Abstract
Acute lung injury is a common disorder with a high mortality rate, but previous efforts to develop drugs to treat this disorder have been unsuccessful. In an effort to develop more effective treatments, we have been studying the molecular pathways that regulate the dysfunction of alveolar epithelial cells and endothelial cells that serve as a final common pathway leading to alveolar flooding. Using integrin subunit knockout mice and antibodies we developed by immunizing these mice, we have found important and distinct roles for the αvβ6 integrin on epithelial cells and the αvβ5 integrin on endothelial cells in mediating increases in alveolar permeability in multiple models of acute lung injury. We have also found therapeutic effects of αvβ5 inhibition in two models of septic shock even when the antibody was administered to animals that were obviously ill. These results identify αvβ6 and αvβ5 as promising therapeutic targets for the treatment of acute lung injury and septic shock.
Keywords: integrin, acute lung injury, endothelium
Acute lung injury is characterized by increased alveolar epithelial and endothelial permeability and impaired uptake of fluid from the alveolar space (1). The cumulative effects of these abnormalities result in alveolar flooding, impaired gas exchange, and hypoxic respiratory failure. Because these events are usually triggered by epithelial and/or endothelial injury and resultant inflammation, most efforts to develop pharmacologic interventions for acute lung injury have focused on inhibition of inflammatory mediators or leukocyte recruitment. None of these strategies has been effective in patients, perhaps because of the redundant cells and mediators that can affect epithelial and endothelial permeability and alveolar fluid reabsorption. An alternative strategy that might be more promising is to target the final common pathways in the alveolar epithelial cells and pulmonary endothelial cells whose dysfunction leads to alveolar flooding. With that idea in mind, we have been focusing on integrins, a family of transmembrane, heterodimeric receptors that play critical roles in many aspects of cellular homeostasis (2). Using mice, we and others, lacking individual integrin subunit genes and blocking antibodies that we generated by immunizing these integrin knockout mice, have identified critical roles for three integrins (αvβ3, αvβ5, and αvβ6) in regulating alveolar endothelial and epithelial permeability and a role for one of these in impairing fluid reabsorption in the setting of acute lung injury. These results have identified αvβ5 and αvβ6 as potential therapeutic targets and raised questions about the safety of drugs currently under development to inhibit αvβ3.
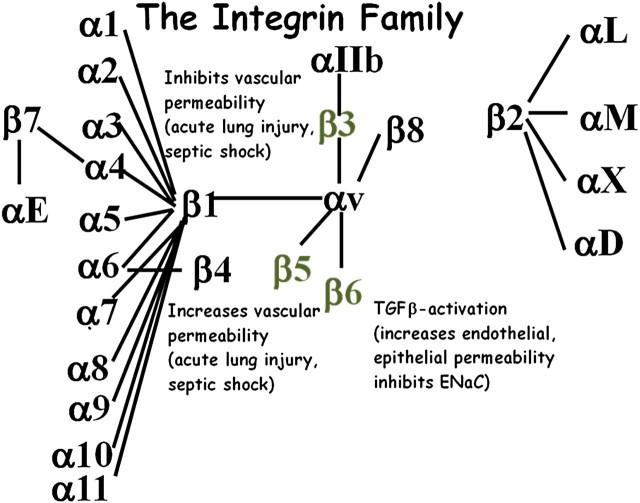
The mammalian integrin family includes 24 receptors, each containing a single α subunit and a single β subunit (Figure 1). Both subunits are type 1 transmembrane proteins containing a large extracellular domain and a relatively short cytoplasmic domain. Adjacent surfaces of the extracellular domain of each subunit directly interact with short linear peptides on integrin ligands, while the cytoplamic domains nucleate large multiprotein signaling complexes that modulate a wide variety of signaling events and cell behaviors. Adaptor proteins present in these complexes directly connect integrins to the actin cytoskeleton, positioning these receptors as critical detectors of mechanical forces and as modulators of cytoskeletal remodeling and transmission of contractile forces to adjacent cells and the extracellular matrix (2). Most cells express multiple members of the integrin family, but studies inactivating individual integrin subunit genes have identified a surprising number of nonredundant functions for individual integrins (3).
