Abstract
OBJECTIVE:
Osteoarthritis (OA) is a chronic progressive degenerative disease of weight-bearing joints and the leading cause of disability in elderly. Current medical management of OA is mostly palliative with nonsteroidal anti-inflammatory drugs (NSAIDs) being the mainstay of therapy. Reports of gastrointestinal adverse effects with traditional NSAIDs and cardiovascular adverse effects associated with selective cyclooxygenase-2 (COX-2) inhibitors have prompted the hunt for a better NSAID with no or minimal adverse effects. This study compares the clinical effectiveness and safety of newer NSAIDS etodolac and lornoxicam to diclofenac which has been a standard therapy in patients of OA of knee joint.
MATERIALS AND METHODS:
It was a randomized, prospective, open-label, parallel-group study conducted in 90 patients of OA of knee joint diagnosed according to the American College of Rheumatology criteria. After obtaining the informed consent, they were randomized in three groups of 30 patients each who received tablet etodolac 400 mg b.i.d, tablet lornoxicam 8 mg b.i.d, and tablet diclofenac sodium 50 mg t.i.d, respectively. The duration of the study was 12 weeks. Data were tabulated and analyzed using analysis of variance (ANOVA) test, and level of significance was determined by its P value.
RESULTS:
After 12 weeks of treatment, pain intensity and functional indices in terms of visual analog scale and Western Ontario and McMaster Universities Osteoarthritis score were significantly better (P < 0.05) in lornoxicam group as compared to etodolac or diclofenac group along with lesser rate of adverse effects.
CONCLUSION:
It was concluded that lornoxicam was more effective and better tolerated NSAID than etodolac and diclofenac in treatment of knee joint OA.
Keywords: Diclofenac, etodolac, lornoxicam, osteoarthritis knee, visual analog scale, Western Ontario and McMaster Universities Osteoarthritis Score
Introduction
Osteoarthritis (OA) is a chronic progressive disease of the weight-bearing joints characterized by degeneration of articular cartilage, subchondral sclerosis, osteophyte, and cyst formation. These pathological changes result clinically in joint pain, stiffness, crepitus, swelling, limited movement leading to significant disability, loss of productivity, and impaired quality of life.[1,2]
Treatment for OA aims at reducing pain, maintaining mobility, and minimization of disability. Current medical management of OA is mostly palliative with nonsteroidal anti-inflammatory drugs (NSAIDs) being the mainstay of therapy.[3] Reports of gastrointestinal (GI) adverse effects with traditional NSAIDs and cardiovascular adverse effects associated with selective cyclooxygenase-2 (COX-2) inhibitors have prompted the quest for a better-tolerated NSAID.
Results from the published studies suggest that lornoxicam is a potent NSAID with balanced cycloxygenase-1/-2 inhibion which offers faster and better pain relief as compared to diclofenac with improved GI tolerability.[4,5,6] In vitro studies of etodolac have demonstrated that there was no alteration in cartilage repair response as the collagen phenotype was preserved and proteoglycan and DNA synthesis was not affected in human chondrocytes grown in a culture in the presence of etodolac as compared to other NSAIDs.[7,8] Hence, the present study was planned to compare the clinical effectiveness and safety of etodolac and lornoxicam to diclofenac in patients of OA of knee joint.
Materials and Methods
This was a comparative, randomized, prospective, open-label, parallel-group study in patients of knee joint OA diagnosed according to the American College of Rheumatology (ACR) criteria. The study protocol was approved by the institutional thesis committee and the institutional ethics committee before the study was initiated. Informed consent was obtained from all patients included in the study after being informed about the nature of the study. This study was conducted in accordance with the Principles of Good Clinical Practice and Declaration of Helsinki.
The investigational drugs for this study were:
Tablet etodolac 400 mg b.i.d
Tablet lornoxicam 8 mg b.i.d
Tablet diclofenac sodium 50 mg t.i.d.
