Abstract
OBJECTIVES:
Although cardioprotective effects of telmisartan are well explored, its effects on epigenetic alterations associated with type 2 diabetic (T2D) cardiomyopathy remain unmapped. Thus, the present study was designed to evaluate the potential of esculetin and telmisartan combination to reverse histone posttranslational modifications (PTMs) in curbing T2D cardiomyopathy.
MATERIALS AND METHODS:
T2D was induced by high-fat diet feeding along with low dose of streptozotocin (35 mg/kg, I.P) in male Wistar rats. T2D rats were treated with either telmisartan (10 mg/kg/day, P.O) or esculetin (50 mg/kg/day doses, P.O) or their combination for 2 weeks. Biochemical estimations, vascular reactivity, immunohistochemistry, and western blotting experiments were performed to evaluate the effects of the treatment in T2D cardiomyopathy.
RESULTS:
Esculetin and telmisartan combination alleviated the pathological features of T2D cardiomyopathy including metabolic perturbations, morphometric alterations, altered vascular reactivity, increased Keap1 and fibronectin expression more effectively than their respective monotherapy. This is the first report showing that telmisartan attenuates increased level of histone PTMs such as H3K9me2, H3K9Ac, H2AK119Ub, and H2BK120Ub in heart of T2D rats. The combination regimen showed a more significant reduction in augmented histone PTMs associated with T2D cardiomyopathy than their independent treatments.
CONCLUSIONS:
The present study demonstrates that esculetin and telmisartan combination can be an advanced pharmacological approach to ameliorate T2D cardiomyopathy which could be partially attributed to its ability to reverse the epigenetic alterations.
Keywords: Epigenetics, esculetin, telmisartan, type 2 diabetic cardiomyopathy
Introduction
The cardiovascular complications associated with diabetes are considered to be one of the leading causes of morbidity and mortality in diabetic patients. The manifestation of microvascular and macrovascular complications associated with diabetes is very complex, and upregulation of the renin-angiotensin system (RAS) is one of the main offenders for these complications.[1] Angiotensin II is a key player of RAS, mediating its physiological and pathological effects through AT1 Rs and AT2 Rs (Ang II type 1 and 2 receptors) which play a pivotal role in pathogenesis of cardiovascular dysfunctions associated with diabetes.[2] Hence, drugs acting on RAS such as AT1R blockers (ARBs) or angiotensin-converting enzyme (ACE) inhibitors are the drugs of choice for diabetic cardiovascular complications.[2] The experimental and epidemiological studies suggest that telmisartan – an ARBs with partial peroxisome proliferator-activated receptor-γ (PPAR-γ) agonistic activity – has cardioprotective effect in type 2 diabetic (T2D) condition.[3] On the other hand, the combinations of ARBs with natural products such as curcumin and resveratrol have been found to be more effective in reducing metabolic perturbation, inflammation, insulin resistance (IR), hypertension, and many other complications associated with diabetes.[4,5] Recently, we have reported that combination of telmisartan with a functional food ingredient, esculetin-prevented oxidative stress, cardiac fibrosis, and vascular stiffness in high-fat diet (HFD) induced IR condition.[6]
It is a well-known fact that in order to understand pathogenesis of T2D completely, the genetic penchant alone is insufficient, so we have to decode information present as the epigenetic code.[7] Among all epigenetic alterations, histone posttranslational modifications (PTMs) are the most widely studied in T2D. Usually, augmented permissive histone PTMs (e.g., H3K9Ac, H3K14Ac, H3K27Ac, H3S10phospo, H3S28phospo, H3K4me, H3K36me, and H3K79me) and abridged repressive histone PTMs (e.g., H3K9me and H3K27me) lead to chromatin opening leading to its transcriptionally active state.[8,9] Recently, we have demonstrated increase in histone H2A/H2B ubiquitination (H2AK119Ub and H2BK120Ub) and other permissive histone H3 modifications in the heart of T2D rats which could be reversed successfully by esculetin (100 mg/kg/day, P.O.) treatment.[10]
However, there is no report demonstrating the effect of telmisartan and its combination with esculetin on epigenetic alterations associated with diabetic cardiomyopathy. Thus, in the present study, we aimed to investigate the effectiveness of this combination in curbing T2D cardiomyopathy and its associated epigenetics changes by utilizing nongenetic experimental model of T2D–HFD feeding and low dose of streptozotocin (STZ) (35 mg/kg, I.P) treated rats.
Materials and Methods
Chemicals
Esculetin and STZ were procured from Sigma-Aldrich. Spectrophotometric kits were purchased from Accurex Biomedical Pvt. Ltd. (Mumbai, Maharashtra, India) and ultra-sensitive rat insulin kit was obtained from Crystal Chem (Downers Grove, IL, USA). Antibodies against fibronectin and Keap1 were obtained from Santa Cruz Biotechnology (Dallas, TX, USA) and the rest of the antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Enhanced chemiluminescence (ECL) reagent was purchased from Thermo Fisher Scientific (MI, USA). All the other chemicals were purchased from Sigma (St. Louis, MO, USA) unless otherwise mentioned.
