Notice
The Indian Society of Organ Transplantation clinical practice guideline document is designed to provide information and assist decision-making in relation to kidney-paired donation (KPD). It does not intend to define a standard of care. Variations in practice will inevitably occur when clinicians take into account the needs of individual patients, available resources, and limitations unique to a clinical situation. The working group acknowledged the lack of high-quality evidence on the issue from India, on which to base our recommendations.
Nomenclature and Description for Rating Guideline Recommendations
We have used the terminology similar to Kidney Disease Improving Global Outcomes (KDIGO) guidelines [Table 1]. We have avoided further subdivisions into A, B, C, and D due to paucity of literature available in the Indian context.
Table 1.
Nomenclature for guideline statements

Summary of Recommendations and Suggestions
Keys to successful KPD program are awareness, counseling, dedicated team, nonanonymous allocation, exchange of kidneys of similar quality, simultaneous transplant surgeries, preference for sensitized, and difficult-to-match donor–recipient pair (DRP). There is no statistically significant difference in short-term outcome of KPD compared to human leukocyte antigen (HLA)-matched living-related donor kidney transplantation. Waiting time in KPD is significantly less for easy-to-match pairs compared to deceased donor kidney transplantation (DDKT). Significant benefits can be achieved by providing better-matched donors for HLA-mismatched compatible DRP through KPD. The quality of matching and number of KPD will be better in “National” KPD program compared to single-center program. The lack of KPD program will affect the poor the most as they cannot afford expensive desensitization therapy and also as very few transplant centers in India have the facility for the same. KPD transplant is legal, cost-effective, and rapidly expanding modality with good long-term outcome to increase the number of kidney transplantation in India and take care of some of the organ shortage.
Evidence-based recommendations, suggestions, and expert consensus statements in this document aim to expand KPD and may serve as a model for other developing countries. For these guidelines, all reference articles in the English literature related to KPD transplantation in India from MEDLINE (PubMed from 2000 to 2017) database were included and reviewed.
We recommend that each potential DRP should be educated, encouraged, and counseled about KPD transplant in an easy-to-understand format as early as possible in the process of chronic kidney disease (CKD) care.
We recommend that all the transplant team members including transplant coordinator in addition to other regular training should also be trained for counseling about risk, benefits of KPD, nonexchange options, consent process, financial screening of DRP, data entry-related issues of KPD, and overall support for KPD.
We recommend that a standard written informed consent should be obtained from each DRP. We suggest that DRP should be given information about expected waiting time before transplantation, and every attempt should be made to reduce waiting time, particularly for hard-to-match pairs with the innovative ways in KPD matching.
We suggest that easy-to-match pairs (A donor and B recipient and vice versa) and sensitized pairs should be encouraged for KPD over ABO-incompatible kidney transplantation (ABOiKT) and desensitization protocol.
We recommend that all types of KPD should be practiced only after legal permission as per the existing transplant law.
We suggest that three-way exchange has optimum quality and quantity of matching.
We suggest that potential KPD transplant centers should study the key elements of success of other successful KPD program.
We suggest that computerized algorithms should be encouraged over manual allocation.
We recommend that all patients should be screened for pretransplant immunological risk, occult infections, and other risk factors to prevent and reduce posttransplant unequal outcome due to patient-related factors.
We suggest that the age difference between KPD donors should not be the key issue in allocation and better immunological match may counteract the effect of higher donor–recipient age difference.
We recommend that participating transplant teams should make the decision by consensus about kidney donor travel versus kidney transport as per local resources and logistics, though donor travel rather than kidney transport is likely to be simple.
We suggest that transplant surgery should be performed at the place where patient is evaluated, admitted, and willing to do posttransplant follow-up and simultaneous rather than sequential surgery should be preferred.
We recommend that the formation of KPD registry is one of the principal strategies to improve the quality of matching and number of KPD.
We suggest that DRP needs to be cognizant of transcultural, language, and legal barriers in national program when patients and their donors may belong to different regions or states of India.
Introduction
The Indian CKD registry in 2010 reported that at the time of enrolment in registry, 61% of end-stage renal disease (ESRD) patients were not on any form of renal replacement therapy (RRT), while 32% were on hemodialysis, 5% on peritoneal dialysis, and only 2% were being worked up for kidney transplantation.[1] There is a gross disparity between supply and demand of the transplant organs across the world, including India. All efforts are to be made to increase the supply of quality organs to the waiting transplant recipients. KPD is one such process for increasing supply of organs to patients waiting for transplant. ABO-compatible living donor kidney transplant (LDKT) is the ideal and cost-effective RRT modality for ESRD patients in resource-limited developing country such as India, where morbidity and mortality on long-term dialysis is unacceptably high. Access to RRT is mainly prevented by paucity of facilities and affordability. Up to 80% of kidney donors are living donors, while DDKT programs are still evolving in most parts of India.
