain-Stabilization during Organic Semiconductor Thin Film Deposition. Sci. Rep. 2016, 6, 32620. 10.1038/srep32620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Diao Y.; Zhou D.; Mao Y.; Giri G.; Chen W.; Liu N.; Mannsfeld S. C. B.; Xue G.; Bao Z. Probing the Interfacial Molecular Packing in TIPS-Pentacene Organic Semiconductors by Surface Enhanced Raman Scattering. J. Mater. Chem. C 2014, 2, 2985–2991. 10.1039/c3tc32581d. [DOI] [Google Scholar]
- Cour I.; Chinta P. V.; Schlepütz C. M.; Yang Y.; Clarke R.; Pindak R.; Headrick R. L. Origin of Stress and Enhanced Carrier Transport in Solution-Cast Organic Semiconductor Films. J. Appl. Phys. 2013, 114, 093501. 10.1063/1.4820384. [DOI] [Google Scholar]
- Miskiewicz P.; Mas-Torrent M.; Jung J.; Kotarba S.; Glowacki I.; Gomar-Nadal E.; Amabilino D. B.; Veciana J.; Krause B.; Carbone D.; Rovira C.; Ulanski J. Efficient High Area OFETs by Solution Based Processing of a π-Electron Rich Donor. Chem. Mater. 2006, 18, 4724–4729. 10.1021/cm060675m. [DOI] [Google Scholar]
- Galindo S.; Tamayo A.; Leonardi F.; Mas-Torrent M. Control of Polymorphism and Morphology in Solution Sheared Organic Field-Effect Transistors. Adv. Funct. Mater. 2017, 27, 1700526. 10.1002/adfm.201700526. [DOI] [Google Scholar]
- Lee W.-Y.; Oh J. H.; Suraru S.-L.; Chen W.-C.; Würthner F.; Bao Z. High-Mobility Air-Stable Solution-Shear-Processed N-Channel Organic Transistors Based on Core-Chlorinated Naphthalene Diimides. Adv. Funct. Mater. 2011, 21, 4173–4181. 10.1002/adfm.201101606. [DOI] [Google Scholar]
- Pisula B. W.; Menon A.; Stepputat M.; Lieberwirth I.; Kolb U.; Tracz A.; Sirringhaus H.; Pakula T.; Müllen K. A Zone-Casting Technique for Device Fabrication of Field-Effect Transistors Based on Discotic Hexa-Peri-Hexabenzocoronene. Adv. Mater. 2005, 17, 684–689. 10.1002/adma.200401171. [DOI] [Google Scholar]
- Kotarba S.; Jung J.; Kowalska A.; Marszalek T.; Kozanecki M.; Miskiewicz P.; Mas-Torrent M.; Rovira C.; Veciana J.; Puigmarti-Luis J.; Ulanski J. Anisotropy in Structural and Physical Properties in Tetrathiafulvalene Derivatives-Based Zone-Cast Layers as Seen by Raman Spectroscopy, UV-Visible Spectroscopy, and Field Effect Measurements. J. Appl. Phys. 2010, 108, 014504. 10.1063/1.3311554. [DOI] [Google Scholar]
- Temiño I.; Del Pozo F. G.; Ajayakumar M. R.; Galindo S.; Puigdollers J.; Mas-Torrent M. A Rapid, Low-Cost, and Scalable Technique for Printing State-of-the-Art Organic Field-Effect Transistors. Adv. Mater. Technol. 2016, 1, 1600090. 10.1002/admt.201600090. [DOI] [Google Scholar]
- Brillante A.; Bilotti I.; Della Valle R. G.; Venuti E.; Milita S.; Dionigi C.; Borgatti F.; Lazar A. N.; Biscarini F.; Mas-Torrent M.; Oxtoby N. S.; Crivillers N.; Veciana J.; Rovira C.; Leufgen M.; Schmidt G.; Molenkamp L. W. The Four Polymorphic Modifications of the Semiconductor Dibenzo-Tetrathiafulvalene. CrystEngComm 2008, 10, 1899. 10.1039/b810993a. [DOI] [Google Scholar]
- Landau L.; Levich B. Dragging of a Liquid by a Moving Plate. Acta Physicochim. URSS 1942, 17, 42. [Google Scholar]
- Dixit H. N.; Homsy G. M. The Elastic Landau–Levich Problem. J. Fluid Mech. 2013, 732, 5–28. 10.1017/jfm.2013.382. [DOI] [Google Scholar]
- Grosso D. How to Exploit the Full Potential of the Dip-Coating Process to Better Control Film Formation. J. Mater. Chem. 2011, 21, 17033. 10.1039/c1jm12837j. [DOI] [Google Scholar]
