Abstract
Exosomes are extracellular vesicles involved in cell-to-cell communication. Previous large scale proteomics revealed that they contain SLC proteins. However, no data on the function of exosomal SLCs is available, so far. An SLC localized in exosomes was here characterized for the first time: the carnitine transporter OCTN2 (SLC22A5). The protein was detected by Western Blot analysis in HEK293 exosomes. To investigate the functional properties of the exosomal OCTN2, the proteins extracted from vesicles were reconstituted into proteolipsomes and the transport function was measured as uptake of 3H-carnitine. Transport was stimulated by sodium and was dependent on pH. 3H-carnitine uptake was inhibited by Acetyl-carnitine, but not by Asn, Gln and Arg thus excluding interference by ATB0,+, an amino acid transporter which also recognizes carnitine. Cardiolipin failed to stimulate transport, excluding the activity of the mitochondrial Carnitine/acylcarnitine transporter. Increased level of exosomal OCTN2 was induced by treatment of HEK293 with the pro-inflammatory cytokine INFγ. All data concurred to demonstrate that OCTN2 present in exosomes is fully functional and is in its native conformation. Functional OCTN2 was detected also in human urinary exosomes, thus suggesting the OCTN2 exosomal protein as a candidate biomarker for inflammation related pathologies.
Introduction
Several studies showed that eukaryotic cells release different types of vesicles into the extracellular environment among which exosomes represent a sub set with definite physicochemical and biochemical characteristics. They are 20–100 nm in size with a density of about 1.10 g × ml−1 and are enriched in specific proteins such as TGS101 and CD9, which are acknowledged as exosomal markers1,2. A growing number of evidences suggest that exosomes play an important role in cell-to-cell communication under both physiological and pathological conditions. Indeed, exosomes are involved in immune response, angiogenesis, inflammation, cell death, neurodegenerative diseases and cancer malignancy3. Knowledge of exosomal cargo composition opens new perspectives in the field of diagnostics. Indeed, exosomes collected from body fluids such as blood, urine, ascites, cerebrospinal fluid and milk, could be used as biomarkers of the tissues from which they originate. Large-scale proteomics have been previously performed to search for exosomal molecular cargos. Interestingly, these studies revealed the presence of several proteins belonging to SLC families4, which include over 400 membrane transporters that are fundamental players in cell metabolisms5. Despite the important role of the transporters in cell life, the physiological significance of the presence of this class of protein in exosomes is unknown. Indeed, apart from the proteomic data, few information is available about the function of SLCs in this exocellular location. In this study, the first characterization of an SLC member present in exosomes is described. SLC22A5, also known as OCTN2, is a sodium dependent carnitine transporter belonging to the sub-family of Organic Cation Transporters Novel and widely expressed in human tissues6,7. In cells, OCTN2 plays a crucial role in the cellular uptake of L-carnitine, which is an essential co-factor for the mitochondrial β-oxidation pathway8. Indeed, humans can synthesize only a relatively small fraction of the required L-carnitine that, hence, has to be absorbed from diet9. For this reason, OCTN2 is essential for completion of the β-oxidation pathway as demonstrated by occurrence of a severe disease, known as primary carnitine deficiency10, caused by OCTN2 gene defects. The pathology is characterized by impaired intestinal and muscular uptake and increased renal loss of carnitine9. Recent studies demonstrated that expression level of OCTN2 is altered in many human cancers11. This alteration, which is induced by DNA methylation12, may cause impairing of the mitochondrial β-oxidation in cancer growth and progression. This serves for switching the cancer cells from lipid to glycolytic metabolism according to Warburg effect13. Very interestingly, OCTN2 is implicated in the inflammatory response. Its gene is located on the “Inflammatory Bowel Diseases 5” risk region (IBD). Moreover, Mikihiro Fujiya et al.14 demonstrated that epithelial OCTN2 expression is increased by inflammation, through increased levels of pro-inflammatory cytokines such as INF-γ. Therefore, investigation of OCTN2 association with exosomes was of interest for human physio/pathology. OCTN2 and exosomes share similar links to human pathology, thus increasing the interest in characterizing the transporter in this alternative location.