Animal studies
The male adult Wistar rats (130–150 g) were procured from the central animal facility of the institute, Birla Institute of Technology and Science Pilani (BITS Pilani). Animals were maintained under standard environmental conditions and provided with feed and water ad libitum. Our animal protocol is in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Environment, Government of India. Rules of CPCSEA are laid down as per ILAR (Institute of Laboratory Animal Resources, USA) guidelines, and prior permit was sought from the Institutional Animal Ethics Committee, BITS Pilani, for conducting the study. All experimental procedures had been approved (IAEC/RES/17/03/Rev-2/19/35) by the local government authorities.
Development of nongenetic model of type 2 diabetes
T2D was induced by HFD feeding and low dose of STZ (35 mg/kg, I.P) as per the protocol described previously.[10] Briefly, after 1 week of normal pellet diet (NPD) feeding, rats were allocated to two dietary regimens either NPD (n = 6) or HFD (n = 24) ad libitum, respectively, for an initial period of 2 weeks. After 2 weeks of dietary manipulation, the rats from the HFD-fed group were injected with a low dose of STZ (35 mg/kg, I.P) to induce T2D, whereas the respective control rats were given vehicle citrate buffer (pH 4.4). Animals were fed on respective diets till the end of the study.
Telmisartan and esculetin treatment
After 4 weeks, T2D control (T2DC) rats were treated with either esculetin (50 mg/kg/day) or telmisartan (10 mg/kg/day) or their combination and respective control animals were treated with vehicle (0.5% sodium carboxymethyl cellulose) for 2 weeks and the rats were allowed to feed on their respective diets till the end of the study.[6] Body weight (BW), biochemical estimations, and blood pressure measurements were performed at the end of 6 weeks.
Assessment of type 2 diabetes and hemodynamic changes
The blood samples were collected, plasma was separated, and fasting plasma glucose (PGL), triglycerides (PTGs) and total cholesterol (PTC) levels were estimated as per manufacturer's instructions using commercially available spectrophotometric kits. Plasma insulin (PI) was estimated by ultrasensitive rat insulin ELISA kit. Systolic blood pressure (SBP) was recorded on the last day of the treatment in all groups using noninvasive blood pressure measurement system (AD Instruments, Australia).[10]
Vascular reactivity experiments
Vascular reactivity experiments were performed as described previously.[6,10] Briefly, aortic ring was suspended using a pair of stainless steel hooks in water-jacketed organ bath (UGO Basile, Varese, Italy), and cumulative contractile responses to increasing concentrations of Ang II (1nM to 30 μM) were measured by using isometric force transducer (7005F, UGO Basile), and the Concentration response curve (CRC) was recorded. For measurement of acetylcholine-mediated relaxation, the aortic ring was precontracted with 100 nmol/L phenylephrine after which the CRC to acetylcholine (1 nM to 30 μM) was recorded. The responses were normalized to cross-sectional area of the tissue and the tension developed was calculated.
Histopathology and immunohistochemistry
Histopathology and immunohistochemistry (IHC) were performed as per the protocol described earlier.[6,10] Briefly, from each rat, a portion of heart tissue was fixed in 10% (v/v) formalin in phosphate-buffered solution, embedded in paraffin after completing the routine processing and 5 μm sections were taken. For histopathology, the sections were stained with hematoxylin/eosin (H and E), and for IHC, the following primary antibodies were used: anti-fibronectin (rabbit, 1:50 dilution; Santa Cruz Biotechnology), anti-Keap1(rabbit, 1:50 dilution; Cell Signaling Technology), and horseradish peroxidase (HRP)-linked anti-rabbit secondary antibody, followed by detection with diaminobenzidine as a chromogen. At least 25 heart sections from each group were observed under microscope (Olympus BX41, NY, USA) and images were captured. All the images were subjected to semiquantitative analysis by Image J software for the measurement of % nuclei positive area, % fibronectin positive area, and % Keap1 positive area; the data were further analyzed by Prism software (version 5.0; GraphPad, San Diego, CA, USA).
Histone isolation and western blotting
Heart tissues were dissected manually, and histone isolation and western blotting were performed as per the protocol described earlier.[10] Immunoblot analysis was performed using rabbit monoclonal antibodies against histone H3 acetylation (H3K9Ac), dimethylation (H3K9 me2), histone ubiquitination - H2AK119Ub and H2BK120Ub; all antibodies were supplied by Cell Signaling Technology and used in 1:1000 dilution. As secondary anti-rabbit IgG, HRP-linked antibody was used in dilution 1:20000 (Cell Signaling Technology). Proteins were detected by the ECL system and ECL Hyperfilm (Amersham Biosciences). Immunoblots were quantified by densitometric analysis using Image J software and the exposures were in linear dynamic range, each modification was normalized by respective total H3 blot; then, data analysis was performed using Prism software (version 5.0; GraphPad, San Diego, CA, USA) and results were expressed as fold over normal control (NC).