- Laquindanum J. G.; Katz H. E.; Lovinger A. J. Synthesis, Morphology, and Field-Effect Mobility of Anthradithiophenes. J. Am. Chem. Soc. 1998, 120, 664–672. 10.1021/ja9728381. [DOI] [Google Scholar]
- Horowitz G.; Hajlaoui M. E. Grain Size Dependent Mobility in Polycrystalline Organic Field-Effect Transistors. Synth. Met. 2001, 122, 185–189. 10.1016/s0379-6779(00)01351-5. [DOI] [Google Scholar]
- Shtein M.; Mapel J.; Benziger J. B.; Forrest S. R. Effects of Film Morphology and Gate Dielectric Surface Preparation on the Electrical Characteristics of Organic-Vapor-Phase-Deposited Pentacene Thin-Film Transistors. Appl. Phys. Lett. 2002, 81, 268–270. 10.1063/1.1491009. [DOI] [Google Scholar]
- Letizia J. A.; Facchetti A.; Stern C. L.; Ratner M. A.; Marks T. J. High Electron Mobility in Solution-Cast and Vapor-Deposited Phenacyl-Quaterthiophene-Based Field-Effect Transistors: Toward N-Type Polythiophenes. J. Am. Chem. Soc. 2005, 127, 13476–13477. 10.1021/ja054276o. [DOI] [PubMed] [Google Scholar]
- Puigdollers J.; Della Pirriera M.; Marsal A.; Orpella A.; Cheylan S.; Voz C.; Alcubilla R. N-Type PTCDI–C13H27 Thin-Film Transistors Deposited at Different Substrate Temperature. Thin Solid Films 2009, 517, 6271–6274. 10.1016/j.tsf.2009.02.113. [DOI] [Google Scholar]
- Locklin J.; Roberts M. E.; Mannsfeld S. C. B.; Bao Z. Optimizing the Thin Film Morphology of Organic Field-Effect Transistors: The Influence of Molecular Structure and Vacuum Deposition Parameters on Device Performance. J. Macromol. Sci., Polym. Rev. 2006, 46, 79–101. 10.1080/15321790500471244. [DOI] [Google Scholar]
- Janneck R.; Vercesi F.; Heremans P.; Genoe J.; Rolin C. Predictive Model for the Meniscus-Guided Coating of High-Quality Organic Single-Crystalline Thin Films. Adv. Mater. 2016, 28, 8007–8013. 10.1002/adma.201602377. [DOI] [PubMed] [Google Scholar]
- Chen J.; Anthony J.; Martin D. C. Thermally Induced Solid-State Phase Transition of Bis(triisopropylsilylethynyl) Pentacene Crystals. J. Phys. Chem. B 2006, 110, 16397–16403. 10.1021/jp0627877. [DOI] [PubMed] [Google Scholar]
- Diao Y.; Lenn K. M.; Lee W.-Y.; Blood-Forsythe M. A.; Xu J.; Mao Y.; Kim Y.; Reinspach J. A.; Park S.; Xue G.; Clancy P.; Bao Z.; Mannsfeld S. C. B. Understanding Polymorphism in Organic Semiconductor Thin Films through Nanoconfinement. J. Am. Chem. Soc. 2014, 136, 17046–17057. 10.1021/ja507179d. [DOI] [PubMed] [Google Scholar]
- Chen J.; Shao M.; Xiao K.; Rondinone A. J.; Loo Y.-L.; Kent P. R. C.; Sumpter B. G.; Li D.; Keum J. K.; Diemer P. J.; Anthony J. E.; Jurchescu O. D.; Huang J. Solvent-Type-Dependent Polymorphism and Charge Transport in a Long Fused-Ring Organic Semiconductor. Nanoscale 2014, 6, 449–456. 10.1039/c3nr04341j. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Peng B.; Ji X.; Pei K.; Chan P. K. L. Marangoni-Effect-Assisted Bar-Coating Method for High-Quality Organic Crystals with Compressive and Tensile Strains. Adv. Funct. Mater. 2017, 27, 1703443. 10.1002/adfm.201703443. [DOI] [Google Scholar]
- Smith J.; Hamilton R.; McCulloch I.; Stingelin-Stutzmann N.; Heeney M.; Bradley D. D. C.; Anthopoulos T. D. Solution-Processed Organic Transistors Based on Semiconducting Blends. J. Mater. Chem. 2010, 20, 2562. 10.1039/b921674j. [DOI] [Google Scholar]