Results
Exosomes harbor functional OCTN2
To detect the presence of OCTN2 in exosomes and to gain further insights into the possible role of the transporter, exosomes from HEK293 were isolated according to the well assessed method of ultracentrifugation15. The enrichment of exosomes in the preparation was confirmed by WB analysis using typical exosome markers that is CD9 and TSG101. Figure 1 shows immunodetection of the two markers in the exosome preparation, while no or very few reaction was observed in the cell extract. This data confirmed that the isolated vesicles were, indeed, exosomes. The purity of the preparation was assessed by Anti-Tom 20 and Anti-Golga2, that is antibodies against specific markers of mitochondria and Golgi, respectively16,17. In this case, the absence of immunostaining in the exosomal extract allowed us to exclude contaminations by mitochondria and Golgi. As expected, positive reaction by Anti-Tom 20 and Anti-Golga2 was observed in the cell extract (Fig. 1). Then, the exosome fraction, was used to perform WB analysis with the Anti-OCTN2 antibody. A band was detected confirming the presence of OCTN2 in exosomes isolated from HEK293 medium. The transporter was also detected in the cell extract, in line with its native location in the plasma membrane (Fig. 1). By comparison with standard proteins, the apparent molecular mass of OCTN2 was about 70 kDa in both the exosomal and the cell fractions. The specificity of the antibody for OCTN2 was assessed as shown in Supplementary Fig. 1.
Figure 1.
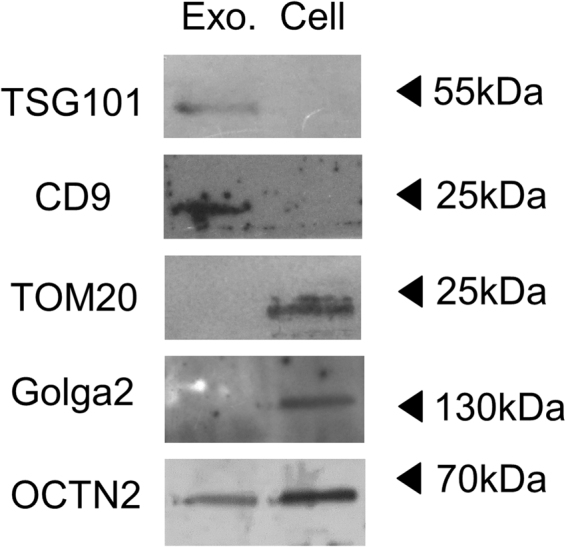
Immunoblot analysis of exosomes isolated from HEK293. Proteins from exosomes (left line) or cell extract (right line) were separated on SDS-PAGE gels and blotted. Then, the membrane was cut for incubation with the antibodies against TSG101, CD9 (conventional exosomal marker), TOMM20, Golga2 (mitochondrial and Golgi marker respectively) and OCTN2. The Molecular Mass of standard proteins (Thermo Scientific PageRuler Plus Prestained Protein Ladder) is indicated by arrows. The image is representative of three independent experiments.
To ascertain whether the transporter present in exosome was functional, HEK293-derived vesicles were solubilized with Triton X-100 and reconstituted into proteoliposomes. The transport function was measured as uptake of 3H-carnitine. The time course in Fig. 2a shows accumulation of labeled carnitine in proteoliposomes which was stimulated by externally added NaCl. Indeed, activity was dependent on NaCl concentration (Fig. 2b). NaCl concentration higher than 80 mM did not further stimulate transport (Supplementary Fig. 2a). Similar results were observed when using 1 µM carnitine instead of 50 µM (Supplementary Fig. 2b). Na-gluconate exerted the same effect (not shown) thus excluding the influence of Cl− on transport. It should be noted that a small amount of Sodium (about 10 mM) is present in the buffer used for reconstitution and transport, since it cannot be substituted by TRIS or Potassium which negatively affect transport. Therefore the actual stimulation by Sodium is higher than that observed. This finding suggests that the carnitine uptake into proteoliposomes was mediated by a functional OCTN2. To definitively assess the nature of the transporter, some other functional properties were tested. Exosome protein extract was reconstituted using buffers at different pH. As shown in Fig. 3 the carnitine uptake increased from pH 6.0 to pH 8.0. The same behavior was found in HEK293 extract, reconstituted in proteoliposomes (not shown) and, previously, in intact cells. Indeed, this is a typical feature of Organic Cation Transporters18. Furthermore, the inhibitory effect of Acetyl-carnitine and Asn, Gln, Arg and Ala on carnitine transport was tested in order to exclude that the carnitine uptake was due to ATB0,+ (SLC6A14), an amino acids transporter which also recognizes carnitine (Fig. 4). Acetyl-carnitine inhibited the transport activity by about 75%, while the amino acids showed poorly or not significant inhibition. This behavior is completely different from the inhibition pattern previously described for ATB0,+19. This confirms that the observed transport function can be ascribed to OCTN2.
Figure 2.