Figure 1.
Organization of the integrin family. Each integrin is formed by a single α subunit and a single β subunit. Not all theoretically possible α and β subunit pairs form. Actual heterodimer pairs are shown by connecting lines. The three integrins shown in gray have been shown to modulate acute lung injury as described in the text.
We have been especially interested in a subset of integrins that share the promiscuous α subunit, αv. There are five members of this subfamily: αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8. Each of these integrins recognizes the same core linear tripetide, arginine-glycine-aspartic acid (RGD), but adjacent amino acids dramatically alter the relative affinity of each αv integrin for specific ligands. αvβ6 and αvβ8 bind with highest affinity to the RGD site in the latency-associated peptide of transforming growth factors 1 and 3 (4, 5), αvβ1 preferentially binds the extracellular matrix protein fibronectin (6), αvβ5 preferentially binds the serum protein vitronectin, and αvβ3 promiscuously binds to many proteins containing an exposed RGD sequence. However, in most cases, these differences in ligand preference are relative rather than absolute, so which integrin–ligand interactions are involved in many integrin-mediated events remains controversial.
Every integrin subunit gene has been inactivated in mice (globally and in some cases conditionally), and these knockout mice have been useful for identifying important roles for individual integrins in development, homeostasis, and models of human disease (3). Among the αv subfamily, this approach has identified a number of unique roles for αvβ3, αvβ5 (7–10), αvβ6 (5, 11), and αvβ8 (12–14). The in vivo functions of αvβ1 have remained elusive because both subunits are present in multiple integrins and because there are no effective specific inhibitors of αvβ1.
Work from several laboratories over the past decade has suggested roles for a number of integrins in acute lung injury and responses to sepsis. The earliest work implicated leukocyte integrins that share the integrin β2 subunit because these integrins mediate firm arrest of circulating leukocytes at sites of injury or infection (15). Several more recent studies have continued to explore the roles these integrins play in regulating acute lung injury and septic shock (16–18). The αvβ3 integrin has also been shown to be up-regulated in the lungs of septic humans (19). Recently, the α6β4 integrin has also been identified as an important regulator of vascular endothelial permeability (20).
Integrin-Mediated Activation Of Tgf-β
More than a decade ago, we identified activation of latent TGF-β as the principal in vivo function of the αvβ6 integrin, based on the phenotype of β6 subunit knockout mice, which develop exaggerated inflammation in response to normally trivial injuries (21) but are protected from tissue fibrosis in multiple epithelial organs (5, 22). We and others have subsequently shown that the closely related integrin αvβ8 (4) can also activate latent TGF-β and that most, if not all, of the in vivo pheotypes that result from inactivation of αvβ6 and αvβ8 can be explained by a loss of TGF-β activity. Combined inhibition of these two integrins from birth phenocopies all of the developmental effects of loss of TGF-β1 and -3 (23), suggesting that these two integrins are required for all biologically relevant TGF-β1 and -3 activation during development. Neither of these integrins can activate TGF-β2, and the mechanisms of TGF-β2 activation in vivo remain largely unexplored. Furthermore, it is likely that additional mechanisms, perhaps involving the lower-affinity interactions of other αv integrins with TGF-β LAP, are involved in the exaggerated TGF-β activation that occurs in the setting of chronic diseases.
Although αvβ6 and αvβ8 can bind to the same RGD sequence and activate TGF-β1 and -3, they are expressed on different cells and activate TGF-β by different mechanisms. αvβ6 is largely restricted to a subset of epithelial cells (24), and its expression is dramatically up-regulating in the setting of epithelial injury and inflammation (25, 26). This integrin activates TGF-β by transmitting retractile force to the tethered latent complex (27), a process that has been confirmed by the recently solved crystal structure of latent TGF-β combined with electron microscopy of integrin/TGF-β complexes (28). In contrast, αvβ8 appears to principally activate TGF-β on the surface of fibroblasts and dendritic cells by presenting the latent complex to transmembrane metaloproteases, which cleave the latency-associated peptide to release the active cytokine (4).