- Park B.; Jeon H. G.; Choi J.; Kim Y. K.; Lim J.; Jung J.; Cho S. Y.; Lee C. High-Performance Organic Thin-Film Transistors with Polymer-Blended Small-Molecular Semiconductor Films, Fabricated Using a Pre-Metered Coating Process. J. Mater. Chem. 2012, 22, 5641–5646. 10.1039/c2jm16007b. [DOI] [Google Scholar]
- Campos A.; Zhang Q.; Ajayakumar M. R.; Leonardi F.; Mas-Torrent M. High Performance Organic Field-Effect Transistor with a Solid and Aqueous Dielectric Based on a Solution Sheared Sulfur-Bridged Annulene Derivative. Adv. Electron. Mater. 2017, 1700349. 10.1002/aelm.201700349. [DOI] [Google Scholar]
- Hunter S.; Mottram A. D.; Anthopoulos T. D. Temperature and Composition-Dependent Density of States in Organic Small-Molecule/Polymer Blend Transistors. J. Appl. Phys. 2016, 120, 025502. 10.1063/1.4955282. [DOI] [Google Scholar]
- Lee J.; Jung J. Y.; Kim D. H.; Kim J.-Y.; Lee B.-L.; Park J.-I.; Chung J. W.; Park J. S.; Koo B.; Jin Y. W.; Lee S. Enhanced Electrical Stability of Organic Thin-Film Transistors with Polymer Semiconductor-Insulator Blended Active Layers. Appl. Phys. Lett. 2012, 100, 083302. 10.1063/1.3688177. [DOI] [Google Scholar]
- Miskiewicz P.; Kotarba S.; Jung J.; Marszalek T.; Mas-Torrent M.; Gomar-Nadal E.; Amabilino D. B.; Rovira C.; Veciana J.; Maniukiewicz W.; Ulanski J. Influence of SiO2 Surface Energy on the Performance of Organic Field Effect Transistors Based on Highly Oriented, Zone-Cast Layers of a Tetrathiafulvalene Derivative. J. Appl. Phys. 2008, 104, 054509. 10.1063/1.2968441. [DOI] [Google Scholar]
- Janssen D.; De Palma R.; Verlaak S.; Heremans P.; Dehaen W. Static Solvent Contact Angle Measurements, Surface Free Energy and Wettability Determination of Various Self-Assembled Monolayers on Silicon Dioxide. Thin Solid Films 2006, 515, 1433–1438. 10.1016/j.tsf.2006.04.006. [DOI] [Google Scholar]
- Giri G.; Park S.; Vosgueritchian M.; Shulaker M. M.; Bao Z. High-Mobility, Aligned Crystalline Domains of Tips-Pentacene with Metastable Polymorphs through Lateral Confinement of Crystal Growth. Adv. Mater. 2014, 26, 487–493. 10.1002/adma.201302439. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos S.; del Pozo F. G.; Mas-Torrent M. Flexible Organic Transistors Based on a Solution-Sheared PVDF Insulator. J. Mater. Chem. C 2015, 3, 12199–12202. 10.1039/c5tc02488a. [DOI] [Google Scholar]
- Lai S.; Temiño I.; Cramer T.; del Pozo F. G.; Fraboni B.; Cosseddu P.; Bonfiglio A.; Mas-Torrent M. Morphology Influence on the Mechanical Stress Response in Bendable Organic Field-Effect Transistors with Solution-Processed Semiconductors. Adv. Electron. Mater. 2017, 1700271. 10.1002/aelm.201700271. [DOI] [Google Scholar]
- Liu C.; Xu Y.; Noh Y.-Y. Contact Engineering in Organic Field-Effect Transistors. Mater. Today 2015, 18, 79–96. 10.1016/j.mattod.2014.08.037. [DOI] [Google Scholar]
- Niazi M. R.; Li R.; Abdelsamie M.; Zhao K.; Anjum D. H.; Payne M. M.; Anthony J.; Smilgies D.-M.; Amassian A. Contact-Induced Nucleation in High-Performance Bottom-Contact Organic Thin Film Transistors Manufactured by Large-Area Compatible Solution Processing. Adv. Funct. Mater. 2016, 26, 2371–2378. 10.1002/adfm.201502428. [DOI] [Google Scholar]