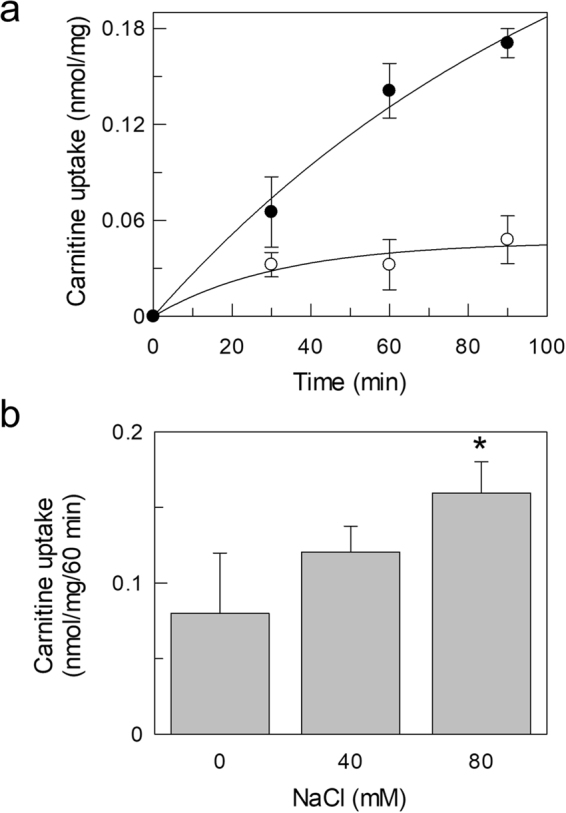
Sodium dependent carnitine uptake in proteoliposomes by exosomal OCTN2. Proteoliposomes were obtained by reconstitution of exosomal protein extract as described in Materials and Methods. (a) Transport activity was started adding 50 µM 3H-carnitine to proteoliposomes and terminated at the indicated times loading the samples on cold columns containing Sephdex G-75. The uptake was measured in presence (⦁) or absence (○) of external 80 mM NaCl. (b) NaCl at the indicated concentrations was added to proteoliposomes. After 2 min of incubation, carnitine transport was started adding 50 µM 3H-carnitine and stopped after 60 min. The osmolarity of each sample was balanced by substituting the NaCl with an appropriate concentration of sucrose. The values are the mean ± SD from three experiments. (*) Significantly different as estimated by the Student’s t test (p < 0.05).
Figure 3.
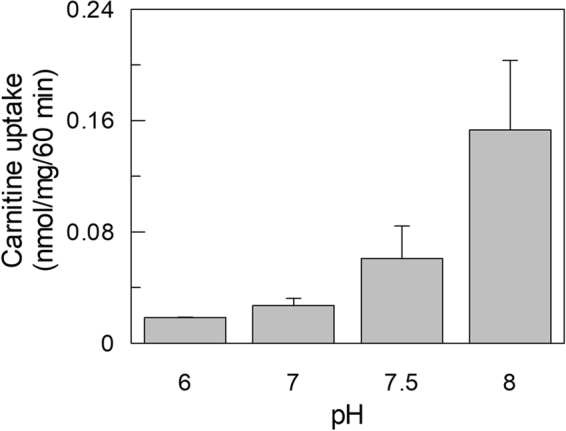
pH dependence of carnitine uptake in proteoliposomes. Proteoliposomes were obtained by reconstitution of exosomal protein extract at the indicated pH, as described in Materials and Methods. Transport activity was started adding 50 µM 3H-carnitine to proteoliposomes and terminated at 60 min as in Fig. 2. The values are the mean ± S.D. from three independent experiments.
Figure 4.
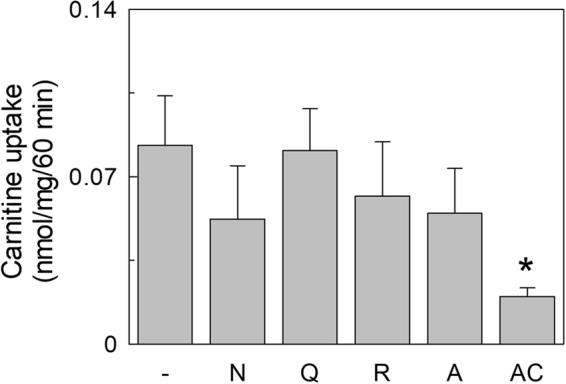
Inhibition of carnitine uptake by amino acids and acetylcarnitine. 2.5 mM Asn, Gln, Arg, Ala or Acetyl-carnitine were added to proteoliposomes, obtained as in Fig. 2, together with 10 µM 3H-carnitine. Transport was stopped after 60 as in Fig. 2. The data represent means ± S.D. of three independent experiments. (*) Significantly different as estimated by the Student’s t test (p < 0.05).