Roles of Integrin-Mediated Tgf-β Activation in Models of Acute Lung Injury
Microarrays we performed at various time points after treatment of wild-type mice with bleomycin revealed increased expression of TGF-β–inducible genes at early time points associated with the well characterized acute lung injury that is caused by bleomycin (29). We therefore examined bleomycin-induced acute lung injury in β6 knockout mice and found that these mice were completely protected from increased albumin permeability despite an increase in bleomycin-induced alveolar inflammation (11). This protective effect appeared to be due to a loss of TGF-β activation because inhibition of TGF-β with a TGF-βRII-Fc fusion protein completely protected wild-type mice from the increased albumin permeability induced by bleomycin. Furthermore, loss or blockade of αvβ6 or TGF-β also protected mice from the increased permeability induced by LPS or large tidal volume ventilation, suggesting that blocking this integrin might be generally useful for preventing or treating acute lung injury.
αvβ6 is only expressed on epithelial cells, so we explored how TGF-β activation on the surface of alveolar epithelial cells might contribute to the accumulation of fluid in the alveolar space. We and others found that TGF-β directly increases the permeability across the endothelial and the epithelial barriers (11, 30). TGF-β also induces the rapid removal of the α subunit of the apical sodium channel ENac from the cell surface of alveolar epithelial cells (31), potentially further exaggerating alveolar flooding by impairing the removal of salt, and thus water, from the alveolar space.
Role of the αVβ5 Integrin in Modulating Acute Lung Injury and Septic Shock
The αvβ5 integrin is expressed in virtually every cell, and expression is tightly regulated during development. Experiments blocking this integrin in various cell types have suggested critical roles for αvβ5 in development, wound healing, and angiogenesis, so we were initially disappointed when the mice we generated lacking the integrin β5 subunit lacked any obvious phenotype (10). The first phenotype we observed in the mice was protection from the increase in cutaneous and cerebral vascular permeability induced by direct administration of vascular endothelial growth factor (VEGF) (9). This phenotype was shown to have potential therapeutic relevance because β5 knockout mice were also protected from brain infarction in a model of ischemic stroke induced by carotid artery occlusion and reperfusion, presumably due to the contribution of cerebral edema to infarct size in the closed intracerebral compartment.
Based on in vitro biochemical studies of endothelial cells treated with αvβ5-blocking antibodies, we initially concluded that this effect of αvβ5 was due to specific interaction between the integrin and the VEGF signaling pathway. To extend these observations to the lung, we examined the effects of a monoclonal antibody we generated by immunizing β5 knockout mice with murine fibroblasts on the increase in lung permeability induced by pulmonary ischemia and reperfusion. Forty-five minutes of occlusion of one pulmonary artery in rats caused a dramatic increase in albumin permeability in that lung, and this effect was substantially inhibited by treatment with αvβ5-blocking antibody (32). As expected, ischemia-reperfusion–induced acute lung injury was mediated by VEGF because the increase in permeability could also be inhibited by treatment with an adenovirus encoding a chimeric protein composed of the extracellular domain of VEGFR2 fused to Ig-Fc.
To assess whether the protective effects of blocking αvβ5 would be relevant in other models of acute lung injury, we examined the effects of the αvβ5 antibody or knockout of the β5 subunit on the increase in albumin permeability induced by large tidal volume ventilation. The αvβ5 antibody largely inhibited large tidal volume–induced acute lung injury, whereas the increase in permeability was absent in β5 knockout mice (32). Increased permeability in this model was not inhibited by VEGFR2-fc but appeared to be principally due to activation of latent TGF-β, suggesting that inhibition of αvβ5 protects against increases in vascular permeability through mechanisms that are downstream of and independent of VEGFR signaling.
