Summary
Overactive p53 has been proposed as an important pathophysiological factor for bone marrow failure syndromes, including Fanconi anemia (FA). Here, we report a p53-dependent effect on hematopoietic stem and progenitor cell (HSPC) proliferation in mice deficient for the FA gene Fanca. Deletion of p53 in Fanca−/− mice leads to replicative exhaustion of the hematopoietic stem cell (HSC) in transplant recipients. Using Fanca−/− HSCs expressing the separation-of-function mutant p53515C transgene, which selectively impairs the p53 function in apoptosis but keeps its cell-cycle checkpoint activities intact, we show that the p53 cell-cycle function is specifically required for the regulation of Fanca−/− HSC proliferation. Our results demonstrate that p53 plays a compensatory role in preventing FA HSCs from replicative exhaustion and suggest a cautious approach to manipulating p53 signaling as a therapeutic utility in FA.
Keywords: p53, bone marrow failure, Fanconi anemia, hematopoietic stem and progenitor cells, apoptosis, cell cycle, proliferation
Highlights
-
•
Loss of p53 in Fanca−/− mice leads to progressive decline of HSC reservoir
-
•
p53 deficiency leads to proliferative exhaustion of Fanca−/− HSCs
-
•
The cell-cycle function of p53 is required for preventing Fanca−/− HSC exhaustion
In this article, Pang and colleagues demonstrate a p53-dependent HSPC proliferation regulation in mice deficient for the Fanca gene in the Fanconi anemia (FA) pathway. They show that the p53 cell-cycle function is specifically required for the regulation of FA HSC proliferation. These results suggest that overactive p53 may represent a compensatory checkpoint mechanism for FA HSC proliferation.
Introduction
The tumor suppressor p53 is a key component of the DNA damage-response network that activates vital damage-control procedures to restrict aberrant cell growth in response to DNA damage, oncogene activation, and loss of normal cell contacts, by maintaining the balance between cell survival and apoptosis (Levine and Oren, 2009, Murray-Zmijewski et al., 2008). Although p53 mutations are common in solid tumors, such mutations are found at a lower frequency in hematologic malignancies (Krug et al., 2002, Prokocimer and Rotter, 1994). In addition, these mutations may abolish some, but not necessarily all, of the functions of the p53 protein (Abbas et al., 2011, Krug et al., 2002, Pant et al., 2012, Prokocimer and Rotter, 1994). Recently, several studies using mouse models suggest a critical role for p53 in hematopoietic stem cell (HSC) self-renewal and quiescence (Liu et al., 2009, TeKippe et al., 2003). Therefore, precise regulation of p53 activity is likely to be important in determining the response of HSCs to cellular stresses. Insufficient p53 activation would favor cell survival, but puts cells at risk for loss of genomic integrity. In contrast, excessive p53 activation could compromise steady-state hematopoiesis and its recovery following exogenous marrow insult by causing too many cells to be eliminated (Wang et al., 2011). In the context of Fanconi anemia (FA), a genetic disorder characterized by a variety of symptoms including skeletal and developmental defects, bone marrow failure (BMF), and a high predisposition to cancer (Bagby, 2003, Kottemann and Smogorzewska, 2013, Mamrak et al., 2017), emerging evidence suggests that p53 deficiency may increase cancer development in patients with FA and FA mice (Ceccaldi et al., 2011, Houghtaling et al., 2005). Conversely, recent studies show that overactive p53 could cause hematopoietic stem and progenitor cell (HSPC) depletion in the bone marrow (BM) of FA patients (Ceccaldi et al., 2012). These studies corroborate a critical role of the FA proteins in cooperating with p53 in apoptosis and cell-cycle regulation after DNA damage induced by the physiologic stress response.