To exclude that the carnitine uptake could be due in part to the presence of contamination by the mitochondrial carnitine carrier (CACT), the dependence on cardiolipin (DPG), which specifically stimulates the activity of the mitochondrial transporter20, was checked. To this aim, cardiolipin was included into the proteoliposome membrane as previously reported21. No effect was observed upon addition of cardiolipin, excluding the presence of the mitochondrial transporter (Fig. 5). To further substantiate the absence of CACT contaminations, the inhibition of carnitine uptake by N-Ethylmaleimide (NEM), a strong inhibitor of the mitochondrial transporter, was tested. The response to NEM was very poor compared with the well described inhibition of the mitochondrial transporter (Fig. 5b)22. As a control, cardiolipin was included into proteoliposomes reconstituted with HEK293 mitochondria extract and the CACT activity assayed. As expected, a clear activation was observed (Fig. 5). Taken together, the data confirms the presence of functional OCTN2 into HEK 293 derived exosomes.
Figure 5.

Effect of cardiolipin (DPG) and NEM on carnitine uptake in proteoliposomes. (a) Exosomal extract (grey bar) or mitochondrial extract (white bar) was reconstituted in proteoliposomes with or without cardiolipin (see Materials and Methods). The black bar shows the inhibition by 0.1 mM NEM of carnitine uptake into proteoliposome reconstituted with exosomal extract. (b) Mitochondrial extract (white bars) was reconstituted in proteoliposomes with or without cardiolipin. The black bars show the inhibition by 0.1 mM NEM of carnitine uptake into proteoliposome with or without cardiolipin. Transport was started adding 50 µM 3H-carnitine to proteoliposomes and stopped after 60 min. The values are the mean ± S.D. from three independent experiments. (*) Significantly different as estimated by the Student’s t test (p < 0.05).
Increase of OCTN2 level in exosomes
To gain some insights in the possible mechanism of OCTN2 insertion in exosomes, the expression of the transporter in HEK293 cells was increased by Interferon-γ (INFγ) treatment, as previously reported14. Indeed, incubation of HEK293 cells with INFγ for 48 h led to increase of OCTN2 expression in the cells, as evidenced by WB analysis (Fig. 6a). Exosomes deriving from treated cells also contained higher amount of OCTN2 (Fig. 6a). The activity of the OCTN2 extracted from the exosome derived from the medium of treated or untreated cells was tested in reconstituted proteoliposomes to confirm the data obtained by WB. An increase of activity of about 40% was found after treatment with INFγ (Fig. 6b). The activity of OCTN2 extracted from cells, not treated or treated with INFγ, was also measured. An increased transport activity was observed which correlated well with the increased expression.
Figure 6.

Effect of IFN-γ on OCTN2 level in cell and exosomes. HEK293 cells were treated with 50ng/ml IFN-γ for 48 hours. After incubation, cells were harvested. Exosomes were isolated from the medium of the same culture. (a) Both cell (upper panel) and exosomal (lower panel) protein extracts deriving from IFN-γ treated (right line) or untreated (left line) culture was analyzed by WB using the antibody against OCTN2. The immunostaining of cell extract with the antibody against actin was used as loading control. Exosomal OCTN2 was normalized using TSG101 by loading on SDS-PAGE an equal amount of the same exosomal extracts used to test the OCTN2 expression. Cell lysate and exosomal extract were subjected to WB. The membrane was cut for incubation with the different antibodies (b) Transport was started adding 50 µM 3H-carnitine to proteoliposomes and stopped after 60 min. Proteoliposome was reconstituted using either exosomal (gray bar) or cell (white bar) protein extracts. The values are the mean ± S.D. from three independent experiments. (*) Significantly different as estimated by the Student’s t test (p < 0.05).
OCTN2 in urinary exosomes
To characterize OCTN2 in urinary exosomes, the vesicles were extracted from human urine. In WB analysis, immunostaining of OCTN2 was observed in these vesicles (Fig. 7a). To ascertain the functionality of the protein, the uptake in reconstituted proteoliposomes was detected in the absence or presence of 80 mM NaCl (Fig. 7b). Uptake was stimulated by Na+ confirming the presence of functional OCTN2 also in human urine exosomes.
Figure 7.