KPD transplant enables two incompatible DRP to receive more compatible kidneys. In this, a living kidney donor who is otherwise incompatible with the recipient exchanges kidneys with another DRP. KPD can be performed at any transplant center that is doing kidney transplantation without the need of extra facilities as required for ABOiKT and transplant with desensitization protocol.
Kidney-Paired Donation in India
In India, in the absence of national KPD program, only single-center KPD is practiced.[2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] Table 2 shows the key elements of success of single-center KPD program at the Institute of Kidney Diseases and Research Center, Dr. HL Trivedi Institute of Transplantation Sciences, Ahmedabad, India, a center which has performed 300 KPD transplants in India.[2,3,4,5,6,7,8,9,10]
Table 2.
Key elements of success of our single-center kidney-paired donation program

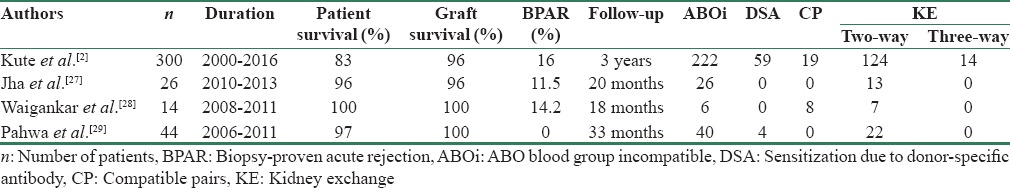
KPD led to an increase in LDKT rate by 25% in 1 year in this single-center KPD program in India.[5] KPD programs are likely to be facilitated more if there is a national KPD program. Table 3 shows the outcome of KPD from India.[2,3,4,5,6,7,8,9,10,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] Waigankar et al.[28] reported inferior outcome of ABOiKT compared to KPD.
Table 3.
Outcome of Indian studies on kidney-paired donation transplant
There has been a rapid expansion of KPD programs worldwide.[33,34,35,36,37,38] The national KPD program, with better quality matching and more numbers, is likely to provide affordable KPD transplantation with good long-term outcome.
Legal Aspect of Kidney-Paired Donation in India
Who can donate kidney in kidney-paired donation?
The Transplantation of Human Organ Act (THOA) and rules in India were promulgated in 1994 and subsequently amended in 2008 and 2011 to streamline organ donation and transplantation activities, including KPD.[32] The recent amendment in THOA in 2014 permitted authorization committee to give permission for KPD transplantation. As of now, only first-degree near relatives can donate kidney under this program.
Which recipient can participate in kidney-paired donation?
The potential KPD recipient must be medically fit and eligible to receive a kidney transplant. The recipient should have a willing living donor who is otherwise medically suitable, but unable to donate because of incompatible blood type or positive cross-match test and gives an informed consent to participate in KPD. DRP pairs agree to adherence with the recipient's transplant program.
Legal Issues Related to Kidney-Paired Donation in Transplantation of Human Organ and Tissue Act, India
There is a need to form an expert committee to study successful KPD models across the world and suggest measures to be incorporated in the Transplantation of Human Organ and Tissue Act, India if any, for further improving KPD.[2,3,4,5,6,7,8,9,10,32] KPD can be more successful and can obviate some of the current organ shortage through innovative strategies that include living–deceased donor list exchange,[39] international KPD,[40] nonsimultaneous chains/dominos,[41] global kidney exchange,[42] and advanced donation strategy, in which a donor provides a kidney before their recipient is matched, or even in need of a kidney transplant.[43] KPD should also include extended family members (such as cousins, uncles, and aunts) to donate. The THOA would require relook of its provisions from this point of view. These modern approaches are being practiced and legally allowed in KPD programs of developed world.
Education and Awareness for Kidney-Paired Donation
We recommend that each potential DRP should be given education, awareness, encouragement, and counseling about KPD transplant in an easy-to-understand format as early as possible in the process of CKD care. We recommend that a standard written informed consent should be obtained from each DRP.
A recent single-center cohort study[4] reported that 90% of incompatible DRP were not aware about KPD as a cost-effective LDKT options with good long-term outcome. This counseling can be performed by any member of transplant team, depending on the local resources at any time during the care of patient of CKD-ESRD. The transplanted patients and kidney donors can share their experience of KPD transplant and kidney donation within ethical and regulatory framework and can also help in increasing awareness and counseling.
Transplant Team and Transplant Coordinator Responsibilities for Kidney-Paired Donation
We recommend that all the transplant team including transplant coordinator in addition to other regular training should also be trained for counseling about risk, benefits of KPD, nonexchange options, consent process, financial screening of DRP, data entry-related issues, and overall support for KPD.
Every attempt should be made to prevent unequal and untoward outcome after kidney transplant due to donor-, patient-, transplant surgery-, and transplant center-related factors.
Consent Process for Kidney-Paired Donation
We recommend that standard written informed consent should be obtained from each DRP.
For any KPD transplant, the transplant hospital is responsible for obtaining and documenting informed consent from each DRP.