In the present study, we demonstrate a p53-dependent HSPC proliferation regulation in mice deficient for the Fanca gene in the FA pathway. Using Fanca−/− HSCs deleted for the entire p53 gene or expressing the p53515C transgene, which selectively impairs the p53 function in apoptosis while keeping its cell-cycle checkpoint activities intact, we show that the p53 cell-cycle function is specifically required for the regulation of FA HSC proliferation. Our findings suggest that overactive p53 may represent a compensatory checkpoint mechanism for FA HSC proliferation.
Results
Loss of p53 in Fanca−/− Mice Leads to Increased HSPC Pool but Progressive Decline of HSC Reservoir
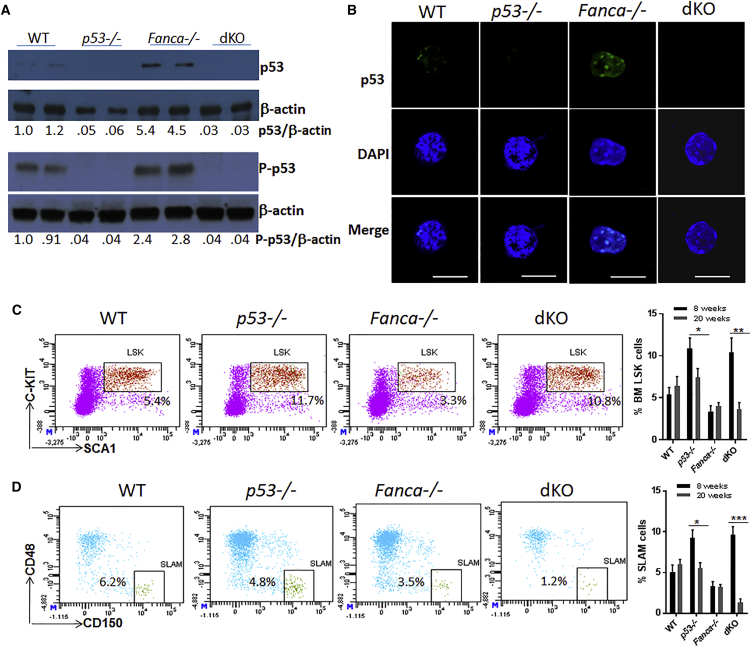
Recent studies showed that p53 is upregulated in HSPCs of FA patients, and postulated that overactive p53 response to DNA damage might be responsible for HSC depletion in FA (Ceccaldi et al., 2012). To study the in vivo effect of p53 on the maintenance of FA HSCs, we deleted the p53 gene in a murine model of FA (Fanca−/−) mice (Wong et al., 2003). The levels of both total and phosphorylated p53 proteins were higher in Lin−SCA-1+C-KIT+ (LSK) cells, a population containing HSCs and multipotential progenitors, of Fanca−/− mice than those of wild-type (WT) mice (Figure 1A). To examine the p53 protein in phenotypic HSCs, we isolated BM CD34− LSK cells, by fluorescence-activated cell sorting for immunostaining with an anti-p53 antibody. Consistent with the western blot results, the level of immunostained p53 was higher in Fanca−/− HSCs compared with WT cells (Figure 1B). We also used the HSCs from p53−/− and double-knockout (dKO) (p53−/−Fanca−/−) mice to verify the specificity of the p53 antibody.
Figure 1.
Loss of p53 in Fanca−/− Mice Leads to Increased HSPC Pool but Progressive Decline of HSC Reservoir
(A) Elevated p53 protein level in Fanca−/− HSPCs. BM LSK (Lin−SCA-1+C-KIT+) cells were isolated from mice with the indicated genotype, and cell lysates were subjected to immunoblot analysis using antibodies specific for total p53, phosphor-p53 (P-p53), or β-actin. The relative levels of total p53 or of P-p53 to β-actin are indicated below the blot. Each lane contains proteins from ∼30,000 LSK cells.
(B) Immunostaining of p53 protein in phenotypic HSCs. Freshly isolated CD34− LSK cells from mice with the indicated genotype were immunostained to detect p53 (green). Nuclei were visualized using DAPI (blue). Scale bars, 10 μm.