A total of 90 patients of OA of knee joint visiting the OPD/wards of Department of Orthopedics were recruited for the present study after the inclusion criteria were fulfilled.
Inclusion criteria
Patients who were found fit in baseline examination were included in the present study as per the criteria given below:
Patients who had symptoms of OA for >3 months, whose diagnosis of knee OA had been verified by the clinical examination and X-rays of knee joint according to the ACR criteria
Patients of OA of knee joint who were already on different NSAIDs or other analgesic medications were included after a washout period of 2 weeks.
Exclusion criteria
Age <50 years or >75 years
Patients having hepatic or renal impairment or concomitant active gastroduodenal ulcers, within last 30 days before receiving the drug under study
Patients with known hypersensitivity or contraindication to NSAIDs
Concomitant therapy with warfarin or heparin or high-dose aspirin (>1000 mg/day).
Women either pregnant or lactating or on oral contraceptive pills
Patients with established cardiovascular disorder or uncontrolled hypertension or ischemic heart disease or patients who had undergone coronary artery bypass graft or angioplasty
Recent history of stroke, myocardial infarction, or transient ischemic attacks within previous 2 years
Patients diagnosed with any other arthritis, gout, or sustained acute trauma to knee, hip, or spine within 3 months
History of arthroscopy of affected knee within 6 months prior to entry in the study
History of acute meniscal injury or ligamentous injury to study joint within previous 2 years
Advanced renal insufficiency (creatinine clearance <30 ml/min) or severe hepatic insufficiency (Child-Pugh score >9) or increased aspartate transaminase or alanine aminotransferase >3 times of their normal values
Patients with severe knee deformity.
Consenting patients fulfilling the eligibility criteria, previously diagnosed with OA and currently taking NSAIDs were given a washout period of 2 weeks. After enrollment, patients were assessed and randomly allocated to receive per oral (p.o.) either tablet etodolac 400 mg b.i.d in group A, or tablet lornoxicam 8 mg b.i.d in group B, or tablet diclofenac sodium 50 mg t.i.d in group C. Drug treatment history, past, personal, and family history, any concomitant illness, vital signs were recorded. Patients were reassessed at 3, 6, and 12 weeks after starting treatment according to the Visual Analog Scale (VAS), Western Ontario and McMaster Universities Osteoarthritis index (WOMAC), X-rays, and physician's and patient's global assessment. The total period of treatment was 12 weeks.
The primary endpoint was clinical improvement in pain, disability, and range of movement. The secondary endpoint was patient's and physician's assessment, VAS and WOMAC composite scores. Adverse events and concomitant use of drugs if any were recorded throughout the study. For each adverse event, the severity, outcome, and causal relationship to treatment were recorded. All the data generated from the study were tabulated and expressed as mean ± standard deviation, and then analyzed statistically using analysis of variance (ANOVA) test. The Statistical analysis of the data was performed using the SPSS statistical package (version 17.0; SPSS Inc., Chicago, IL, USA). The level of significance was determined by its P value. P < 0.05 was considered to be statistically significant.
Results
Out of total 125 patients screened, 110 were found eligible to be included in the study and were randomized to either of Group A, B, or C receiving p.o. etodolac 400 mg b.i.d, lornoxicam 8 mg b.i.d, or diclofenac 50 mg t.i.d, respectively. A total of 30 patients in each group completed the treatment period of 12 weeks to form the per-protocol population.
The reduction in mean VAS scores in etodolac group was by 15.66 (21.95%) at week 3, 25.66 (35.97%) at week 6, and 39 (54.67%) at week 12. In lornoxicam group, the reduction in mean VAS scores was by 22.33 (31.97%) at week 3, 37.50 (53.70%) at week 6, and 53.33 (76.37%) at week 12. In diclofenac group, the reduction in mean VAS scores was by 15.33 (20.76%) at week 3, 25.50 (34.53%) at week 6, and 32.66 (44.23%) at week 12. At the end of study period of 12 weeks, there was a highly significant (P <0.001) decrease in VAS scores in all the three groups [Figure 1].