Because loss or blockade of αvβ5 had no effect on lung inflammation in the ischemia-reperfusion or in the ventilator-induced lung injury model, we hypothesized that this integrin might regulate alveolar permeability through direct effects on alveolar epithelial cells or endothelial cells, each of which expresses αvβ5 at high levels. We found no effects of loss of αvβ5 on epithelial cells. However, treatment of confluent monolayers of pulmonary endothelial cells with αvβ5-blocking antibody prevent the increases in permeability induced in these cells by treatment with a variety of edemagenic agonists, including VEGF, TGF-β, and thrombin (32). Because each of these agonists increases permeability by activating different types of signaling receptors (VEGF through tyrosine kinase receptors, TGF-β through serine-threonine kinase receptors, and thrombin through G protein–coupled receptors), these results suggest that αvβ5 modulates endothelial permeability through a final common pathway downstream of these divergent initiating events. Endothelial permeability is known to be regulated by reorganization of the actin cytoskeleton into actin-myosin cables called stress fibers, which increase paracellular permeability by exerting retractile force on endothelial cell–cell junctions. Each of the agonists studied rapidly induced the formation of actin stress fibers in pulmonary endothelial cells. These effects were abrogated in endothelial cells treated with αvβ5-blocking antibody.
Having determined that αvβ5 can modulate the permeability of pulmonary endothelial cells downstream of multiple edemagenic agonists, we sought to determine whether a similar effect would have relevance in the systemic circulation. We were especially interested in septic shock, a common disorder with a high mortality rate and few therapeutic options. We began using a simple model, administering high doses of intraperitoneal LPS to control of β5 knockout mice. β5 knockout mice had a dramatic and significant survival advantage in this model and were protected from increased endothelial permeability in multiple organs, an effect that could be well visualized by examining extravasation of FITC-dextran from mesenteric blood vessels.
Although this result was biologically interesting, we could not be sure that it was therapeutically relevant because αvβ5 was inhibited in these experiments before the onset of illness. We therefore sought to determine whether treatment with αvβ5-blocking antibody could affect survival after treatment with LPS if we waited to initiate therapy until some mice had died and the remaining mice were moribund. We found that blockade of αvβ5 as late as 24 hours after administration of LPS significantly shifted the survival curve. Because LPS is a highly artificial model, we also examined a more physiologically relevant model, cecal ligation and puncture, which causes septic shock as a result of polymicrobial intraperitoneal sepsis. αvβ5 antibody also significantly improved survival in this model even when given 24 hours after initiation of the model at a time when all of the treated mice were obviously ill. Taken together, these results suggest that inhibition of αvβ5 might have therapeutic utility for treatment of acute lung injury and septic shock. We are therefore in the process of humanizing our αvβ5-blocking antibody for preclinical studies in large animals and clinical studies in patients.
Reciprocal Role of the αVβ3 Integrin in Regulating the Endothelial Actin Cytoskeleton And Modulating Acute Lung Injury and Septic Shock
Most cells express multiple members of the integrin family. Endothelial cells also express the closely related integrin αvβ3, which has overlapping ligand specificity with αvβ5. We therefore wondered why αvβ3 was apparently unable to substitute for αvβ5 in inducing actin stress fibers and mediating induced increases in endothelial permeability. Surprisingly, treatment of pulmonary endothelial cells with an αvβ3-blocking antibody had the opposite effect on induced permeability, increasing rather than decreasing increases induced by edemagenic agonists (33). Endothelial permeability is known to be reciprocally regulated by two different modes of actin organization. Actin stress fibers mediate increases in permeability, but these effects are normally antagonized by organization of subcortical actin rings, termed cortical actin, which stabilize cell–cell contacts and prevent transmission of retractile force. Cortical actin can be induced in endothelial cells by a variety of barrier-enhancing agonists, including the lipid phosphate sphingosine-1 phosphate. We found that αvβ3 and αvβ5 mediate different and antagonistic effects on the actin cytoskeleton. In contrast to αvβ5, blockade of αvβ3 has no effect on the formation of actin stress fibers (or perhaps enhances their formation). However, αvβ3 blockade prevents sphingosine-1 phosphate–induced enhancement of cortical actin, whereas αvβ5 blockade has no effect on cortical actin (Figure 2).