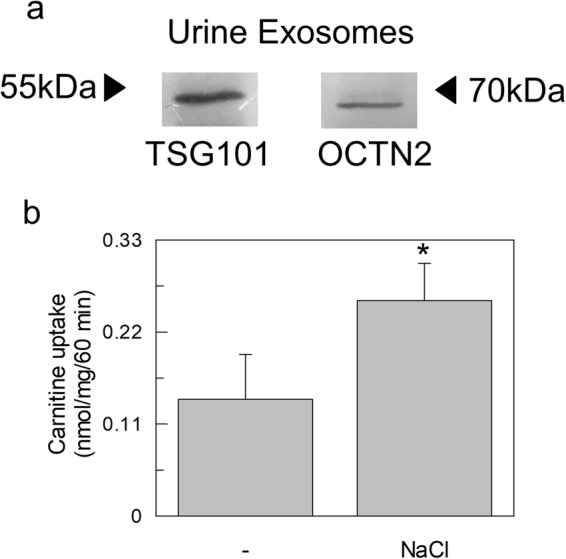
OCTN2 in human urine exosomes. (a) Proteins from human urine derived exosomes were separated on SDS-PAGE gels, and blotted. Then, the membrane was cut for incubation with the antibodies against TSG101and OCTN2. The Molecular Mass of standard proteins (Thermo Scientific PageRuler Plus Prestained Protein Ladder) is indicated by arrows. (b) Transport activity was started adding 50 µM 3H-carnitine to proteoliposomes and stopped after 60 min as in Fig. 2. The uptake was measured in presence or absence of external 80 mM NaCl. The values are the mean ± SD from three experiments. (*) Significantly different as estimated by the Student’s t test (p < 0.05).
Discussion
Initial studies showed that eukaryotic vesicles are used to remove obsolete cellular components. More recently, the hypothesis that exosomes play a pivotal role in intercellular communication by carrying functional proteins and nucleic acids has been considered3. However, molecular and functional characterization of cargos is still poor especially to what concerns membrane transporters. The interest in studying exosomal OCTN2 derives from its crucial role in cell homeostasis and from the association of transporter defects to human diseases, such as inflammatory diseases, including IBD. This is even more relevant in the frame of the findings that also exosomes have been linked to IBD etiology23,24. Previous proteomic studies revealed the presence of amino acid sequences belonging to OCTN2, among others SLC, in human urine exosomes25. In line with this, the results here described highlight that exosomes collected from human urine contain functional OCTN2. This correlates well with the high expression level of OCTN2 in kidney, besides other tissues18. Investigating SLC members could help to determine the cellular origin of urine exosomes. To characterize in more details the human exosomal OCTN2, we have exploited HEK293 cells. It was reported that these immortalized cells maintain some features of renal cells and are able to release exosomes26,27, even though, they show also some properties of neuronal cells. Noteworthy, exosomes deriving from a human kidney cell line are more appropriate to study OCTN2 compared to exosomes collected from urine of animal model such as rat or mouse. Indeed, these animals harbor different patterns of carnitine transport and metabolism with respect to humans. As an example, murine genome encodes for the third carnitine transporter (OCTN3)28, which is not present in humans7. Biochemical characterization of OCTN2 suggested that the protein in exosomes, derived from HEK293 cells, is in a glycosylation state equal to that of native plasma membrane. This is indicated by the apparent molecular mass of 70 kDa (see Fig. 1 and Fig. 7) which is much higher than the theoretical mass of the protein or than the apparent molecular mass of the OCTN2 expressed in bacteria that cannot assemble glycosylation moieties (see Supplementary Fig. 1)29. The glycosylation state of OCTN2 suggests a correct folding in the exosomal membrane that was indeed confirmed by the activity assay. Thanks to the proteoliposome approach, the protein extracted from both cells and exosomes could be assayed. It is worth noting that the transport activity of exosomal OCTN2 is enriched if compared with the cellular one. This probably depends on the fact that the exosomal extract contains less total proteins than the cell extract. Indeed, in some previous studies, the activity of OCTN2 was detectable in intact cells only after the induction of the overexpression of the transporter by transfection18. The measured carnitine uptake using exosomal extracts showed all the main features of OCTN2 located at the plasma membrane18. Importantly, interferences by other transporters on the observed carnitine uptake activity was excluded. In particular, the mitochondrial carnitine carrier could be excluded by the lack of cardiolipin dependence and NEM sensitivity20. Another transporter which was shown to exhibit specificity for carnitine is ATB0,+, whose main function consists in mediating amino acid transport. Its presence could be excluded by absence of specificity towards amino acids transported by ATB0,+ (see Fig. 4). OCTN2 expression in exosomes is regulated by one of the same factors that regulates its expression in cells, i.e., the pro-inflammatory cytokine INFγ. This suggests that the abundance of OCTN2 in exosomes relates to expression in cells. This finding may suggest a relevance of the exosomal OCTN2 in pathological contexts such as inflammation.