- Gundlach D. J.; Royer J. E.; Park S. K.; Subramanian S.; Jurchescu O. D.; Hamadani B. H.; Moad A. J.; Kline R. J.; Teague L. C.; Kirillov O.; Richter C. A.; Kushmerick J. G.; Richter L. J.; Parkin S. R.; Jackson T. N.; Anthony J. E. Contact-Induced Crystallinity for High-Performance Soluble Acene-Based Transistors and Circuits. Nat. Mater. 2008, 7, 216–221. 10.1038/nmat2122. [DOI] [PubMed] [Google Scholar]
- Ward J. W.; Loth M. A.; Kline R. J.; Coll M.; Ocal C.; Anthony J. E.; Jurchescu O. D. Tailored Interfaces for Self-Patterning Organic Thin-Film Transistors. J. Mater. Chem. 2012, 22, 19047–19053. 10.1039/c2jm33974a. [DOI] [Google Scholar]
- Li R.; Ward J. W.; Smilgies D.-M.; Payne M. M.; Anthony J. E.; Jurchescu O. D.; Amassian A. Direct Structural Mapping of Organic Field-Effect Transistors Reveals Bottlenecks to Carrier Transport. Adv. Mater. 2012, 24, 5553–5558. 10.1002/adma.201201856. [DOI] [PubMed] [Google Scholar]
- Diemer P. J.; Lyle C. R.; Mei Y.; Sutton C.; Payne M. M.; Anthony J. E.; Coropceanu V.; Brédas J.-L.; Jurchescu O. D. Vibration-Assisted Crystallization Improves Organic/Dielectric Interface in Organic Thin-Film Transistors. Adv. Mater. 2013, 25, 6956–6962. 10.1002/adma.201302838. [DOI] [PubMed] [Google Scholar]
- Molina-Lopez F.; Yan H.; Gu X.; Kim Y.; Toney M. F.; Bao Z. Electric Field Tuning Molecular Packing and Electrical Properties of Solution-Shearing Coated Organic Semiconducting Thin Films. Adv. Funct. Mater. 2017, 27, 1605503. 10.1002/adfm.201605503. [DOI] [Google Scholar]
- Jones A. O. F.; Chattopadhyay B.; Geerts Y. H.; Resel R. Substrate-Induced and Thin-Film Phases: Polymorphism of Organic Materials on Surfaces. Adv. Funct. Mater. 2016, 26, 2233–2255. 10.1002/adfm.201503169. [DOI] [Google Scholar]
- Shibata Y.; Tsutsumi J.; Matsuoka S.; Minemawari H.; Arai S.; Kumai R. hAS. Unidirectionally Crystallized Stable N-Type Organic Thin-Film Transistors Based on Solution-Processable Donor–Acceptor Compounds. Adv. Electron. Mater. 2017, 3, 1700097. 10.1002/aelm.201700097. [DOI] [Google Scholar]
- Brillante A.; Bilotti I.; Biscarini F.; Della Valle R. G.; Venuti E. Polymorphs of a-Sexithiophene Probed by Lattice Phonon Raman Microscopy. Chem. Phys. 2006, 328, 125–131. 10.1016/j.chemphys.2006.06.018. [DOI] [Google Scholar]
- Pfattner R.; Mas-Torrent M.; Bilotti I.; Brillante A.; Milita S.; Liscio F.; Biscarini F.; Marszalek T.; Ulanski J.; Nosal A.; Gazicki-Lipman M.; Leufgen M.; Schmidt G.; Molenkamp L. W.; Laukhin V.; Veciana J.; Rovira C. High-Performance Single Crystal Organic Field-Effect Transistors Based on Two Dithiophene-Tetrathiafulvalene (DT-TTF) Polymorphs. Adv. Mater. 2010, 22, 4198–4203. 10.1002/adma.201001446. [DOI] [PubMed] [Google Scholar]
- Brillante A.; Bilotti I.; Della Valle R. G.; Venuti E.; Masino M.; Girlando A. Characterization of Phase Purity in Organic Semiconductors by Lattice- Phonon Confocal Raman Mapping: Application to Pentacene. Adv. Mater. 2005, 17, 2549–2553. 10.1002/adma.200501350. [DOI] [Google Scholar]
- Kalihari V.; Ellison D. J.; Haugstad G.; Frisbie C. D. Observation of Unusual Homoepitaxy in Ultrathin Pentacene Films and Correlation with Surface Electrostatic Potential. Adv. Mater. 2009, 21, 3092–3098. 10.1002/adma.200900362. [DOI] [Google Scholar]