Figure 1.
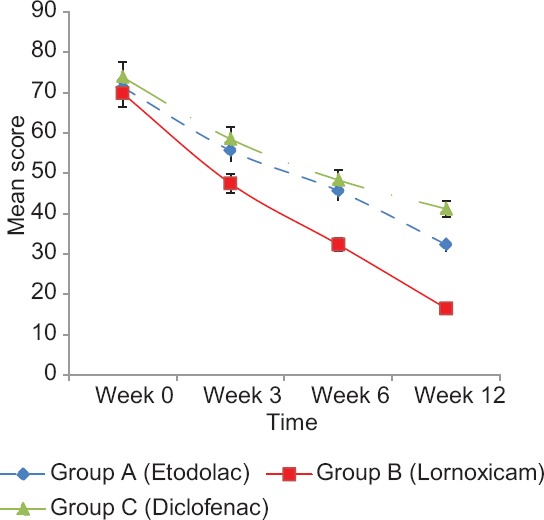
Mean VAS scores at different time intervals in three groups
The reduction in mean WOMAC score (total) in etodolac group was by 15.06 (34.20%) at week 3, 23.73 (53.89%) at week 6, and 30.03 (68.20%) at week 12. In lornoxicam group, the reduction in mean WOMAC score (total) was by 12.93 (31.15%) at week 3, 23 (55.42%) at week 6, and 29.57 (71.20%) at week 12. In diclofenac group, the reduction in mean WOMAC score (total) was by 10.93 (24.89%) at week 3, 21.13 (48.13%) at week 6, and 24.70 (56.26%) at week 12 [Figure 2].
Figure 2.
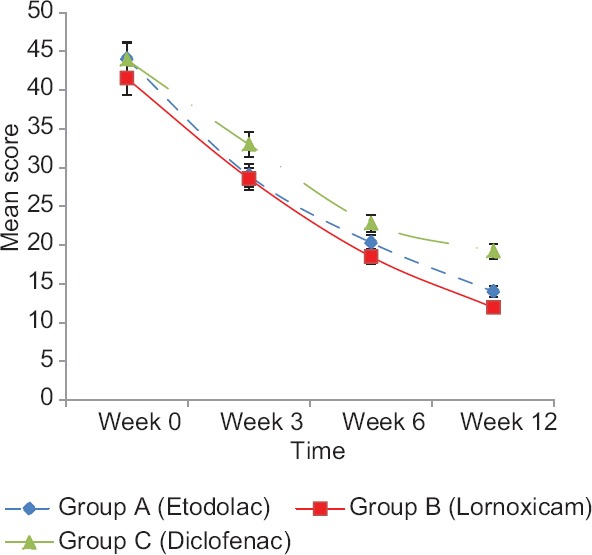
Mean Western Ontario and McMaster Universities Osteoarthritis score (total) at different time intervals in three groups
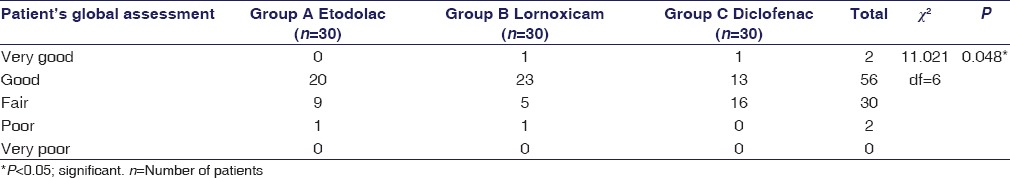
In patient's global assessment at 12 weeks, 1 (3.3%) patient each in lornoxicam group and diclofenac group assessed their condition as “very good,” whereas 20 (66.66%) patients in etodolac group, 23 (76.66%) patients in lornoxicam group, and 13 (43.33%) patients in diclofenac group assessed their condition as “good”. Nine (30.0%) patients in etodolac group, 5 (16.66%) patients in lornoxicam group, and 16 (53.33%) patients in diclofenac group assessed their condition as “fair,” whereas 1 (3.3%) patient each in etodolac and lornoxicam group assessed it as “poor” but none as “very poor” with a significant difference between the groups (P < 0.05) [Table 1].