Several studies showed that OCTN2, its substrate carnitine and exosomes are involved in inflammatory process. On the one hand, carnitine has been associated to inflammation related-diseases including diabetes mellitus30, chronic renal failure, cardiomyopathy, cirrhosis and sepsis8. Indeed, carnitine administration to patient in hemodialysis significantly reduces serum C-Reactive Protein (CRP) and Serum Amyloid A (SAA), two systemic inflammation markers31. On the other hand, OCTN2 is associated with Crohn’s disease and ulcerative colitis32–34. The intestine of neonatal homozygous mouse, in which the OCTN2 is deleted, displayed early signs of inflammation, such as lymphocytic and macrophage infiltration. This is in line with the ability of OCTN2 to mediate the absorption of the pentapeptide Competence and Sporulation Factor (CSF) into the intestinal cells. This factor induces cytoprotective mechanisms, which prevent intestinal epithelial cell injury and loss of barrier function35. Therefore, Shtrichman et al. showed that INFγ, which plays a crucial role in the defense against microbial infections36, increase the OCTN2 expression. This suggests that OCTN2 may participate to restoration of intestinal homeostasis under inflammation stress. Moreover, the protein composition of exosomes can be influenced by pro-inflammatory cytokines as demonstrated in the case of neutral ceramidase (NCDase) whose expression is reduced in exosomes in the presence of high level of pro-inflammatory cytokines and vice versa37. The described results represent an encouraging starting point to further investigate the link between exosomal OCTN2 and inflammatory diseases with important implications in diagnosis.
Methods
Materials
Sephadex G-75 was purchased from Pharmacia, l-[methyl-3H]carnitine from Amersham, egg-yolk phospholipids (l-α-phosphatidylcholine from fresh turkey egg yolk), Ethylenedinitrilo tetraacetic acid (EDTA), 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), 4-(1,1,3,3-Tetramethylbutyl)phenyl-polyethylene (Triton X-100), cardiolipin (DPG) and L-carnitine from Sigma, St. Louis, MO. Human embryonic kidney HEK293 cells were obtained from the American Type Culture Collection (ATCC). Tissue culture media and fetal bovine serum were purchased from Life Technologies. INFγ, SLC22A5 and CD9 Antibodies were purchased from Thermo Fisher Scientific, TSG101, TOMM20 and Golga2 from Santa Cruz Biotechnology.
Cell culture
HEK 293 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 1 mM glutamine, 1 mM sodium pyruvate and Pen/Strep as antibiotics. Cells were grown on 10 cm2 plates at 37 °C in a humidified incubator and a 5% CO2 atmosphere. In case of collection of exosome, conditioned medium was used in place of classical one.
Cytokine treatment
HEK 293 cells were cultured using standard culturing conditions seeded onto 12 well plates up to 50–70% confluence. 50 ng/ml of IFNγ was added for 48 h in a serum free medium according to manufacturer’s conditions.
Exosome isolation
Urine (from a human individual) or culture media was centrifuged at 15,000 g for 10 min to pellet cells and other debris. The supernatant was then centrifuged at 150,000 g for 120 min to pellet the exosomal fraction. The pellet was washed with phosphate-buffered saline (PBS) and then re-centrifuged at 150,000 g for 120 min before final resuspension15. The protocol regarding the collection of human urine was carried out in accordance to the relevant guidelines and regulations. All experimental protocols were approved by the Institutional Ethic Committee of the University of Calabria. The informed consent was obtained from all healthy volunteer subjects.
Exosome and cell solubilisation
Exosomal fraction were resuspended in the lysis buffer made of 3% Triton X-100 in 20 mM Hepes/NaOH pH 7.5 and protease inhibitors. After incubation in ice for 30 min the lysate was centrifuged at 12,000 g for 15 min at 4 °C. The supernatant was used for further analysis. The same procedure and buffer were used to solubilized HEK293 cells pellets which were harvested at the same time of media collection.
Mitochondria isolation from HEK293
HEK293 cells were harvested and resuspended in cold Isolation Buffer (10 mM HEPES, pH 7.4, 300 mM mannitol and 0.2 mM EDTA). After that, cells were homogenized on ice with a 2-ml glass potter and mitochondria was isolated using a differential centrifugation method38.
SDS-PAGE and western blot analysis
SDS-PAGE electrophoresis was performed in the presence of 0.1% SDS according to Laemmli. Stacking gel and separation gel were prepared as 4% and 12% acrylamide respectively (acrylamide/bisacrylamide ratio 30:0.2). Proteins were transferred to nitrocellulose membrane. The membrane was immunostained using anti-OCTN2, anti-CD9, anti-TSG101, anti-Golga2 and anti-TOMM20 antibodies in accordance with the directions manufacturer. The WB images are representative of at least two independent experiments.