(C) Progressive decrease of HSPCs in p53-deficient Fanca−/− mice. Whole bone marrow cells (WBMCs) isolated from mice with the indicated genotype were subjected to flow cytometric analysis for LSK staining. Representative plots for 8 weeks (left) and quantification for both 8 and 20 weeks (right) are shown. Results are means ± SD of three independent experiments (n = 9 per group).
(D) Progressive decrease of HSCs in p53-deficient Fanca−/− mice. WBMCs isolated from mice with the indicated genotype were subjected to flow cytometric analysis for SLAM (LSK CD150+CD48−) staining. Representative plots for 20 weeks (left) and quantification for both 8 and 20 weeks (right) are shown. Results are means ± SD of three independent experiments (n = 9 per group).
∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Consistent with previous reports (Liu et al., 2009), loss of p53 increased both the frequencies of LSK cells (2- to 3-fold) and phenotypic (LSK CD150+ CD48−; SLAM; Kiel et al., 2005) HSCs (∼2-fold) compared with WT mice (Figures 1C and 1D). Interestingly, we found that the expansion of p53−/− SLAM cells in young mice (8 weeks of age) was followed by a significant decline in SLAM frequency at 20 weeks of age (Figure 1D), suggesting a possible replicative exhaustion. Importantly, the dKO (Fanca−/−p53−/−) HSCs showed much more dramatic exhaustion than those deficient for p53 alone at 20 weeks of age. Specifically, at 8 weeks of age, dKO mice showed a significant increase in LSK and SLAM cells. However, at 20 weeks of age, the frequencies of both LSK and SLAM cells in dKO mice declined significantly, compared with those at 8 weeks of age, which was equivalent to or lesser than that of WT or single-knockout (sKO) Fanca−/− mice (Figures 1C and 1D). Furthermore, SLAM cells deficient for Fanca alone did not undergo exhaustion (Figure 1D). These results suggest that the Fanca deficiency may collaborate with p53 loss in HSC replicative exhaustion.
p53 Deficiency Leads to Proliferative Exhaustion of Fanca−/− HSCs
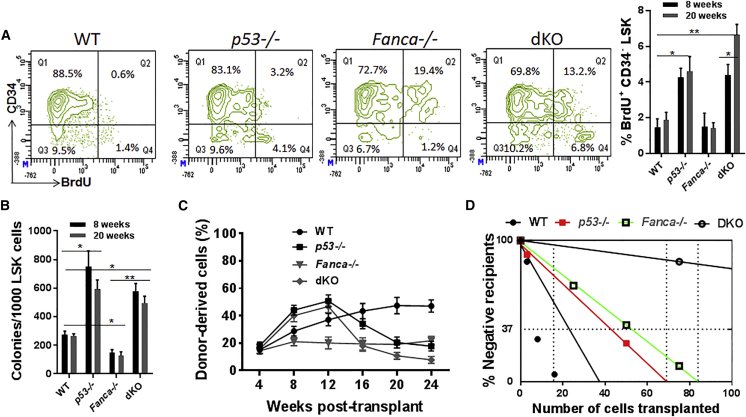
The observation that loss of p53 decreased HSC frequency in Fanca−/− mice prompted us to measure HSC proliferation in dKO mice by bromodeoxyuridine (BrdU) incorporation in vivo. After BrdU administration, we harvested BM cells from the mice and measured the percentage of LSK CD34− HSCs that had incorporated BrdU. Loss of p53 increased the percentage of HSCs incorporating BrdU in both WT and Fanca−/− mice, with approximately 40% (among total 16.8% LSK CD34− cells) of the p53−/− HSCs and 30% (among total 13.7% LSK CD34− cells) of the dKO HSCs incorporating BrdU in vivo at 20 weeks of age (Figure 2A). Similar results were obtained with progenitor proliferation assay, in which p53 deficiency led to more than 2-fold increase in colony formation in both WT and Fanca−/− mice compared with their respective controls at 8 and 20 weeks of age (Figure 2B).