Table 1.
Patient's global assessment at 12 weeks
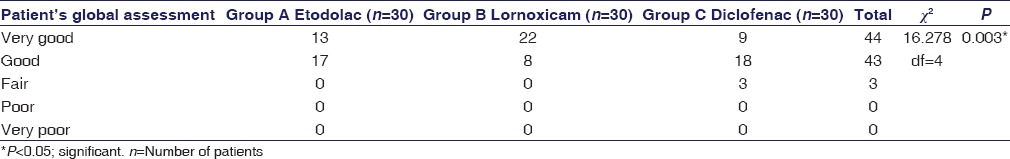
In physician's global assessment at 12 weeks, physician assessed 22 (73.3%) patients in lornoxicam group as “very good” as compared to 13 (43.3%) patients in etodolac group and 9 (30%) patients in diclofenac group. Also, 17 (56.6%) patients in etodolac group, 8 (26.6%) patients in lornoxicam group, and 18 (60%) patients in diclofenac group were assessed as “good” with statistically significant difference between the groups (P < 0.05) [Table 2].
Table 2.
Physician's global assessment at 12 weeks
All the study drugs were well-tolerated with no serious adverse events requiring hospitalization. Adverse events usually of GI tract (GIT) are often a limiting factor for NSAID use, comprising of mild events such as dyspepsia and nausea. At 3 weeks of treatment, 25 (83.3%) patients in diclofenac group, whereas 11 (36.6%) patients in etodolac group and 4 (13.3%) patients in lornoxicam group reported pain abdomen. Dyspepsia was reported by 28 (93.33%) in diclofenac group and 19 (63.33%) patients each in etodolac group and lornoxicam group. Flatulence was complained by 26 (86.66%) patients in diclofenac group as compared to 17 (56.66%) patients in etodolac group and 11 (36.66%) patients in lornoxicam group. Nausea was reported by 21 (70%) patients in diclofenac group but only 9 (30%) patients in etodolac group and 10 (33.33%) patients in diclofenac group [Figure 3].
Figure 3.
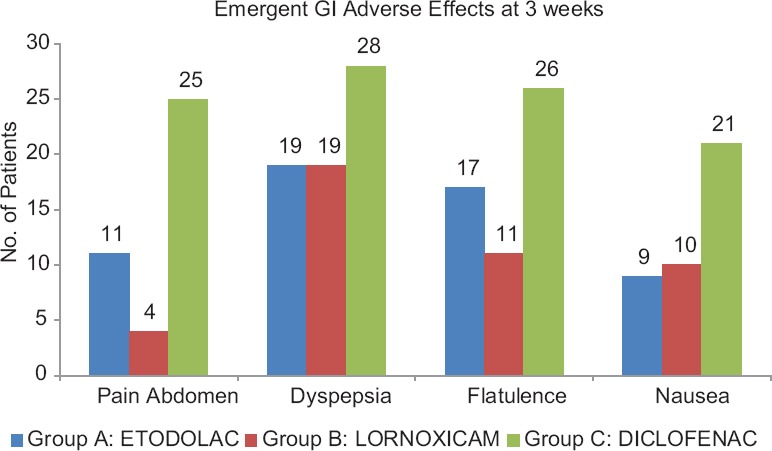
Emergent adverse effects (at 3 weeks) in the three groups
Discussion
Thirty patients in each group completed the treatment period of 12 weeks to form the per-protocol population in Group A, B, and C receiving p.o. etodolac 400 mg b.i.d, lornoxicam 8 mg b.i.d, or diclofenac 50 mg t.i.d, respectively.