Fully documented standard written informed consent should include but not limited to:
Consent for donor nephrectomy and transplant surgery
Risks and benefits of KPD and non-KPD options (ABOiKT, desensitization therapy, DDKT, and maintenance dialysis)
Risks and benefits of kidney donor transport versus kidney transport
DRP willingness to travel to other transplant hospitals/state
Hospital outcomes of matched candidate's transplant
Potential exchange of all medical reports of each other before final allocation and consent
Possibility of untoward outcome after surgery or unexpected transmission of infection, disease, or tumor through the donor kidney, even after due precautions have been taken
Each high-risk patient with preexisting comorbid conditions such as diabetes, heart disease, and infections should be cognizant of unequal outcome after kidney transplant, which can occur due to patient-related factors. In 5%–10% DRP, there is a chance of poor outcome after transplantation due to unpredictable medical complications
Financial conflict of interest in nonanonymous allocation if there is socioeconomic disparity between DRPs
Informed counseling process about age disparity and quality of the kidney in nonanonymous donations.
It should also be on medical record that DRPs have been informed of all of the following elements of KPD program:
The KPD program's matching requirements and allocation process
DRPs do not choose their match
DRPs may decline a match or can withdraw from participation in the KPD program at any time, for any reason
DRPs can meet each other after completing all medical fitness and legal documents and before final allocation.
Waiting Time in Kidney-Paired Donation
We suggest that DRP should be given information about expected waiting time before transplantation and every attempt should be made to reduce waiting time, particularly for hard-to-match pairs with the innovative ways in KPD. We suggest that easy-to-match pairs (A donor and B recipient and vice versa) and sensitized pairs should be encouraged for KPD over ABOiKT and desensitization protocol.
The waiting time in KPD is ≤3 months in an active transplant center doing KPD for easy-to-match DRP (A donor and B recipient and vice versa).[3,4,5] The waiting time is more (>6 months) for difficult-to-match DRP such as O patient and non-O donor. Non-KPD options should be explored at regular intervals to avoid the morbidity and mortality of long-term dialysis. National KPD program can reduce this waiting time for KPD matching. Computing the strict time limit for all DRP is difficult and waiting timeline should be finalized as per the individual DRP requirement.
Prolong duration of pretransplant dialysis has adverse impact on long-term graft survival. Transplant rate for difficult-to-match pairs such as O blood group and sensitized patients can be increased by compatible pairs, longer chain, KPD with desensitization, KPD with ABOiKT, use of A2 donor to O patient, expanding the number of acceptable mismatches, national program, and living–deceased donor list exchange.[11,39,40] Waiting time in KPD is mostly less compared to DDKT.[4,44] ABOiKT is expensive compared to ABO compatible KPD transplant.[4,45,46]
Number of Exchanges in Kidney-Paired Donation
We suggest that all types of KPD [Table 4] should be practiced only after legal permission as per the existing law. We suggest that three-way exchange has optimum quality and quantity of matching.
Table 4.
Types of kidney-paired donation to increase donor pool

In India, two-way KPD is commonly practiced in single-center–based KPD program to avoid logistic problems.[3,4,5] In single-center program when surgical capacity is limited, two-way kidney exchange should be preferred. However, it has limitations in quality and quantity of matching. Longer chain may be considered for transplanting difficult-to-match pairs such as sensitized and O group patients. De Klerk et al.[47] reported that the optimal chain length for living donor KPD programs is 3. Unlimited chain length did not significantly affect the results. Longer chains with their inherent logistic burden do not lead to significantly more transplants.
Computer Versus Manual Allocation
We suggest that computerized algorithms should be encouraged over manual allocation. We suggest that potential KPD transplant centers should study the key elements of success of other successful KPD program.
We suggest that computerized algorithms should be encouraged over manual allocation. However, in the absence of computer allocation system, nonanonymous manual allocation can be considered. The allocation algorithm should be simple to minimize waiting time when donor pool is small like in single-center KPD program. The additional allocation parameters such as HLA matching can be considered when donor pool is large as in any national KPD program.
Patient Evaluation in Kidney-Paired Donation
We recommend that all patients should be screened for pretransplant immunological risk, occult infections, and other risk factors to prevent and reduce posttransplant unequal outcome due to patient-related factors.
Pretransplant immunological risk assessment can include many factors, but there is no clear consensus on to the parameters and its relative importance. If feasible, there should be provision for a centralized laboratory at least at each state level to perform screening cross-matches. The development of blood cryopreservation-based cross-matching accelerates matching process of pairs in a KPD without requiring fresh blood from donors. There should be uniform policy of cross-match by both complement-dependent cytotoxicity and flow cytometry cross-match and donor-specific antibody (DSA) in all transplants. The 2009 KDIGO clinical practice guideline for care of kidney transplant recipients reported that risk factors for acute rejection include the number of HLA mismatches, presence of a DSA, panel reactive antibody test > 0%, and blood group incompatibility (B is the majority agreement). Kute et al.[4] in a retrospective single-center cohort study suggested that pretransplant immunological risk assessment based on a combination of lymphocyte cross-match, flow cross-match, and DSA is intended to reduce posttransplant unequal outcome due to patient-related factors. This should be doubly checked before final allocation and a final cross-match before transplant and should always be practiced. Immunosuppressive regimen should be tailored according to the immunological risk status of individual patients. The blood sample of DRP used for final cross-match should be stored and cryopreserved for future testing, if required.