Statistical analysis
Experimental values were expressed as means ± standard error of mean. Statistical comparison between different groups was performed using one-way analysis of variance and if F value is significant then multiple comparison was done by Tukey test using Prism software (version 5.0; GraphPad, San Diego, CA, USA) for Windows. Data were considered statistically significant if P < 0.05.
Results
Effect of telmisartan and esculetin on metabolic perturbations in type 2 diabetic condition
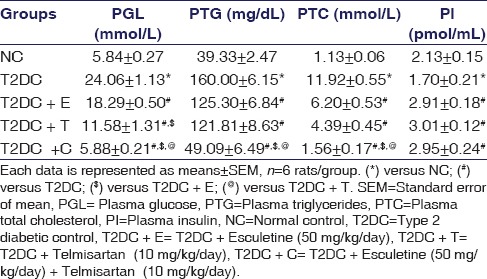
At the end of the experimental period, fasting PGL, PTGs, and PTC levels were significantly higher in T2DC rats compared to NC rats [Table 1]. On the other hand, T2DC rats showed a significant reduction in PI as compared to NC [Table 1], which confirmed the development of T2D in these rats. The treatment with either esculetin or telmisartan reduced PGL, PTGs, and PTC levels in T2D rats. The rats receiving combination treatment showed significant improvement in PGL level and plasma lipid profile as compared to T2DC rats as well as rats treated with either esculetin or telmisartan. Esculetin or telmisartan treatment, alone or in combination, significantly increased PI level as compared to T2DC rats.
Table 1.
Plasma biochemical parameters: Plasma glucose level, triglycerides, total cholesterol, and insulin levels in normal control, type 2 diabetic control, type 2 diabetic control treated with esculetin, telmisartan, and the combination at the end of the experimental period
Effect of telmisartan and esculetin on hemodynamic and morphometric alterations in type 2 diabetic condition
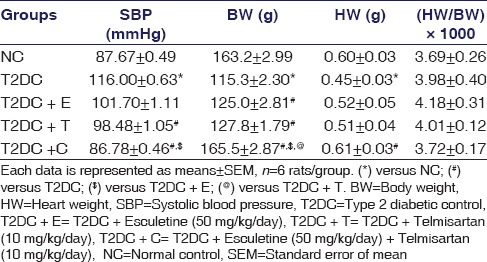
SBP was significantly increased in T2DC as compared to NC rats. In contrast, the combination-treated rats showed significant reduction in SBP, while esculetin alone was unable to reduce SBP, and telmisartan monotherapy showed slight reduction in SBP compared to T2DC rats. Cardiac hypertrophy, a major feature of T2D cardiomyopathy, is indicated by a reduction in cardiac nuclei count. H and E staining showed that the nuclei count in T2D rats' heart was significantly reduced, which was improved significantly by the combination regimen [Figure 1a]. The morphometric parameters, i.e., BW and heart weight (HW), were significantly reduced in T2DC rats as compared to NC rats [Table 2]. BW was significantly increased, while HW remained unaltered by treatment with esculetin or telmisartan alone as compared to T2DC. Interestingly, telmisartan and esculetin combination increased BW as compared to T2D and esculetin or telmisartan monotherapy. In addition, rats receiving combination treatment showed significant increase in HW as compared to T2DC rats [Table 2]. There was no significant difference observed in relative HW ([HW/BW] × 1000) of the animals among all experimental groups.
Figure 1.
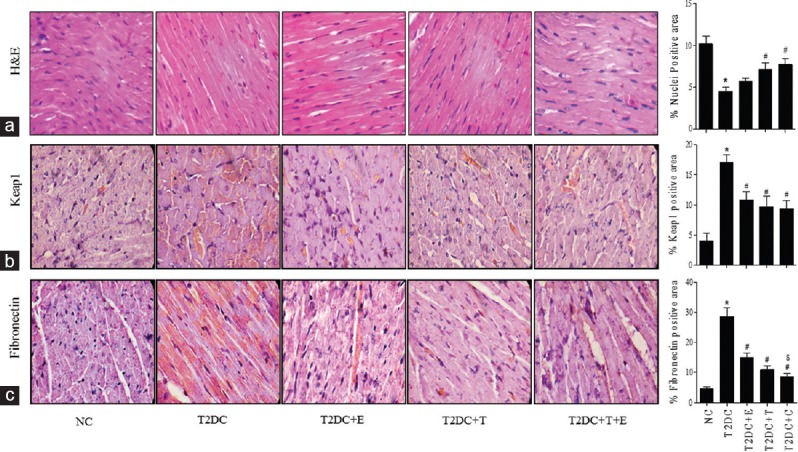
Telmisartan and esculetin combination attenuated increased expression of Keap1 and fibronectin in type 2 diabetic heart. (a) H and E staining, (b) the immunostaining of Keap1, and (c) fibronectin in heart sections, and their respective bar graph for % nuclei positive area, % Keap1 positive area, and % fibronectin positive area calculated semiquantitatively using ImageJ software. All values are represented as means ± standard error of mean from at least 25 sections per group. The scale bar represents 50 μm (original magnification, ×100). Note: Each data is represented as means ± standard error of mean (n = 6). *P < 0.05 versus NC;#P < 0.05 versus T2DC;$P < 0.05 versus T2DC+E;@P < 0.05 versus T2DC +T
Table 2.