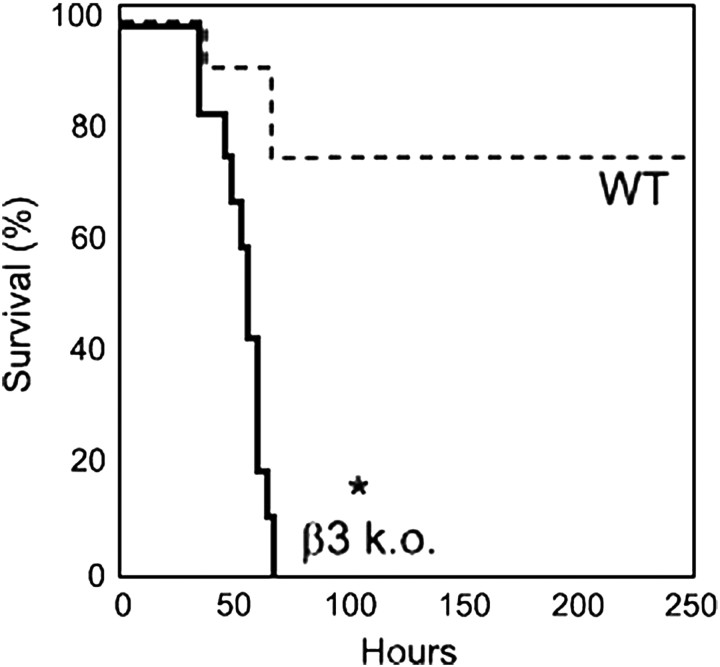
Figure 2.
Survival after administration of intraperitoneal LPS (10 μg/kg) to control of β3 knockout mice. *P < 0.0001 compared with survival of control mice. Reprinted by permission from Reference 33.
Several drugs have been developed to inhibit αvβ3. These drugs have shown promise in preclinical studies of angiogenesis, tumor growth, and osteoporosis, and some are being tested in clinical trials. Our in vitro results suggested that inhibition of αvβ3 could have the unwanted side effect of increasing susceptibility to diseases characterized by increases in endothelial permeability, such as acute lung injury and septic shock. To determine whether this effect might be important in vivo, we examined the responses of β3 knockout mice to LPS-induced acute lung injury and septic shock. We found that these mice had exaggerated increases in albumin extravasation into the lungs (in response to intratracheal LPS) and other organs (in response to intraperitoneal LPS) and had a dramatic increase in mortality in response to intraperitoneal LPS (Figure 2). Because β3 is also expressed on platelets (as αIIbβ3) and leukocytes, it was important to exclude the effects of the knockout on hematopoietic cells. We therefore performed bone marrow chimeras and confirmed that the increase in sepsis mortality was due to loss of β3 from tissue cells and not hematopoietic cells. These results suggest that it will be important to closely monitor patients in clinical trials of αvβ3 inhibitors for the development of acute lung injury and/or septic shock and suggest that it will likely be important to use highly specific αvβ5 and αvβ6 inhibitors that do not crossreact with αvβ3 to optimally realize the therapeutic potential of targeting these integrins.
Conclusions
Epithelial cells and endothelial cells express multiple members of the integrin family, but our results suggest that each of these receptors plays a unique role in modulating the behavior of these critical cells that comprise the gas exchanging apparatus of the lung. Our results suggest that targeting αvβ6 on epithelial cells and αvβ5 on endothelial cells can potently inhibit alveolar flooding in multiple models of acute lung injury, suggesting that either or both of these integrins might be attractive new targets for the treatment of this common, often lethal, and largely untreatable disorder. Our finding that inhibition of the αvβ3 integrin had the opposite effect of increasing susceptibility to acute lung injury and septic shock provides further evidence for the remarkable specificity of integrin function and suggests that drugs targeting integrins for treatment of acute lung injury might need to be highly specific for αvβ5 and/or αvβ6.