The improvement in symptoms and functional status of each patient with these drugs was assessed at 3, 6, and 12 weeks of treatment for different parameters such as respective mean VAS and WOMAC scores and the percentage change in mean scores. Baseline score of VAS and WOMAC was comparable in the three groups with no significant difference (P > 0.05). Over the 12-week study period, all the three drugs provided significant (P < 0.05) sustained relief in symptoms of OA as compared with baseline. The number of patients showing improvement in different parameters was significant in all the three groups (P < 0.05).
After 12 weeks of treatment, the reduction in mean VAS score of lornoxicam group (76.37%) was significantly more than etodolac (54.67%) and diclofenac group (44.23%). At the end of the study, there was a significant (P < 0.05) reduction in overall pain in each of the groups individually. On comparing the three groups together, the reduction in mean VAS score was highly significant (P < 0.001) between diclofenac and lornoxicam group but significant (P < 0.05) between diclofenac and etodolac group [Figure 1]. The results of our study are comparable to those obtained in a randomized, controlled clinical trial comparing efficacy, safety, and cost-effectiveness of lornoxicam with diclofenac sodium in patients of OA of knee joint which reported 80.89% reduction in pain in lornoxicam group as compared to 45.45% reduction in diclofenac group at 3 months (P < 0.001). Furthermore, the mean pain score of lornoxicam group was less than the diclofenac group after 2 and 3 months of treatment.[6] Similar results were also obtained in another 12-week study in 135 patients of arthritis where those treated with lornoxicam 4 mg t.i.d. and 8 mg b.i.d. showed same clinical efficacy (P < 0.05) as diclofenac 50 mg t.i.d and the clinical effects remained stable, with a further pain reduction of up to 23% in the course of a subsequent 40-week follow-up treatment in lornoxicam group.[9]
In our study, mean WOMAC Score (TOTAL) after 12 weeks of treatment in lornoxicam group (11.93 ± 7.97) was significantly less than etodolac (14.00 ± 5.90) and diclofenac group (19.20 ± 7.73) [Figure 2]. The mean reduction in WOMAC scores (total) as compared to baseline was 30.03 (68.20%) in etodolac group, 29.57 (71.20%) in lornoxicam group, and 24.70 (56.26%) in diclofenac group. On comparison of three groups, the decrease in WOMAC scores (total) were significant between group C and A, group C and B but not between Group A and B [Figure 2]. The reduction in WOMAC scores (total) of our study are in consonance with the results of another study conducted to compare clinical effectiveness and tolerability of oral lornoxicam 8 mg b.i.d. and diclofenac 50 mg t.i.d. in adult Indian patients with OA knee or hip joint.[5] The results of etodolac-treated group in our study show conformity with the results of three double-blind clinical trials of six to 12 weeks duration comparing etodolac (200 mg t.i.d or 300 mg b.i.d.) with piroxicam (20 mg u.i.d.), diclofenac (50 mg t.i.d.), or naproxen (500 mg b.i.d.) in patients with OA of knee with evaluation as compared to baseline every 2 weeks in terms of night pain, pain intensity, WOMAC score, and patient's and physician's assessment. Response rates were etodolac (66%) versus diclofenac (56%) suggesting that the efficacy of etodolac compares favorably with that of other NSAIDs in the treatment of knee OA.[10] Similar results were obtained in another 6 weeks double-blind, parallel trial, in which etodolac (300 mg b.i.d.)-treated patients reported significantly greater (P ≤ 0.05) improvement from baseline than for indomethacin.[11]
On analysing the data at 12 weeks, significant difference (P < 0.05) for scores of VAS and WOMAC was observed in both etodolac group and lornoxicam group as compared to diclofenac group with maximum reduction VAS and WOMAC scores in lornoxicam group. Similar results were obtained in another study conducted to compare effectiveness and tolerability of oral lornoxicam 8 mg b.i.d. and diclofenac 50 mg t.i.d. in Indian patients of OA of the knee or hip joint.[5]
Patient's global assessment was similar (P > 0.05) in the three treatment groups at baseline evaluation which improved progressively over the 12 week study period. At 12 weeks, assessments were in favor of lornoxicam group with 23 (76.6%) patients assessing their condition as “good” as compared to 20 (66.6%) in etodolac group and 13 (43.33%) patients in diclofenac group. However, 9 (30%) patients in etodolac group, 5 (16.6%) patients in lornoxicam group, and 16 (53.3%) patients in diclofenac group assessed their condition as “fair” and 1 (3.3%) patient each in etodolac and lornoxicam group as “poor,” whereas none as “very poor” [Table 1]. Pair-wise comparisons revealed significantly more improvement from baseline scores (P < 0.05) for lornoxicam and etodolac group than in diclofenac group with significant intergroup difference (P < 0.05).