Hemodynamic and morphometric measures: Systolic blood pressure, body weight, heart weight, and relative heart weight (heart weight/body weight) of rats of all the experimental groups at the end of study
Effect of telmisartan and esculetin on the vascular responsiveness to Angiotensin II and acetylcholine challenges in type 2 diabetic condition
Aortic ring of T2D rats showed exaggerated vascular responsiveness to Ang II as indicated by increased maximal contractile response to Ang II challenges, which was depicted by upward shift of the cumulative concentration-response curves (CRCs) as compared to NC. Treatment with telmisartan or esculetin alone ameliorated the vascular hyperresponsiveness to Ang II in T2D rats to some extent, but even better improvement was observed in case of the rats receiving the combination treatment regimen. There was no significant difference among all the groups in terms of the pD2 value, which indicated the similar sensitivity of aortic rings from different groups to Ang II [Figure 2a]. T2DC rats showed impaired vascular endothelial-dependent relaxation in response to acetylcholine. The monotherapy with esculetin was unable to make it better while telmisartan alone alleviated acetylcholine-mediated vascular relaxation to some extent. The combination-treated rats showed improvement in acetylcholine-mediated endothelial dependent relaxation as compared to T2DC rats and monotherapy [Figure 2b].
Figure 2.
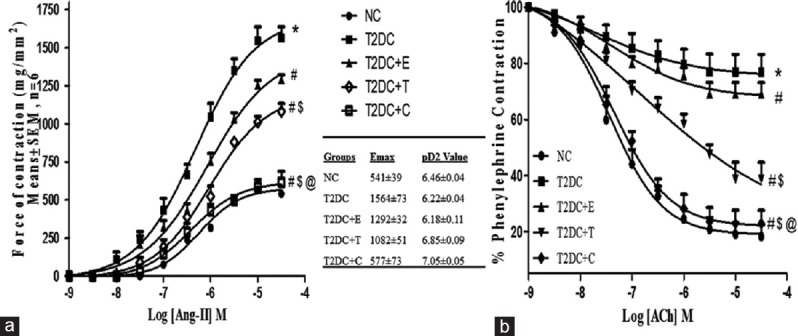
Telmisartan and esculetin combination prevented hyperresponsiveness to Ang II and restored endothelial-dependent relaxation. (a) Ang II and (b) acetylcholine-mediated cumulative concentration response curve (CRC) in aortic ring obtained from normal control; normal control rats, type 2 diabetic control; type 2 diabetic control, T2DC+E; type 2 diabetic control treated with esculetin, T2DC+T; type 2 diabetic control treated with telmisartan, T2DC+C; type 2 diabetic control treated with combination of esculetin and telmisartan. Note: Each data is represented as means ± standard error of mean (n = 6). *P < 0.05 versus NC;#P < 0.05 versus T2DC;$P < 0.05 versus T2DC+E;@P < 0.05 versus T2DC+T
Effect of telmisartan and esculetin on Keap1 and fibronectin expression in type 2 diabetic heart
The oxidative stress is considered to be one the culprits for cell death, and the Keap1/Nrf2 complex is an important regulator of antioxidant gene expression which plays a crucial role in pathogenesis of diabetes and its complications. Hence, we have analyzed Keap1 protein levels by IHC, and T2DC rats showed increased Keap1 level in the heart as compared to NC rats. Esculetin- or telmisartan-treated rats showed decreased Keap1 expression in the heart as compared to T2DC rats. The rats receiving combination regimen showed better reduction in Keap1 level but was not statistically significant as compared to monotherapy [Figure 1b].
T2D is known to be associated with myocardial fibrosis and concomitant increase in expression of fibrotic markers such as fibronectin. Hence, to further evaluate the effect of esculetin and telmisartan on cardiac fibrosis, the expression of fibronectin was checked using IHC. T2DC animals showed increased expression of fibronectin in heart as compared to NC, which was significantly reduced by esculetin and telmisartan monotherapy. The rats receiving the combination showed even more significant decrease in fibronectin level in heart as compared to T2DC rats and monotherapy [Figure 1c].