Reconstitution in proteoliposomes
Total protein extract from exosomes or HEK293 cell lysate was reconstituted by removing the detergent from mixed micelles containing detergent, protein and phospholipids. The composition of the initial mixture was: 200 μg of total proteins, 75 μl of 10% Triton X-100, 110 μl of 10% egg yolk phospholipids in the form of sonicated liposomes 20 mM Hepes (pH 7.5) in a final volume of 700 μl.
Transport measurements
550 μl of proteoliposomes were passed through a Sephadex G-75 column. The first 600 μl of the turbid eluate from the Sephadex column were collected, transferred to reaction vessels (100 μl each), and readily used for transport measurement. Transport was performed at 25 °C and started by adding 0.05 mM 3H-carnitine to proteoliposomes. Transport assay was terminated at the required time interval. Finally, the external substrate was removed by chromatography on Sephadex G-75 columns, and the radioactivity in the liposomes was measured.
Statistical test
A two tailed t-tests was performed to calculate the significant difference between samples.
Electronic supplementary material
Acknowledgements
This work was supported by funds from MIUR (Ministery of Instruction, University and Research): Programma Operativo Nazionale [01_00937] — “Modelli sperimentali biotecnologici integrati per lo sviluppo e la selezione di molecole di interesse per la salute dell’uomo”.
Author Contributions
L.C. planned, performed experiments and contributed to write the paper; M.S. contributed to carry out experiments and writing; A.T. and N.G. contributed to exosomes isolation; C.I. supervised the entire work and wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22170-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yoshioka, Y. et al. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles2, 10.3402/jev.v2i0.20424 (2013). [DOI] [PMC free article] [PubMed]
- 2.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 3.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Ung TH, Madsen HJ, Hellwinkel JE, Lencioni AM, Graner MW. Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci. 2014;105:1384–1392. doi: 10.1111/cas.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.César-Razquin A, et al. A Call for Systematic Research on Solute Carriers. Cell. 2015;162:478–487. doi: 10.1016/j.cell.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Molecular aspects of medicine. 2013;34:413–435. doi: 10.1016/j.mam.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Pochini L, Scalise M, Galluccio M, Indiveri C. OCTN cation transporters in health and disease: role as drug targets and assay development. Journal of biomolecular screening. 2013;18:851–867. doi: 10.1177/1087057113493006. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond) 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaz FM, Wanders RJ. Carnitine biosynthesis in mammals. The Biochemical journal. 2002;361:417–429. doi: 10.1042/bj3610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmieri F. Diseases caused by defects of mitochondrial carriers: a review. Biochim Biophys Acta. 2008;1777:564–78. doi: 10.1016/j.bbabio.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Scalise M, et al. Human OCTN2 (SLC22A5) is down-regulated in virus- and nonvirus-mediated cancer. Cell Biochem Funct. 2012;30:419–425. doi: 10.1002/cbf.2816. [DOI] [PubMed] [Google Scholar]
- 12.Qu Q, et al. Different involvement of promoter methylation in the expression of organic cation/carnitine transporter 2 (OCTN2) in cancer cell lines. PLoS One. 2013;8:e76474. doi: 10.1371/journal.pone.0076474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. The Journal of general physiology. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiya M, et al. Cytokine regulation of OCTN2 expression and activity in small and large intestine. Inflamm Bowel Dis. 2011;17:907–916. doi: 10.1002/ibd.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter. 2006;3:Unit3.22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 16.Paech F, Bouitbir J, Krähenbühl S. Hepatocellular Toxicity Associated with Tyrosine Kinase Inhibitors: Mitochondrial Damage and Inhibition of Glycolysis. Front Pharmacol. 2017;8:367. doi: 10.3389/fphar.2017.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sin AT, Harrison RE. Growth of the Mammalian Golgi Apparatus during Interphase. Mol Cell Biol. 2016;36:2344–2359. doi: 10.1128/MCB.00046-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamai I, et al. Molecular and functional characterization of organic cation/carnitine transporter family in mice. J Biol Chem. 2000;275:40064–40072. doi: 10.1074/jbc.M005340200. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi T, et al. Na+- and Cl–coupled active transport of carnitine by the amino acid transporter ATB(0, +) from mouse colon expressed in HRPE cells and Xenopus oocytes. J Physiol. 2001;532:297–304. doi: 10.1111/j.1469-7793.2001.0297f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonazzi A, Giangregorio N, Console L, Indiveri C. Mitochondrial carnitine/acylcarnitine translocase: insights in structure/ function relationships. Basis for drug therapy and side effects prediction. Mini Rev Med Chem. 2015;15:396–405. doi: 10.2174/138955751505150408142032. [DOI] [PubMed] [Google Scholar]