At baseline evaluation, the physician's global assessment was similar (P > 0.05) in the three treatment groups which improved progressively over 12 weeks. Most of the patients had “poor” or “fair” condition at baseline with no significant intergroup difference (P > 0.05). At 12 weeks, physician's assessment was in favor of lornoxicam group with 22 (73.3%) assessed as “very good” as compared to 13 (43.3%) patients in etodolac group and 9 (30%) patients in diclofenac group. A total of 17 (56.6%) patients in etodolac group, 8 (26.6%) patients in lornoxicam group, and 18 (60%) patients in diclofenac group were assessed as “good” with statistically significant difference between the groups (P < 0.05) [Table 2].
Significant improvement from baseline was reported in all patients of the three groups (P < 0.05). At the end of study period, physician's and patient's assessment were in favor of lornoxicam-treated group as compared to etodolac- and diclofenac-treated group with significant difference between the three groups (P < 0.05). The results of our study are in accordance with a previous 12-week multicenter, randomized, parallel group study done to compare the efficacy and tolerability of lornoxicam and diclofenac in treatment of patients with OA of hip and/or knee with significant intergroup equivalence (P < 0.033) in terms of improvements in disease activity (about 46%), pain intensity (42%–48%), patient's and physician's global assessments.[9] Similar results were seen in other double-blind studies where lornoxicam was compared to other NSAIDs such as diclofenac, piroxicam, and indomethacin in patients with knee OA in terms of physician's and patient's overall assessments.[11,12] Furthermore, the results in etodolac group were in conformity with a previous study, in which patient's and physician's global evaluation had improved by at least 64% of all etodolac-treated patients and 62% of all active-reference preparation-treated patients by the end of the study.[13]
In our study, all the study drugs were well-tolerated with no serious adverse events requiring hospitalization. Adverse events of the GIT are often a limiting factor for NSAID use such as mild dyspepsia and nausea which are eventually associated with reduced tolerability and noncompliance to the treatment protocol.[14] Better tolerability is likely to result in high compliance which was 93% in our study as confirmed by tablet counts and empty blister packs. There were no statistically significant intergroup differences in adverse experiences at baseline evaluation. The most common adverse effects reported includes mild-to-moderate degree GIT disturbances usually manifested as pain abdomen, dyspepsia, flatulence, nausea with mild headache, or insomnia which were significantly (P < 0.05) less in lornoxicam and etodolac group than in diclofenac group [Figure 3]. None of the patients reported cardiovascular adverse drug reactions (e.g., increased blood pressure or edema) or any hepatic function abnormality. A total of 11 patients discontinued the treatment because of adverse experiences out of which 4 (10.81%) occurred in etodolac-treated patients, 2 (5.4%) in lornoxicam-treated patients, and 5 (13.5%) in diclofenac-treated patients.