Effect of telmisartan and esculetin on histone H3 posttranslational modifications type 2 diabetic heart
To evaluate effect of esculetin and telmisartan on histone H3 PTMs in T2D, we profiled well-studied PTMs, namely, H3K9me2 (panel a) and H3K9Ac (panel b). It was found that H3K9me2 and H3K9Ac levels were significantly increased in T2DC rats as compared to NC [Figure 3]. Esculetin monotherapy reduced only H3K9Ac level significantly. Telmisartan-treated T2D rats' heart showed significant decrease in H3K9me2 and H3K9Ac levels as compared to T2DC. The combination regimen reduced H3K9me2 and H3K9Ac more effectively as compared to monotherapy [Figure 3].
Figure 3.
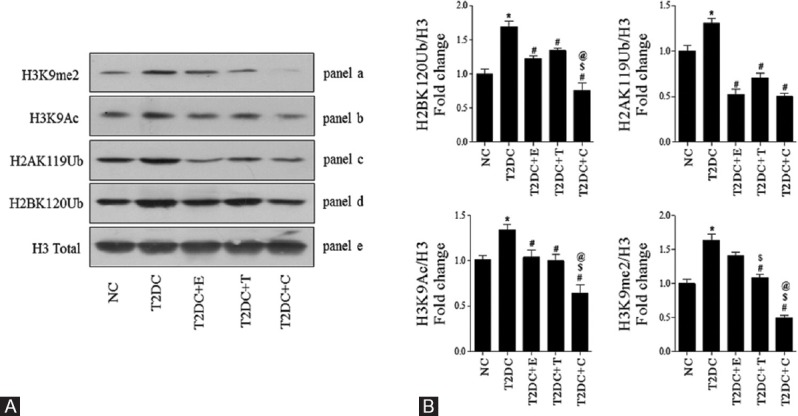
Telmisartan and esculetin combination alleviated histone posttranslational modifications – H3K9 me2, H3K9Ac, H2AK119Ub, and H2BK120Ub in type 2 diabetic heart Immunoblotting in total heart from all groups for PTHMs-H3K9 me2 (panel a), H3K9Ac (panel b), H2AK119Ub (panel c), and H2BK120Ub (panel d) were performed and a representative of three different blots were shown in (A); Proteins level were quantified by scanning densitometry using Image J software (B) Each data point is represented as mean ± standard error of mean, n = 3 blots/protein *P < 0.05 versus NC;#P < 0.05 versus T2DC;$P < 0.05 versus T2DC+E;@P < 0.05 versus T2DC+T
Effect of telmisartan and esculetin on histone H2A and H2B ubiquitination in T2D heart
To the best of our knowledge, the effects of telmisartan and its combination with esculetin on histone H2A and H2B ubiquitination in T2D cardiomyopathy have not yet been documented. Hence, in addition to histone H3 PTMs, we examined H2AK119Ub (panel c) and H2BK120Ub (panel d) [Figure 3], and we found that in the hearts of T2DC rats, levels of H2AK119Ub and H2BK120Ub were significantly higher than NC rats [Figure 3]. Treatment with esculetin and telmisartan significantly reduced H2AK119Ub and H2BK120Ub as compared to T2DC. However, the rats subjected to combination therapy have shown more pronounced reduction in H2AK119Ub and H2BK120Ub levels as compared to monotherapy [Figure 3].
Discussion
In the current study, we found that esculetin and telmisartan combination may play a significant role in reversing the posttranslational histone modifications including H3K9 dimethylation, H3K9 acetylation, H2AK119 monoubiquitination, and H2BK120 monoubiquitination more significantly than their individual treatments. The combination also ameliorates biochemical, morphometric, and hemodynamic alterations linked with diabetic cardiomyopathy along with the reduction in oxidative stress and fibrosis, which may be associated with its ability to reverse the aforementioned histone covalent modifications.
In T2D, hyperglycemia is considered to be a main culprit for the development of cardiovascular complications. Hyperglycemia is responsible for altered insulin receptor signaling cascades and overactivation of RAS which in turn increases oxidative stress, inflammation, endothelial dysfunctions, vascular stiffness, cardiomyocytes hypertrophy, and cardiac fibrosis.[11] Hyperglycemia along with other concomitant risk factors (e.g. hypertension, IR, and altered plasma lipid profile) accelerate the rate of the development of cardiovascular diseases (e.g. coronary heart disease, stroke, and peripheral vascular disease.[12] Hence, drugs which provide good glycemic control and/or act on RAS either by inhibiting ACE or by blocking AT1R remain major focuses for the prevention of cardiovascular dysfunctions in patients with T2D.
ARB telmisartan remains drug of choice among physician for the management of diabetic cardiomyopathy. Telmisartan possess partial agonistic activity on peroxisome PPAR-γ, which plays an important role in regulation of carbohydrate and lipid metabolism.[3] The existing literature suggests that cardiovascular protective effects of telmisartan were markedly increased when it is used in combination with either ACE inhibitor or other molecule which acts through a different mechanism to give an additive therapeutic effects.[4,5] Esculetin, a natural coumarin derivative, has been known to possess antioxidant, antihypertrophic, anti-inflammatory, antiallergic, antiobesity, and insulin-sensitizing properties.[6,7,10] The current study demonstrated that rats receiving esculetin and telmisartan combination therapy showed more pronounced alleviation in morphometric, biochemical, or hemodynamic alterations as compared to the rats treated with individual drugs. Furthermore, we found that the cardiomyocyte nuclei count went drastically low in the diabetic rats, whereas the relative HW did not show any major alterations as compared to the normal rats, which indicates cardiac hypertrophy rather than proliferation.