- 21.Tonazzi A, Console L, Giangregorio N, Indiveri C, Palmieri F. Identification by site-directed mutagenesis of a hydrophobic binding site of the mitochondrial carnitine/acylcarnitine carrier involved in the interaction with acyl groups. Biochimica Et Biophysica Acta-Bioenergetics. 2012;1817:697–704. doi: 10.1016/j.bbabio.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Tonazzi A, Giangregorio N, Console L, De Palma A, Indiveri C. Nitric oxide inhibits the mitochondrial carnitine/acylcarnitine carrier through reversible S-nitrosylation of cysteine 136. Biochim Biophys Acta. 2017;1858:475–482. doi: 10.1016/j.bbabio.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, et al. Exosomes released by granulocytic myeloid-derived suppressor cells attenuate DSS-induced colitis in mice. Oncotarget. 2016;7:15356–15368. doi: 10.18632/oncotarget.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu AT, Lu JT, Ran ZH, Zheng Q. Exosome in intestinal mucosal immunity. J Gastroenterol Hepatol. 2016;31:1694–1699. doi: 10.1111/jgh.13413. [DOI] [PubMed] [Google Scholar]
- 25.Gonzales PA, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20:363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- 27.Serban AI, Stanca L, Geicu OI, Dinischiotu A. AGEs-Induced IL-6 Synthesis Precedes RAGE Up-Regulation in HEK 293 Cells: An Alternative Inflammatory Mechanism? Int J Mol Sci. 2015;16:20100–20117. doi: 10.3390/ijms160920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scalise M, Galluccio M, Pochini L, Indiveri C. Over-expression in Escherichia coli, purification and reconstitution in liposomes of the third member of the OCTN sub-family: the mouse carnitine transporter OCTN3. Biochemical and biophysical research communications. 2012;422:59–63. doi: 10.1016/j.bbrc.2012.04.105. [DOI] [PubMed] [Google Scholar]
- 29.Indiveri C, Galluccio M, Scalise M, Pochini L. Strategies of bacterial over expression of membrane transporters relevant in human health: the successful case of the three members of OCTN subfamily. Mol Biotechnol. 2013;54:724–736. doi: 10.1007/s12033-012-9586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mamoulakis D, Galanakis E, Dionyssopoulou E, Evangeliou A, Sbyrakis S. Carnitine deficiency in children and adolescents with type 1 diabetes. J Diabetes Complications. 2004;18:271–274. doi: 10.1016/S1056-8727(03)00091-6. [DOI] [PubMed] [Google Scholar]
- 31.Khalatbari-Soltani S, Tabibi H. Inflammation and L-carnitine therapy in hemodialysis patients: a review. Clin Exp Nephrol. 2015;19:331–335. doi: 10.1007/s10157-014-1061-3. [DOI] [PubMed] [Google Scholar]
- 32.Girardin M, et al. Expression and functional analysis of intestinal organic cation/L-carnitine transporter (OCTN) in Crohn’s disease. J Crohns Colitis. 2012;6:189–197. doi: 10.1016/j.crohns.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Peltekova VD, et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet. 2004;36:471–475. doi: 10.1038/ng1339. [DOI] [PubMed] [Google Scholar]
- 34.Silverberg MS, et al. Refined genomic localization and ethnic differences observed for the IBD5 association with Crohn’s disease. Eur J Hum Genet. 2007;15:328–335. doi: 10.1038/sj.ejhg.5201756. [DOI] [PubMed] [Google Scholar]
- 35.Fujiya M, et al. The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe. 2007;1:299–308. doi: 10.1016/j.chom.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Shtrichman R, Samuel CE. The role of gamma interferon in antimicrobial immunity. Curr Opin Microbiol. 2001;4:251–259. doi: 10.1016/S1369-5274(00)00199-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Q, et al. Low-dose cytokine-induced neutral ceramidase secretion from INS-1 cells via exosomes and its anti-apoptotic effect. FEBS J. 2014;281:2861–2870. doi: 10.1111/febs.12826. [DOI] [PubMed] [Google Scholar]
- 38.Console L, Giangregorio N, Indiveri C, Tonazzi A. Carnitine/acylcarnitine translocase and carnitine palmitoyltransferase 2 form a complex in the inner mitochondrial membrane. Mol Cell Biochem. 2014;394:307–314. doi: 10.1007/s11010-014-2098-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