In our study, lornoxicam proved to be effective alternative in the treatment of OA, as illustrated by significantly better improvement in pain and functional indices of VAS and WOMAC, limited use of rescue medication, and satisfactory patient's and physician's global assessment. Physician's assessment differed slightly from patient's assessments, although both were in favor of lornoxicam over etodolac and diclofenac, along with lesser rate of adverse events. The rapid and long-lasting relief from OA symptoms with lornoxicam 16 mg/day suggests that it has good potential to reduce the disability due to the disease and improve the productivity.
Better improvement in symptomatology in lornoxicam group could be due to its potential to inhibit migration and release of superoxide from polymorphonuclear (PMN)-leukocytes, release of platelet derived growth factor (PDGF) from human platelets and stimulate the synthesis of proteoglycans in cartilage in tissue culture in addition to cyclo-oxygenase inhibition. Furthermore, it has been demonstrated in animal studies that lornoxicam prevents the bone destruction which occurs in OA in the adjuvant polyarthritic rat thereby accounting for some disease-modifying property of lornoxicam.[15] Lesser rate of adverse effects is due to the reason that unlike other NSAIDs, inhibition of cyclooxygenase by lornoxicam does not lead to an increase in leukotriene formation, as arachidonic acid is not moved to the 5-lipoxygenase cascade, in addition to its short plasma elimination half-life of 4 h further contributing to the minimization of the risk of adverse events.[15,16,17,18]
Etodolac has also compared favorably with lornoxicam over diclofenac. The dose of etodolac in our study was an important distinction from the previous studies. The daily dose of etodolac investigated in European or South American studies was 600 mg.[19] Consistent with prescribing trends in the United States, a higher dose of 800 mg/day was administered in our study.[20] The results of our study show that etodolac is also an effective, well-tolerated alternative to traditional NSAIDs in the treatment of OA of the knee joint.
Conclusion
The present work has concluded that etodolac, lornoxicam, and diclofenac are useful drugs in the treatment of OA, but lornoxicam led to significantly better improvements in pain and functional indices of WOMAC as well as overall patient and physician assessment compared to etodolac as well as diclofenac along with lesser rate of adverse events.
As pain is often the limiting factor in degenerate forms of arthritis, the main focus of treatment of OA is on provision of pain relief by NSAIDs. The outcome of our study that patients report significantly better pain relief during lornoxicam treatment is particularly important in the future trends of management of OA. The limitation of our study is it's open-label design. Hence, the results of present study need to be confirmed in larger double-blind, multicentric trials of longer duration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;350:503–8. doi: 10.1016/S0140-6736(97)07226-7. [DOI] [PubMed] [Google Scholar]
- 2.Brandt KD. Osteoarthritis. Clin Geriatr Med. 1988;4:279–93. [PubMed] [Google Scholar]
- 3.Fawaz-Estrup F. The osteoarthritis initiative: An overview. Med Health R I. 2004;87:169–71. [PubMed] [Google Scholar]