Hypernutrition and obesity often lead to IR. Substantial clinical evidences suggested that β-cell mass is increased in obese nondiabetic humans compared with lean controls and is decreased in obese patients with impaired fasting glucose or type 2 diabetes. Similarly, β-cell apoptosis is increased in obese humans with glucose intolerance or diabetes. Further, in both animal models and humans, the triggering factor in transforming from IR to type 2 diabetes is β-cell failure, which involves a decrease in β-cell mass and deterioration of key β-cell functions such as glucose-stimulated insulin secretion. Current research literatures suggest that β-cell failure occurs as a combined consequence of metabolic overload, oxidative stress, increased rates of apoptosis, and loss of expression of fundamental components of the insulin granule secretory machinery.[13] Based on such explored mechanisms, the effective therapeutic targets in the treatment of diabetes could be either direct insulin or noninsulin related. Partially reduction in oxidative stress and exaggerated inflammatory machineries could help in deduction in β-cell apoptosis and improve the β-cell mass which results into improved insulin production. In this context, numerous studies have demonstrated that telmisartan at the cellular level increases the activation of PPAR-γ target genes in human preadipocytes; PPAR-g is an important mediator in the pathogenesis of IR leading into reduction of serum glucose, insulin, and PTG levels and of weight gain in rats which were fed with a high-fat diet. Further, telmisartan activates the IGF-1 receptor expression in human skeletal muscle cells, the improvement of insulin sensitivity in Hep3B cells, and the enhanced fatty acid oxidation in rat skeletal muscle. Furthermore, telmisartan was reported for inhibition of tumor necrosis factor-induced interleukin-6 expression in rat vascular smooth muscle cells, transforming growth factor-stimulated accumulation of extracellular matrix, downregulation of advanced glycation end products (AGE) receptor inhuman mesangial cells and cellular damage in human endothelial cells, and inhibition of AGE-induced monocyte chemoattractant protein-1 expression. This conclude that temisartan not only improves insulin sensitivity but also paralyze the complex inflammatory cascade under diabetes.[14] Further, telmisartan combination with potent antioxidant, in this study esculetin, could be functionalized pleotropic manner which resulted in tweaked insulin levels in T2D animals.
Telmisartan and esculetin combination was found to ameliorate the cardiac hypertrophy more efficiently than their respective individual treatments. The observed additive improvement in metabolic and morphometric alterations by combination treatment might be due to concomitant obstruction of pathogenesis by two different mechanisms; telmisartan acts by AT1R blockage and PPAR-γ partial agonism while esculetin acted by reducing oxidative stress and inflammation.[7,15]
The exacerbated vascular responsiveness to Ang II challenges and impaired endothelial depended acetylcholine-mediated relaxation are the major contributors for the diminished cardiovascular functions in IR, obesity, and T2D.[16,17] It has been reported that telmisartan attenuated aortic stiffening and vascular remodeling in diabetic condition.[18] Previously, we had demonstrated that esculetin improved vascular endothelial functions in IR and T2D.[16] In the present study, we observed most significant improvement in Ang II-mediated vascular reactivity and endothelial relaxation in case of rats receiving combination regimen.[6] The mechanism behind this might be increased nitric oxide synthase by additive reduction in the oxidative stress and Ang II level by combination treatment.[7,10,19]
The Keap1/Nrf2 pathway, a regulator of the oxidative stress, is an important target for treatment of diabetes and metabolic syndrome.[20] In T2D heart, we found an increase in Keap1 level which depicts imbalanced antioxidative stress and increased free radicals generation.[20] The combination treatment markedly reduced Keap1 level as compared with T2DC and monotherapy and this is might be due to antioxidant potential of both telmisartan and esculetin.[15,21] The pathogenesis of myocardial fibrosis and thereby diabetic cardiomyopathy is strongly associated with increased expression of fibronectin.[22] Furthermore, we observed significantly increased expression of fibronectin in T2D heart. Telmisartan and esculetin combination could attenuate fibronectin expression more significantly than monotherapy, which might be a reason for better cardioprotective effect of the combination regimen in T2D.