Medical fitness should be completed for kidney transplant and kidney donation by a multidisciplinary team consisting of but not limited to transplant physician, transplant surgeon, anesthetist, psychiatrist, gynecologist, HLA laboratory person, and other medical experts such as cardiologist and infectious disease physician as per the standard guidelines. The complete medical records and consent form should be maintained and submitted by transplant center before final donor allocation and double checked by appropriate authorized person. The information of DRP should remain confidential. The recent Indian study of 300 KPD transplant showed that short-term donor survival is 100%.[4]
Human Leukocyte Antigen Matching, Donor Age, and Long-Term Outcome in Kidney-Paired Donation
We suggest that the age difference between KPD donors should not be the key issue in allocation and better immunological match may counteract the effect of higher donor–recipient age difference.
Compatible DRP is expected to expand KPD transplant. Compatible pairs can increase the quality and quantity of KPD matching even in the single-center program with less logistics and improve long-term patient survival, graft survival, and outcome. The increasing enrolment of compatible pairs in KPD will shorten the waiting time for incompatible pairs with hard-to-match blood type combinations.
HLA matching has been best prognostic marker for long-term graft survival based on multiple regressions.[48,49] Basu et al. reported that significant benefits, better long-term survival, and lower infections due to less potent immunosuppression in Indian environment can be achieved by providing better-matched donors for HLA-mismatched compatible pairs through KPD.[25] Kidney transplant recipient of older DDKT has decreased long-term kidney graft survival. However, the impact of donor–recipient age difference on LDKT outcomes, where donors are older than recipients, remains unclear.
Kute et al.[9] reported a study examining the association of the difference in donor and recipient age on outcomes following living kidney donation. The authors reported no significant difference in recipient outcomes based on this age mismatch, and this supports the use of KPD in age mismatched pairs too. Given the limited number of first-degree relatives available as possible donors in a small family, it is far more relevant that older donors (usually within families) are just as good as younger ones.
The analysis using data from the Australia and New Zealand Dialysis and Transplant Registry, the US Renal Transplant Data System, and Indian experience showed that living kidney donor aged between 18 and 65 years has little or no impact on long-term outcome of LDKT.[9,50,51] This finding is useful in single-center KPD program when donor pool is small. These findings are of relevance when considering KPD program because the chance of finding a suitable match should not be limited by restrictions on the perceived disadvantage of high donor–recipient age difference.[9,50,51] There is gender imbalance in Indian transplant program.[2,3,4,5,6,7,8,9,10] The majority of transplant recipients are males and majority of kidney donors are females. KPD has the potential to increase transplant rate for female recipients.
Transplant Surgery in Kidney-Paired Donation
We recommend that participating transplant teams should make the decision by consensus about kidney donor travel versus kidney transport as per the local resources and logistics, though donor travel rather than kidney transport is likely to be logistically simpler to execute in the Indian situation. We suggest that transplant surgery should be performed at the place where patient is evaluated, admitted, and willing to do posttransplant follow-up and simultaneous rather than sequential surgery should be preferred.
Gill et al.[52] reported that cold ischemia time ≤16 h has little impact on LDKT outcomes. The one option is for the kidney donor to travel to the center where recipient is admitted. The transplant team should manage the logistics for transport of donor or kidney. Apex swap transplant registry, Mumbai, reported that the risk involved in transport of kidney donor is less than transport of kidney.[26] Transplant team should discuss the best option with the DRP as per available resources. The participating transplant centers and DRP should decide about transport of donor or kidney.
Multicenter simultaneous surgery should be encouraged over single-center nonsimultaneous surgery due to risk of donor renege. A recent study also reported willingness of Indian DRP for simultaneous surgery,[4] although another study suggested that nonsimultaneous KPD can be performed in some instances in carefully selected DRP.[8] Before nonsimultaneous multicenter KPD transplant, surgeon-to-surgeon communication should occur before starting donor or transplant surgery. In case where surgeries are not simultaneous, the patient must receive the kidney first before his/her donor donates the kidney. If available, deceased donor kidney can be utilized to initiate nonsimultaneous living donor chains rather than KPD living donors.[39] A highly sensitized difficult-to-match patient can be allocated standard criteria DDKT on priority without waiting list when living donor KPD is not feasible and his/her living donor can initiate nonsimultaneous KPD chain. Donor renege was nil in the recent Indian study.[4] A total of 1748 KPD transplants were performed in National Kidney Registry from 2008 to May 2016 and broken chains were infrequent and were rarely due to lack of donor motivation (n = 6).[53] Every precaution should be taken to avoid risk of donor renege.