- 4.Yakhno N, Guekht A, Skoromets A, Spirin N, Strachunskaya E, Ternavsky A, et al. Analgesic efficacy and safety of lornoxicam quick-release formulation compared with diclofenac potassium: Randomised, double-blind trial in acute low back pain. Clin Drug Investig. 2006;26:267–77. doi: 10.2165/00044011-200626050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Goregaonkar A, Mathiazhagan KJ, Shah RR, Kapoor PS, Taneja P, Sharma A, et al. Comparative assessment of the effectiveness and tolerability of lornoxicam 8 mg BID and diclofenac 50 mg TID in adult indian patients with osteoarthritis of the hip or knee: A 4-week, double-blind, randomized, comparative, multicenter study. Curr Ther Res Clin Exp. 2009;70:56–68. doi: 10.1016/j.curtheres.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vadgama VK, Gharia R, Mehta K, Tripathi CB. A randomized controlled clinical trial comparing efficacy safety and cost effectiveness of lornoxicam with diclofenac sodium in patients of osteoarthritis knee. Internet J Med Update. 2011;6:25–9. [Google Scholar]
- 7.Goldring MB, Sohbat E, Elwell JM, Chang JY. Etodolac preserves cartilage-specific phenotype in human chondrocytes: Effects on type II collagen synthesis and associated mRNA levels. Eur J Rheumatol Inflamm. 1990;10:10–21. [PubMed] [Google Scholar]
- 8.Henrotin Y, Bassleer C, Reginster JY, Franchimont P. Effects of etodolac on human chondrocytes cultivated in three dimensional culture. Clin Rheumatol. 1989;8(Suppl 1):36–42. doi: 10.1007/BF02214108. [DOI] [PubMed] [Google Scholar]
- 9.Kidd B, Frenzel W. A multicenter, randomized, double blind study comparing lornoxicam with diclofenac in osteoarthritis. J Rheumatol. 1996;23:1605–11. [PubMed] [Google Scholar]
- 10.Platt PN. Recent clinical experience with etodolac in the treatment of osteoarthritis of the knee. Clin Rheumatol. 1989;8(Suppl 1):54–62. doi: 10.1007/BF02214110. [DOI] [PubMed] [Google Scholar]
- 11.Karbowski A. Double-blind, parallel comparison of etodolac and indomethacin in patients with osteoarthritis of the knee. Curr Med Res Opin. 1991;12:309–17. doi: 10.1185/03007999109112666. [DOI] [PubMed] [Google Scholar]
- 12.Freitas GG. A double-blind comparison of etodolac and piroxicam in the treatment of osteoarthritis. Curr Med Res Opin. 1990;12:255–62. doi: 10.1185/03007999009111655. [DOI] [PubMed] [Google Scholar]
- 13.Bacon P. Worldwide experience with etodolac (Lodine) 300 mg b.i.d. In the treatment of osteoarthritis. Rheumatol Int. 1993;13:S7, 12. doi: 10.1007/BF00290278. [DOI] [PubMed] [Google Scholar]
- 14.Singh G, Triadafilopoulos G. Epidemiology of NSAID induced gastrointestinal complications. J Rheumatol Suppl. 1999;56:18–24. [PubMed] [Google Scholar]
- 15.Pruss TP, Stroissnig H, Radhofer-Welte S, Wendtlandt W, Mehdi N, Takacs F, et al. Overview of the pharmacological properties, pharmacokinetics and animal safety assessment of lornoxicam. Postgrad Med J. 1990;66(Suppl 4):S18–21. [PubMed] [Google Scholar]
- 16.Radhofer-Welte S, Rabasseda X. Lornoxicam, a new potent NSAID with an improved tolerability profile. Drugs Today (Barc) 2000;36:55–76. doi: 10.1358/dot.2000.36.1.566627. [DOI] [PubMed] [Google Scholar]
- 17.Adams SS. Non-steroidal anti-inflammatory drugs, plasma half-lives, and adverse reactions. Lancet. 1987;2:1204–5. doi: 10.1016/s0140-6736(87)91335-3. [DOI] [PubMed] [Google Scholar]
- 18.Skjodt NM, Davies NM. Clinical pharmacokinetics of lornoxicam. A short half-life oxicam. Clin Pharmacokinet. 1998;34:421–8. doi: 10.2165/00003088-199834060-00001. [DOI] [PubMed] [Google Scholar]
- 19.Chikanza IC, Clarke B, Hopkins R, MacFarlane DG, Bird H, Grahame R, et al. Acomparative study of the efficacy and toxicity of etodolac and naproxen in the treatment of osteoarthritis. Br J Clin Pract. 1994;48:67–9. [PubMed] [Google Scholar]
- 20.Palmoski MJ, Brandt KD. In vivo effect of aspirin on canine osteoarthritic cartilage. Arthritis Rheum. 1983;26:994–1001. doi: 10.1002/art.1780260808. [DOI] [PubMed] [Google Scholar]