Accumulating evidences suggest a potential role of epigenetics in pathogenesis of various diseases.[8,18,23,24] The histone PTMs regulate the translational outcomes of several pathological genes associated with diabetic cardiovascular complications.[8,18,23,24] In T2D patients, expression of histone methyltransferase Set7 and H3K4me1 were found to be augmented which is involved in the regulation of transcription factor nuclear factor kappa B, inflammation, oxidative stress, and endothelial dysfunction.[18] It has been reported that in T2D db/db mice, renal failure increased level of histone H3 PTMs such as H3K9Ac, H3K23Ac, H3K4me2, and H3S10phospho in heart which might be a reason for increased expression of genes related to cardiac hypertrophy and fibrosis (e.g., myosin light chain 3 [Myl3], myosin heavy chain 3, 6, and 7 [Myh3, Myh6, and Myh7], matrix metallopeptidase 1b [Mmp1b], and Tgfb).[25] In nongenetic model of IR and T2D, levels of H3K9/14Ac, H3S10phospho, and H3K4me2 were increased in heart which indicates the chromatin opening state and ease the access of transcriptional machinery which ultimately results into alteration in expression of fibrillin 1 and collagen type III α1 gene expression.[23] Earlier, we have reported increase in permissive histone H3 PTMs, including lysine dimethylation, serine phosphorylation and acetylation and histone H2A/H2B monoubiquitination in diabetic cardiomyopathy.[10] The present study shows that permissive histone modifications such as H3K9Ac and H2BK120Ub and repressive modifications such as H3K9me2 and H2AK119Ub were increased in the T2D heart as compared to NC.
Here, we have observed that esculetin (50 mg/kg/day) attenuated increased level of H3K9Ac, H2AK119Ub, and H2BK120Ub significantly but did not affect the H3K9me2 level in T2D heart. Losartan reversed histone H3 PTMs-H3K9/14Ac and H3K36me3 by inhibiting histone acetyltransferases and H3K36-methyltransferase, respectively, at plasminogen activator inhibitor-1 (Pai1) and receptor for advanced glycation end product (Rage) in T2D nephropathy.[9] However, until now, the effect of telmisartan on epigenetic alterations related with T2D cardiomyopathy is unknown. To the best of our knowledge, this is the first report which shows that telmisartan treatment inhibited histone H3 PTMs (e.g., H3K9me2 and H3K9Ac) and histone H2A/H2B ubiquitination (e.g., H2AK119Ub and H2BK120Ub) in T2D heart. Moreover, the rats receiving combination regimen showed more significant reduction in H3K9me2, H3K9Ac, H2AK119Ub, and H2BK120Ub level in T2D cardiomyopathy.
Conclusions
Results obtained from the current study suggest that the combination of esculetin and telmisartan ameliorated the pathological features of T2D cardiomyopathy by attenuating metabolic perturbations, reducing oxidative stress, improving vascular functions, diminishing fibronectin expression, and alleviating epigenetic alterations. Hence, it can be concluded that the combination of telmisartan and esculetin may serve as a better therapeutic intervention for treatment of T2D cardiomyopathy.
Financial support and sponsorship
Author sincerely acknowledges the financial support obtained from the Science and Engineering Research Board – Department of Science and Technology (SERB-DST), Government of India (SB/EMEQ-053/2013) and University Grant Commission – Major Research Project (UGC-MRP) [F.NO.42-702/2013 (SR)] for this work.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank other members of Laboratory of Molecular Pharmacology (Department of pharmacy, BITS-Pilani, India) Nisha Sharma, Shreyas M Bagal and Priyank Raj for their valuable assistance in Vascular reactivity, Histopahology and immunohistochemisrty experiments.
References
- 1.Barzilay JI, Whelton PK, Davis BR. Does renin angiotensin system blockade deserve preferred status over other anti-hypertensive medications for the treatment of people with diabetes? Ann Transl Med. 2016;4:202. doi: 10.21037/atm.2016.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnier M, Zanchi A. Blockade of the renin-angiotensin-aldosterone system: A key therapeutic strategy to reduce renal and cardiovascular events in patients with diabetes. J Hypertens. 2006;24:11–25. doi: 10.1097/01.hjh.0000191244.91314.9d. [DOI] [PubMed] [Google Scholar]
- 3.Goyal BR, Parmar K, Goyal RK, Mehta AA. Beneficial role of telmisartan on cardiovascular complications associated with STZ-induced type 2 diabetes in rats. Pharmacol Rep. 2011;63:956–66. doi: 10.1016/s1734-1140(11)70611-9. [DOI] [PubMed] [Google Scholar]
- 4.Allah OM, EL-Debakey F. Prophylactic resveratrol and telmisartan combination ameliorates experimentally-induced diabetic nephropathy in rats, focus on the pro-sclerotic cytokine, transforming growth factor-ß1 (TGF-ß1) Med J Cairo Univ. 2010;78:705–13. [Google Scholar]