National Kidney-Paired Donation Registry
We recommend that the formation of KPD registry is one of the principal strategies to improve quality of matching and number of KPD. We suggest that DRPs need to be cognizant of transcultural, language, and legal barriers in national program when patients and their donors may belong to different regions or states of India.
KPD can be a single-center program or may be multicenter or national program. Single-center KPD program has advantages that donor transport or transport of kidney is not required, surgical care is uniform, cold ischemia time is less, administrative cost is less, and follow-up of DRP is in familiar hospital.[11] However, multicenter KPD program has advantages of better quality matching and number of KPD due to large donor pool and computer allocation, less stress on the surgical team to carry simultaneous surgery in long chain, and better transplant rate for difficult-to-match DRP.[11]
There should be web-based data entry portal nationwide like National Organ and Tissue Transplant Registry (NOTTR). This should include a clear description of the options available to the patient and some basic information about other available choices such as KPD, ABOiKT, and DDKT. Transplant centers should be encouraged to enroll all of their pairs instead of performing internal exchanges utilizing their easy-to-match pairs. This would increase the quality of matching and number of KPD. It should be necessary to upload the HLA data of incompatible living donor for all potential recipients. Each state should have access to its state data of KPD registry.
KPD database of incompatible DRP should in addition to regular transplant data include information on demographics, physical characteristics, HLA profile, unacceptable and amenable antigens, discretionary exclusion criteria, certification of registration, suspension or withdrawal date and reason, dates of registration and update, contacts at recipient's center, and reason for joining KPD with coregistered recipient (ABO incompatible, lymphocyte cross-match positive, flow cross-match positive, luminex DSA positive, donor is compatible and joined KPD for better HLA/donor age matching, altruistic).[54,55]
Certification of the Patient's and Donor's Registration
An authorized person such as program director in the registering transplant center must attest all documents as per the standard guidelines and regulations.
National Kidney-Paired Donation Program
Guidelines and rules related to all KPD transplant may also be framed and updated by appropriate regulatory authority. KPD transplant should also be considered among unrelated donors (such as cousins, uncles, and aunts). Since unrelated transplants are allowed as per the law, paired donation among unrelated donors should also be allowed
For KPD transplants, there should be provision of clearance by single authority of the transplant center/state at the same time for all the eligible pairs, instead of collecting clearance from individual states of donors/recipients
All KPD activities to be directed and supervised by the state authorization committee and the NOTTO and follow their guidelines
NOTTO website should have more detailed information about KPD, ABOiKT, and DDKT, so that patient can make independent decision
All data of incompatible DRP should flow from the state into NOTTR as mandated under THOA and its rules, in India
All data/registry to be governed by existing law and work within the regulatory framework of NOTTO, regional organ and tissue transplant organization, and state organ and tissue transplant organization
Appropriate government notification or executive orders related to KPD may be issued.
Conclusion
KPD transplant is legal, cost-effective, rapidly expanding modality with good long-term outcome, and being implemented in several centers in India with the potential to increase LDKT by 25%. KPD transplant should be encouraged over ABOiKT and desensitization protocol. The quality of matching and number of KPD will be superior in national program versus single-center program due to large donor pool. Transplant team members, stakeholder, and policy-makers should work together to expand KPD.
Disclosure