- Yang H.; Shin T. J.; Ling M.-M.; Cho K.; Ryu C. Y.; Bao Z. Conducting AFM and 2D GIXD Studies on Pentacene Thin Films. J. Am. Chem. Soc. 2005, 127, 11542–11543. 10.1021/ja052478e. [DOI] [PubMed] [Google Scholar]
- Fritz S. E.; Martin S. M.; Frisbie C. D.; Ward M. D.; Toney M. F. Structural Characterization of a Pentacene Monolayer on an Amorphous SiO2 Substrate with Grazing Incidence X-Ray Diffraction. J. Am. Chem. Soc. 2004, 126, 4084–4085. 10.1021/ja049726b. [DOI] [PubMed] [Google Scholar]
- Dinelli F.; Murgia M.; Levy P.; Cavallini M.; Biscarini F.; de Leeuw D. M. Spatially Correlated Charge Transport in Organic Thin Film Transistors. Phys. Rev. Lett. 2004, 92, 116802. 10.1103/physrevlett.92.116802. [DOI] [PubMed] [Google Scholar]
- Delongchamp D. M.; Kline R. J.; Fischer D. A.; Richter L. J.; Toney M. F. Molecular Characterization of Organic Electronic Films. Adv. Mater. 2011, 23, 319–337. 10.1002/adma.201001760. [DOI] [PubMed] [Google Scholar]
- Kim J. B.; Lee S.; Toney M. F.; Chen Z.; Facchetti A.; Kim Y. S.; Loo Y.-L. Reversible Soft-Contact Lamination and Delamination for Non-Invasive Fabrication and Characterization of Bulk-Heterojunction and Bilayer Organic Solar Cells. Chem. Mater. 2010, 22, 4931–4938. 10.1021/cm101692a. [DOI] [Google Scholar]
- Walter S. R.; Youn J.; Emery J. D.; Kewalramani S.; Hennek J. W.; Bedzyk M. J.; Facchetti A.; Marks T. J.; Geiger F. M. In-Situ Probe of Gate Dielectric-Semiconductor Interfacial Order in Organic Transistors: Origin and Control of Large Performance Sensitivities. J. Am. Chem. Soc. 2012, 134, 11726–11733. 10.1021/ja3036493. [DOI] [PubMed] [Google Scholar]
- Vázquez A. V.; Holden B.; Kristalyn C.; Fuller M.; Wilkerson B.; Chen Z. Surface and Buried Interfacial Structures of Epoxy Resins Used as Underfills Studied by Sum Frequency Generation Vibrational Spectroscopy. ACS Appl. Mater. Interfaces 2011, 3, 1640–1651. 10.1021/am2001899. [DOI] [PubMed] [Google Scholar]
- Richter L. J.; Delongchamp D. M.; Amassian A. Morphology Development in Solution-Processed Functional Organic Blend Films: An In Situ Viewpoint. Chem. Rev. 2017, 117, 6332–6366. 10.1021/acs.chemrev.6b00618. [DOI] [PubMed] [Google Scholar]
- Nowak D. E.; Blasini D. R.; Vodnick A. M.; Blank B.; Tate M. W.; Deyhim A.; Smilgies D.-M.; Abruño H.; Gruner S. M.; Baker S. P. Six-Circle Diffractometer with Atmosphere- and Temperature-Controlled Sample Stage and Area and Line Detectors for Use in the G2 Experimental Station at CHESS. Rev. Sci. Instrum. 2006, 77, 113301. [Google Scholar]
- Wan J.; Li Y.; Ulbrandt J. G.; Smilgies D.-M.; Hollin J.; Whalley A. C.; Headrick R. L. Transient Phases during Fast Crystallization of Organic Thin Films from Solution. APL Mater. 2016, 4, 016103. 10.1063/1.4939464. [DOI] [Google Scholar]
- Smilgies D.-M.; Li R.; Giri G.; Chou K. W.; Diao Y.; Bao Z.; Amassian A. Look Fast: Crystallization of Conjugated Molecules during Solution Shearing Probed in-Situ and in Real Time by X-Ray Scattering. Phys. Status Solidi RRL 2013, 7, 177–179. 10.1002/pssr.201206507. [DOI] [Google Scholar]
- Shin J.; Hong T. R.; Lee T. W.; Kim A.; Kim Y. H.; Cho M. J.; Choi D. H. Template-Guided Solution-Shearing Method for Enhanced Charge Carrier Mobility in Diketopyrrolopyrrole-Based Polymer Field-Effect Transistors. Adv. Mater. 2014, 26, 6031–6035. 10.1002/adma.201401179. [DOI] [PubMed] [Google Scholar]