Abstract
Valproate-related pedal edema is usually regarded as a problem occurring after long-term administration of valproate. Valproate has been a drug of choice for the treatment of generalized or partial seizures as monotherapy or adjunctive therapy, bipolar disorder, for the prophylaxis of migraine headache in adults. This case report described patient-acquiring bilateral pedal edema after long-term use of magnesium valproate. Discontinuing valproate resulted in rapid improvement of the condition. This adverse reaction to the best of our knowledge is first reported a case of bilateral pedal edema cause by magnesium valproate in low dose. The dose of magnesium valproate was 1200 mg/day. No previous case as reported with the same dose.
Keywords: Adverse reaction, bilateral pedal edema, magnesium valproate
Introduction
Valproate is a drug of choice for treatment of epilepsy, bipolar disorder, and for the prophylaxis of migraine headache in adults. Despite the fact that lithium is more efficacious in the maintenance of bipolar disorder, but its use is abated by its adverse effects.[1] Common adverse drug reactions (ADRs) of valproate are gastrointestinal symptoms, sedation, ataxia, tremor, rash, alopecia, appetite stimulation, and rare ones are fulminant hepatitis, pancreatitis, hyperammonemia, and pedal edema. Severe peripheral edema may occur as a result of volume overload, hypoalbuminemia, lymphatic or venous obstruction, congestive heart failure, cirrhosis, and nephrotic syndrome.[2] Avid salt retention by renal tubules triggered by medication can also lead to severe volume overload and peripheral edema. Medications known to cause salt retention and edema are estrogens by hormonal mediation of salt retention and calcium channel blocker's arterial vasodilators such as minoxidil due to renal tubular reaction to altered renal or glomerular hemodynamics. Isolated hormonal activation of tubular sodium retention does not occur as can be observed in patients with primary hyperaldosteronism who do not develop significant edema.[3] In these cases, the mechanism of spontaneous resolution of the edema can occur through aldosterone escape phenomena, secretion of atrial natriuretic peptide, and increased glomerular filtration rate. In addition to all these, a delayed pedal edema on prolonged use of valproate is reported, which cannot be explained by any of these mechanisms.
Case Report
The patient was a 68-year-old female, diagnosed to have bipolar disorder in the year 2002 by a psychiatrist. Psychiatrist advised patient to take the tablet MACORATE® CR 400 mg tds lifelong and was advised to get yearly follow-up.
Patient was being looked after by a primary care physician with consultations of psychiatrist available if required. She was never diagnosed to have any other chronic illness since then. She had a history of annual blood tests being conducted which were reported to be normal. Her bipolar disorder symptoms were reported to be well under control over all these years of treatment. She was taking regular treatment religiously since then with occasional defaults mainly due to supply problems. The advantage of magnesium salt over sodium salt of valproate is not to cause sodium and water retention kept the patient motivated to carry on with the “MACORATE® CR 400.”
Patient reported to have bilateral pedal edema on April 4, 2016, which was steadily increasing [Figure 1]. On examination, the patient had pitting edema over both feet and legs. On blood examination, she had thrombocytopenia, but there were no clinical events suggestive of bleeding disorder. On history, she had a complaint of easy bruising since childhood. There was no evidence of chronic heart failure, hepatic disease, or renal disease clinically, and liver function test/kidney function test were found to be normal to rule out the possibility of having them. Patient discontinued the medicine on April 10, 2016, due to nonavailability of the drug. Patient planned to go for a specialist consultation, but before she could do so, she observed that the pedal edema was decreasing steadily. Thereafter, pedal edema was assessed regularly and was not assessable clinically June 26, 2016. On literature research, the rare ADR of valproate of delayed onset pedal edema was found. The patient and family decided to withhold drug treatment for bipolar disease. We tried to relate the drug exposure with the development of pedal edema using the WHO-Uppsala Monitoring Centre (UMC) causality assessment scale. Causality was “probable” according to WHO-UMC scale. This case was reported to the ADR monitoring center under Pharmacovigilance Programme of India.
Figure 1.
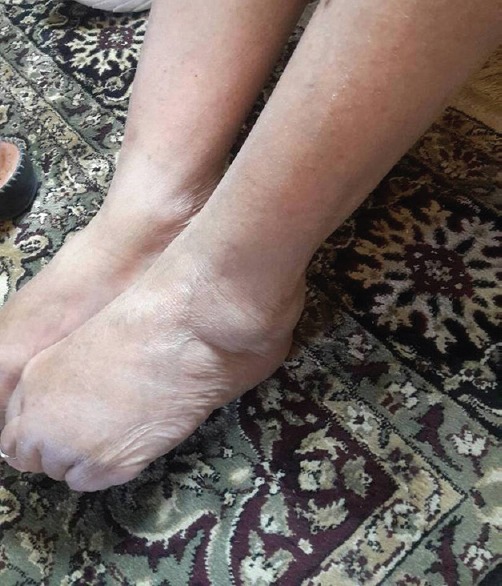
This was the image of the patient who experienced pedal edema after chronic therapy of magnesium valproate
Discussion
Prescribing magnesium and not sodium salt of valproate to avoid salt and water retention is not always successful. A delayed onset reversible pedal edema has also been reported earlier with valproate as a rare ADR, but it was associated with high-dose valproate ≥1500 mg/day which in this case dose was 1200 mg/day.[4] It was unclear whether magnesium valproate played a role in the edema. In several studies, edema has been in the context of valproate-induced hepatic injury.[5] We do not find obvious hepatic disease in our patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kessing LV, Hellmund G, Geddes JR, Goodwin GM, Andersen PK, Valproate V. Lithium in the treatment of bipolar disorder in clinical practice: Observational nationwide register-based cohort study. Br J Psychiatry. 2011;199:57–63. doi: 10.1192/bjp.bp.110.084822. [DOI] [PubMed] [Google Scholar]
- 2.Cho S, Atwood JE. Peripheral edema. Am J Med. 2002;113:580–6. doi: 10.1016/s0002-9343(02)01322-0. [DOI] [PubMed] [Google Scholar]
- 3.Keeley V. Drugs and Lymphedema: Those Which May Cause Edema or Make Lymphedema Worse. Vol. 24. National Lymphedema Network. 2012 [Google Scholar]
- 4.Ettinger A, Moshe S, Shinnar S. Edema associated with long-term valproate therapy. Epilepsia. 1990;31:211–3. doi: 10.1111/j.1528-1167.1990.tb06308.x. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman HJ, Ishak KG. Valproate-induced hepatic injury: Analyses of 23 fatal cases. Hepatology. 1982;2:591–7. doi: 10.1002/hep.1840020513. [DOI] [PubMed] [Google Scholar]