These are recommendation on KPD transplantation after the Indian Society of Organ Transplantation (ISOT) midterm meeting organized at Chennai on March 18, 2017, and 1-day Workshop organized at Hotel Pullman, Aerocity, New Delhi, on April 29, 2017, under the Aegis of ISOT and participation of NOTT organization to discuss various issues related to expanding KPD and starting the National KPD program. Transplant surgeons, physicians, and other stakeholders from major centers across the country participated and had a robust discussion on the related issues.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rajapurkar MM, John GT, Kirpalani AL, Abraham G, Agarwal SK, Almeida AF, et al. What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol. 2012;13:10. doi: 10.1186/1471-2369-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kute VB, Gumber MR, Patel HV, Shah PR, Vanikar AV, Modi PR, et al. Outcome of kidney paired donation transplantation to increase donor pool and to prevent commercial transplantation: A single-center experience from a developing country. Int Urol Nephrol. 2013;45:1171–8. doi: 10.1007/s11255-012-0323-9. [DOI] [PubMed] [Google Scholar]
- 3.Kute VB, Shah PS, Vanikar AV, Gumber MR, Patel HV, Engineer DP, et al. Increasing access to renal transplantation in India through our single-center kidney paired donation program: A model for the developing world to prevent commercial transplantation. Transpl Int. 2014;27:1015–21. doi: 10.1111/tri.12373. [DOI] [PubMed] [Google Scholar]
- 4.Kute VB, Patel HV, Shah PR, Modi PR, Shah VR, Rizvi SJ, et al. Impact of single centre kidney paired donation transplantation to increase donor pool in India: A cohort study. Transpl Int. 2017;30:679–88. doi: 10.1111/tri.12956. [DOI] [PubMed] [Google Scholar]
- 5.Kute VB, Patel HV, Shah PR, Modi PR, Shah VR, Rizvi SJ, et al. Seventy-seven kidney paired donation transplantations at a single transplant centre in India led to an increase in living donor kidney transplantations in 2015. Clin Kidney J. 2017;10:709–14. doi: 10.1093/ckj/sfx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kute VB, Gumber MR, Vanikar AV, Shah PR, Patel HV, Engineer DP, et al. Comparison of kidney paired donation transplantations with living related donor kidney transplantation: Implications for national kidney paired donation program. Ren Fail. 2013;35:504–8. doi: 10.3109/0886022X.2013.773914. [DOI] [PubMed] [Google Scholar]
- 7.Kute VB, Vanikar AV, Shah PR, Gumber MR, Patel HV, Engineer DP, et al. Ten kidney paired donation transplantation on world kidney day 2013: Raising awareness and time to take action to increase donor pool. Ren Fail. 2013;35:1269–72. doi: 10.3109/0886022X.2013.823997. [DOI] [PubMed] [Google Scholar]
- 8.Kute VB, Patel HV, Varyani UT, Shah PR, Modi PR, Shah VR, et al. Six end-stage renal disease patients benefited from first non-simultaneous single center 6-way kidney exchange transplantation in India. World J Nephrol. 2016;5:531–7. doi: 10.5527/wjn.v5.i6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kute VB, Vanikar AV, Shah PR, Gumber MR, Patel HV, Engineer DP, et al. Does donor-recipient age difference matter in outcome of kidney transplantation. Implications for kidney paired donation? Ren Fail. 2014;36:378–83. doi: 10.3109/0886022X.2013.862769. [DOI] [PubMed] [Google Scholar]
- 10.Kute VB, Patel HV, Shah PR, Modi PR, Shah VR, Rizvi SJ, et al. International kidney paired donation transplantations to increase kidney transplant of O group and highly sensitized patient: First report from India. World J Transplant. 2017;7:64–9. doi: 10.5500/wjt.v7.i1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kute VB, Patel HV, Shah PR, Modi PR, Shah VR, Rizvi SJ, et al. Past, present and future of kidney paired donation transplantation in India. World J Transplant. 2017;7:134–43. doi: 10.5500/wjt.v7.i2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kute VB, Vanikar AV, Shah PR, Gumber MR, Patel HV, Engineer DP, et al. Increasing access to kidney transplantation in countries with limited resources: The Indian experience with kidney paired donation. Nephrology (Carlton) 2014;19:599–604. doi: 10.1111/nep.12307. [DOI] [PubMed] [Google Scholar]
- 13.Varyani UT, Kute VB, Patel HV, Shah PR, Vanikar AV, Modi PR, et al. Participation of compatible donor to improve HLA matching can increase kidney transplant rate of o blood group patients. Clin Queries Nephrol. 2015;4:38. [Google Scholar]
- 14.Kute VB, Gumber MR, Shah PR, Patel HV, Vanikar AV, Modi PR, et al. Successful three-way kidney paired donation transplantation: The first Indian report. Indian J Nephrol. 2014;24:45–7. doi: 10.4103/0971-4065.125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kute VB, Vanikar AV, Gumber MR, Shah PR, Patel HV, Engineer DP, et al. Successful three-way kidney paired donation with compatible pairs to increase donor pool. Ren Fail. 2014;36:447–50. doi: 10.3109/0886022X.2013.868294. [DOI] [PubMed] [Google Scholar]