Footnotes
This work was supported by RO1 grant HL083950 and R37 grant HL53949 from the National Heart Lung and Blood Institute.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673–687. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Sheppard D. Identification and molecular characterization of multiple phenotypes in integrin knockout mice. Methods Enzymol 2007;426:291–305. [DOI] [PubMed] [Google Scholar]
- 4.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol 2002;157:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. . The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Morla AO, Vuori K, Bauer JS, Juliano RL, Ruoslahti E. The alpha v beta 1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol 1993;122:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane NE, Yao W, Nakamura MC, Humphrey MB, Kimmel D, Huang X, Sheppard D, Ross FP, Teitelbaum SL. Mice lacking the integrin beta5 subunit have accelerated osteoclast maturation and increased activity in the estrogen-deficient state. J Bone Miner Res 2005;20:58–66. [DOI] [PubMed] [Google Scholar]
- 8.Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med 2004;200:1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol 2002;157:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Griffiths M, Wu J, Farese RV, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol 2000;20:755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, et al. . TGF-beta is a critical mediator of acute lung injury. J Clin Invest 2001;107:1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. . Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 2007;449:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci 2005;25:9940–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest 2010;120:4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Windsor AC, Walsh CJ, Mullen PG, Cook DJ, Fisher BJ, Blocher CR, Leeper-Woodford SK, Sugerman HJ, Fowler AA. Tumor necrosis factor-alpha blockade prevents neutrophil CD18 receptor upregulation and attenuates acute lung injury in porcine sepsis without inhibition of neutrophil oxygen radical generation. J Clin Invest 1993;91:1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuutila J, Jalava-Karvinen P, Hohenthal U, Laitinen I, Kotilainen P, Rajamaki A, Nikoskelainen J, Lilius EM. CRP/CD11b ratio: a novel parameter for detecting gram-positive sepsis. Hum Immunol 2009;70:237–243. [DOI] [PubMed] [Google Scholar]
- 17.Lewis SM, Treacher DF, Bergmeier L, Brain SD, Chambers DJ, Pearson JD, Brown KA. Plasma from patients with sepsis up-regulates the expression of CD49d and CD64 on blood neutrophils. Am J Respir Cell Mol Biol 2009;40:724–732. [DOI] [PubMed] [Google Scholar]
- 18.Asaduzzaman M, Zhang S, Lavasani S, Wang Y, Thorlacius H. LFA-1 and MAC-1 mediate pulmonary recruitment of neutrophils and tissue damage in abdominal sepsis. Shock 2008;30:254–259. [DOI] [PubMed] [Google Scholar]
- 19.Singh B, Janardhan KS, Kanthan R. Expression of angiostatin, integrin alphavbeta3, and vitronectin in human lungs in sepsis. Exp Lung Res 2005;31:771–782. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Garcia JG, Jacobson JR. Integrin beta4 attenuates SHP-2 and MAPK signaling and reduces human lung endothelial inflammatory responses. J Cell Biochem 2010;110:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 1996;133:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, et al. . Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 2007;170:110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 2009;122:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem 1993;41:1521–1527. [DOI] [PubMed] [Google Scholar]
- 25.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, et al. . Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 2008;177:56–65. [DOI] [PubMed] [Google Scholar]
- 26.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev 2005;24:395–402. [DOI] [PubMed] [Google Scholar]
- 27.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol 2004;165:723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature 2011;474:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, Huang X, Sheppard D, Heller RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci USA 2000;97:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurst VI, Goldberg PL, Minnear FL, Heimark RL, Vincent PA. Rearrangement of adherens junctions by transforming growth factor-beta1: role of contraction. Am J Physiol 1999;276:L582–L595. [DOI] [PubMed] [Google Scholar]
- 31.Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, et al. . Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 2003;278:43939–43950. [DOI] [PubMed] [Google Scholar]
- 32.Su G, Hodnett M, Wu N, Atakilit A, Kosinski C, Godzich M, Huang XZ, Kim JK, Frank JA, Matthay MA, et al. . Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am J Respir Cell Mol Biol 2007;36:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su G, Atakilit A, Li JT, Wu N, Bhattacharya M, Zhu J, Shieh JE, Li E, Chen R, Sun S, et al. . Blockade of integrin {alpha}v{beta}3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am J Respir Crit Care Med 2012;185:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author disclosures