- 5.Zhu H, Chen X, Cai G, Zheng Y, Liu M, Liu W, et al. Telmisartan combined with probucol effectively reduces urinary protein in patients with type 2 diabetes: A randomized double-blind placebo-controlled multicenter clinical study. J Diabetes. 2016;8:677–85. doi: 10.1111/1753-0407.12347. [DOI] [PubMed] [Google Scholar]
- 6.Kadakol A, Pandey A, Goru SK, Malek V, Gaikwad AB. Insulin sensitizing and cardioprotective effects of Esculetin and Telmisartan combination by attenuating ang II mediated vascular reactivity and cardiac fibrosis. Eur J Pharmacol. 2015c;765:591–7. doi: 10.1016/j.ejphar.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 7.Sim MO, Lee HI, Ham JR, Seo KI, Lee MK. Long-term supplementation of esculetin ameliorates hepatosteatosis and insulin resistance partly by activating AdipoR2 – AMPK pathway in diet-induced obese mice. J Funct Foods. 2015;15:160–71. [Google Scholar]
- 8.Keating ST, El-Osta A. Epigenetic changes in diabetes. Clin Genet. 2013;84:1–0. doi: 10.1111/cge.12121. [DOI] [PubMed] [Google Scholar]
- 9.Reddy MA, Sumanth P, Lanting L, Yuan H, Wang M, Mar D, et al. Losartan reverses permissive epigenetic changes in renal glomeruli of diabetic db/db mice. Kidney Int. 2014;85:362–73. doi: 10.1038/ki.2013.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadakol A, Malek V, Goru SK, Pandey A, Gaikwad AB. Esculetin reverses histone H2A/H2B ubiquitination, H3 dimethylation, acetylation and phosphorylation in preventing type 2 diabetic cardiomyopathy. J Funct Foods. 2015b;17:127–36. [Google Scholar]
- 11.Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–19. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 12.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A, et al. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–60. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muoio DM, Newgard CB. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 14.Michel MC, Foster C, Brunner HR, Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev. 2013;65:809–48. doi: 10.1124/pr.112.007278. [DOI] [PubMed] [Google Scholar]
- 15.Lakshmanan AP, Watanabe K, Thandavarayan RA, Sari FR, Harima M, Giridharan VV, et al. Telmisartan attenuates oxidative stress and renal fibrosis in streptozotocin induced diabetic mice with the alteration of angiotensin-(1-7) mas receptor expression associated with its PPAR-γ agonist action. Free Radic Res. 2011;45:575–84. doi: 10.3109/10715762.2011.560149. [DOI] [PubMed] [Google Scholar]
- 16.Kadakol A, Malek V, Goru SK, Pandey A, Bagal S, Gaikwad AB, et al. Esculetin attenuates alterations in ang II and acetylcholine mediated vascular reactivity associated with hyperinsulinemia and hyperglycemia. Biochem Biophys Res Commun. 2015;461:342–7. doi: 10.1016/j.bbrc.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Salum E, Butlin M, Kals J, Zilmer M, Eha J, Avolio AP, et al. Angiotensin II receptor blocker telmisartan attenuates aortic stiffening and remodelling in STZ-diabetic rats. Diabetol Metab Syndr. 2014;6:57. doi: 10.1186/1758-5996-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paneni F, Costantino S, Battista R, Castello L, Capretti G, Chiandotto S, et al. Adverse epigenetic signatures by histone methyltransferase set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ Cardiovasc Genet. 2015;8:150–8. doi: 10.1161/CIRCGENETICS.114.000671. [DOI] [PubMed] [Google Scholar]
- 19.Ramalingam L, Menikdiwela K, LeMieux M, Dufour JM, Kaur G, Kalupahana N, et al. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta. 2017;1863:1106–14. doi: 10.1016/j.bbadis.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Chartoumpekis DV, Kensler TW. New player on an old field; the keap1/Nrf2 pathway as a target for treatment of type 2 diabetes and metabolic syndrome. Curr Diabetes Rev. 2013;9:137–45. doi: 10.2174/1573399811309020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prabakaran D, Ashokkumar N. Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie. 2013;95:366–73. doi: 10.1016/j.biochi.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Rajesh M, Bátkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horváth B, et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61:716–27. doi: 10.2337/db11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaikwad AB, Gupta J, Tikoo K. Epigenetic changes and alteration of Fbn1 and Col3A1 gene expression under hyperglycaemic and hyperinsulinaemic conditions. Biochem J. 2010;432:333–41. doi: 10.1042/BJ20100414. [DOI] [PubMed] [Google Scholar]
- 24.Hanson MA, Godfrey KM. Genetics: Epigenetic mechanisms underlying type 2 diabetes mellitus. Nat Rev Endocrinol. 2015;11:261–2. doi: 10.1038/nrendo.2015.31. [DOI] [PubMed] [Google Scholar]
- 25.Gaikwad AB, Sayyed SG, Lichtnekert J, Tikoo K, Anders HJ. Renal failure increases cardiac histone h3 acetylation, dimethylation, and phosphorylation and the induction of cardiomyopathy-related genes in type 2 diabetes. Am J Pathol. 2010;176:1079–83. doi: 10.2353/ajpath.2010.090528. [DOI] [PMC free article] [PubMed] [Google Scholar]