- 16.Modi P, Rizvi SJ, Pal B, Baradwaj R, Gupta S, Shah V, et al. Living donor paired-kidney exchange transplantation: A single institution experience. Indian J Urol. 2010;26:511–4. doi: 10.4103/0970-1591.74446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kute VB, Patel HV, Shah PR, Modi PR, Shah VR, Rizvi SJ, et al. Increasing access to kidney transplantation for sensitized recipient through three-way kidney paired donation with desensitization: The first Indian report. World J Clin Cases. 2016;4:351–5. doi: 10.12998/wjcc.v4.i10.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kute VB, Vanikar AV Patel HV, Shah PR, Gumber MR, Engineer DP, et al. Combining kidney paired donation with desensitization increases renal transplantation rate in highly sensitized patients. Indian J Transplant. 2013;7:109–11. [Google Scholar]
- 19.Kute VB, Patel HV, Shah PR, Modi PR, Shah VR, Trivedi HL, et al. A potential solution to make the best use of a living donor-deceased donor list exchange. Am J Transplant. 2016;16:3580. doi: 10.1111/ajt.13974. [DOI] [PubMed] [Google Scholar]
- 20.Kute V, Jindal RM, Prasad N. Kidney paired-donation program versus global kidney exchange in India. Am J Transplant. 2017;17:2740–1. doi: 10.1111/ajt.14324. [DOI] [PubMed] [Google Scholar]
- 21.Kute VB, Vanikar AV, Shah PR, Gumber MR, Patel HV, Modi PR, et al. Facilitators to national kidney paired donation program. Transpl Int. 2013;26:e38–9. doi: 10.1111/tri.12078. [DOI] [PubMed] [Google Scholar]
- 22.Kute VB, Patel HV, Shah PR, Vanikar AV, Trivedi HL. National kidney paired donation programme in India: Challenges, solution, future direction. Nephrology (Carlton) 2015;20:442. doi: 10.1111/nep.12408. [DOI] [PubMed] [Google Scholar]
- 23.Kute VB, Gumber MR, Dhananjay KL, Vanikar AV, Yadav DK, Patel MP, et al. Living donor exchange programs in renal transplantation: A paradigm ready for broad implementation. Int Urol Nephrol. 2013;45:597–9. doi: 10.1007/s11255-012-0204-2. [DOI] [PubMed] [Google Scholar]
- 24.Kute VB, Patel HV, Shah PR, Modi PR, Shah VR, Kasat GS, et al. Four-way kidney exchange transplant with desensitization increases access to living-donor kidney transplant: First report from India. Exp Clin Transplant. 2017 Sep 26; doi: 10.6002/ect.2017.0089. doi: 10.6002/ect.2017.0089. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Basu G, Daniel D, Rajagopal A, Neelakantan N, John GT. A model for human leukocyte antigen-matched donor-swap transplantation in India. Transplantation. 2008;85:687–92. doi: 10.1097/TP.0b013e318163827e. [DOI] [PubMed] [Google Scholar]
- 26.Travasso C. Five patients benefit from India's first “domino” kidney swap. BMJ. 2013;347:f4260. doi: 10.1136/bmj.f4260. [DOI] [PubMed] [Google Scholar]
- 27.Jha PK, Sethi S, Bansal SB, Jain M, Sharma R, Phanish MK, et al. Paired kidney exchange transplantation: Maximizing the donor pool. Indian J Nephrol. 2015;25:349–54. doi: 10.4103/0971-4065.150721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waigankar SS, Kamat MH, Joshi S, Gandhi BV, Bahadur M, Deshpande RV, et al. Living donor transplant options in end-stage renal disease patients with ABO incompatibility. Indian J Urol. 2013;29:114–8. doi: 10.4103/0970-1591.114031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pahwa M, Saifee Y, Tyagi V, Chadha S, Jauhari H. Paired exchange kidney donation in India: A five-year single-center experience. Int Urol Nephrol. 2012;44:1101–5. doi: 10.1007/s11255-012-0155-7. [DOI] [PubMed] [Google Scholar]
- 30.Chaskar V, Oak S, Kesarwani A, Darshini D, Garasia M. Successful first swap renal transplant in a public hospital. Indian J Anaesth. 2016;60:768–71. doi: 10.4103/0019-5049.191699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gumber MR, Kute VB, Goplani KR, Shah PR, Patel HV, Vanikar AV, et al. Transplantation with kidney paired donation to increase the donor pool: A single-center experience. Transplant Proc. 2011;43:1412–4. doi: 10.1016/j.transproceed.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal SK, Srivastava RK, Gupta S, Tripathi S. Evolution of the transplantation of human organ act and law in India. Transplantation. 2012;94:110–3. doi: 10.1097/TP.0b013e31825ace15. [DOI] [PubMed] [Google Scholar]
- 33.Bingaman A, Kapturczak M, Murphey C, Wright F. Kidney paired donation: A single center approach to increase living donor transplantation. Clin Transpl. 2010:345–52. [PubMed] [Google Scholar]
- 34.de Klerk M, Keizer KM, Claas FH, Witvliet M, Haase-Kromwijk BJ, Weimar W, et al. The Dutch national living donor kidney exchange program. Am J Transplant. 2005;5:2302–5. doi: 10.1111/j.1600-6143.2005.01024.x. [DOI] [PubMed] [Google Scholar]
- 35.Hadaya K, Fehr T, Rüsi B, Ferrari-Lacraz S, Jean V, Ferrari P, et al. Kidney paired donation: A plea for a Swiss National Programme. Swiss Med Wkly. 2015;145:w14083. doi: 10.4414/smw.2015.14083. [DOI] [PubMed] [Google Scholar]
- 36.Cole EH, Nickerson P, Campbell P, Yetzer K, Lahaie N, Zaltzman J, et al. The Canadian kidney paired donation program: A national program to increase living donor transplantation. Transplantation. 2015;99:985–90. doi: 10.1097/TP.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 37.Cantwell L, Woodroffe C, Holdsworth R, Ferrari P. Four years of experience with the Australian kidney paired donation programme. Nephrology (Carlton) 2015;20:124–31. doi: 10.1111/nep.12369. [DOI] [PubMed] [Google Scholar]
- 38.Ferrari P, Weimar W, Johnson RJ, Lim WH, Tinckam KJ. Kidney paired donation: Principles, protocols and programs. Nephrol Dial Transplant. 2015;30:1276–85. doi: 10.1093/ndt/gfu309. [DOI] [PubMed] [Google Scholar]
- 39.Melcher ML, Roberts JP, Leichtman AB, Roth AE, Rees MA. Utilization of deceased donor kidneys to initiate living donor chains. Am J Transplant. 2016;16:1367–70. doi: 10.1111/ajt.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garonzik-Wang JM, Sullivan B, Hiller JM, Cass V, Tchervenkow J, Feldman L, et al. International kidney paired donation. Transplantation. 2013;96:e55–6. doi: 10.1097/TP.0b013e3182a68879. [DOI] [PubMed] [Google Scholar]
- 41.Ashlagi I, Gilchrist DS, Roth AE, Rees MA. Nonsimultaneous chains and dominos in kidney- paired donation-revisited. Am J Transplant. 2011;11:984–94. doi: 10.1111/j.1600-6143.2011.03481.x. [DOI] [PubMed] [Google Scholar]
- 42.Rees MA, Dunn TB, Kuhr CS, Marsh CL, Rogers J, Rees SE, et al. Kidney exchange to overcome financial barriers to kidney transplantation. Am J Transplant. 2017;17:782–90. doi: 10.1111/ajt.14106. [DOI] [PubMed] [Google Scholar]
- 43.Wall AE, Veale JL, Melcher ML. Advanced donation programs and deceased donor initiated chains – 2 innovations in kidney paired donation. Transplantation. 2017;101:2818–2824. doi: 10.1097/TP.0000000000001838. [DOI] [PubMed] [Google Scholar]
- 44.Ferrari P, Fidler S, Woodroffe C, Tassone G, D’Orsogna L. Comparison of time on the deceased donor kidney waitlist versus time on the kidney paired donation registry in the Australian program. Transpl Int. 2012;25:1026–31. doi: 10.1111/j.1432-2277.2012.01541.x. [DOI] [PubMed] [Google Scholar]
- 45.Held PJ, McCormick F. ABO-incompatible kidney transplants: Twice as expensive, half as good. Am J Transplant. 2016;16:1343–4. doi: 10.1111/ajt.13638. [DOI] [PubMed] [Google Scholar]
- 46.Axelrod D, Segev DL, Xiao H, Schnitzler MA, Brennan DC, Dharnidharka VR, et al. Economic impacts of ABO-incompatible live donor kidney transplantation: A National study of medicare-insured recipients. Am J Transplant. 2016;16:1465–73. doi: 10.1111/ajt.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Klerk M, Van Der Deijl WM, Witvliet MD, Haase-Kromwijk BJ, Claas FH, Weimar W, et al. The optimal chain length for kidney paired exchanges: An analysis of the Dutch program. Transpl Int. 2010;23:1120–5. doi: 10.1111/j.1432-2277.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- 48.Milner J, Melcher ML, Lee B, Veale J, Ronin M, D’Alessandro T, et al. HLA matching trumps donor age: Donor-recipient pairing characteristics that impact long-term success in living donor kidney transplantation in the era of paired kidney exchange. Transplant Direct. 2016;2:e85. doi: 10.1097/TXD.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuncer M, Tekin S, Yücetin L, Şengül A, Demirbas A. Comparison of paired exchange kidney transplantations with living related kidney transplantations. Transplant Proc. 2012;44:1626–7. doi: 10.1016/j.transproceed.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 50.Ferrari P, Lim W, Dent H, McDonald SP. Effect of donor-recipient age difference on graft function and survival in live-donor kidney transplantation. Nephrol Dial Transplant. 2011;26:702–8. doi: 10.1093/ndt/gfq383. [DOI] [PubMed] [Google Scholar]
- 51.Chang P, Gill J, Dong J, Rose C, Yan H, Landsberg D, et al. Living donor age and kidney allograft half-life: Implications for living donor paired exchange programs. Clin J Am Soc Nephrol. 2012;7:835–41. doi: 10.2215/CJN.09990911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gill J, Rose C, Joffres Y, Kadatz M, Gill J. Cold ischemia time up to 16 hours has little impact on living donor kidney transplant outcomes in the era of kidney paired donation. Kidney Int. 2017;92:490–6. doi: 10.1016/j.kint.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 53.Cowan N, Gritsch HA, Nassiri N, Sinacore J, Veale J. Broken chains and reneging: A Review of 1748 kidney paired donation transplants. Am J Transplant. 2017;17:2451–7. doi: 10.1111/ajt.14343. [DOI] [PubMed] [Google Scholar]
- 54.Wallis CB, Samy KP, Roth AE, Rees MA. Kidney paired donation. Nephrol Dial Transplant. 2011;26:2091–9. doi: 10.1093/ndt/gfr155. [DOI] [PubMed] [Google Scholar]
- 55.Rees MA, Bargnesi D, Samy K, Reece L. Altruistic donation through the alliance for paired donation. Clin Transpl. 2009:235–46. [PubMed] [Google Scholar]