Figure 2.
p53 Deficiency Leads to Proliferative Exhaustion of Fanca−/− HSCs
(A) p53 deficiency decreases Fanca−/− HSC quiescence. BM cells from mice with the indicated genotype at 8 and 20 weeks of age were gated for CD34− LSK population and analyzed for BrdU incorporation. Representative plots for 20 weeks (left) and quantification for both 8 and 20 weeks (right) are shown. Results are means ± SD of two independent experiments (n = 6 per group).
(B) Loss of p53 promotes Fanca−/− HSPC proliferation. LSK cells isolated from mice with the indicated genotype at 8 and 20 weeks of age were plated in cytokine-supplemented methylcellulose medium. Colonies were enumerated on day 7 after plating. Results are means ± SD of three independent experiments (n = 9 per group).
(C) Loss of p53 causes progressive decline in repopulating potential of Fanca−/− HSCs. 50 SLAM cells from mice with the indicated genotype (CD45.2) at 20 weeks of age, along with 4 × 105 protector BM cells (CD45.1), were transplanted into lethally irradiated Boy J recipients. Donor-derived chimera was detected by flow cytometry at the indicated weeks post-transplant.
(D) Competitive repopulating units (CRUs) determined by limiting dilution BM transplantation assay. Graded numbers of test SLAM cells (CD45.2+) from mice with the indicated genotype at 20 weeks of age were mixed with 4 × 105 protector BM cells (CD45.1) and transplanted into irradiated congenic recipients (CD45.1+). Plotted are the percentages of recipient mice containing less than 1% CD45.2+ blood nucleated cells at 16 weeks after transplantation. The frequency of functional HSCs was calculated according to Poisson statistics.
∗p < 0.05; ∗∗p < 0.01.
To study the effect of p53 deficiency on Fanca−/− HSC proliferation under replicative stress, we performed competitive repopulating experiments by transplanting 50 SLAM cells from WT, sKO, or dKO into lethally irradiated syngeneic recipient mice (CD45.1) along with 4 × 105 protector BM cells (CD45.1). We found that the repopulating abilities of both the p53−/− and dKO cells were higher than either WT or Fanca−/− cells at 8 and 12 weeks post-transplantation (Figure 2C). However, the repopulating capacity of the dKO HSCs underwent progressive decline thereafter, as demonstrated by a gradual decrease in donor-derived nucleated cells in the peripheral blood of the recipient mice (Figure 2C). The donor p53−/− HSCs also experienced a decline in repopulating potential, albeit less dramatic than the dKO HSCs (Figure 2C).
The results described above suggest that the dKO HSCs might have undergone replicative exhaustion in transplant recipients. To test this notion, we performed competitive repopulating unit (CRU) assays with limiting dilutions of SLAM cells from these four genotypes of mice. Various doses of sorted SLAM cells (15, 45, and 150 cells) were mixed with protector BM cells and transplanted into lethally irradiated Boy J mice. Peripheral blood samples were collected at 8 and 16 weeks post-transplantation and analyzed for donor engraftment. Poisson statistical analysis at 8 weeks post-transplant showed >5-fold reduction in CRUs in dKO mice than in WT mice (CRUs: 1 in 20.2 in WT mice; 1 in 24.7 in p53−/− mice; 1 in 26.8 in Fanca−/− mice; and 1 in 139.2 in dKO mice). This reduction in CRUs in dKO mice increased to >30-fold when estimated at 16 weeks post-transplantation (CRUs: 1 in 15.9 in WT mice; 1 in 68.6 in p53−/− mice; 1 in 83.5 in Fanca−/− mice; and 1 in 492 in dKO mice) (Figure 2D). These results indicate more severely impaired HSC functionality caused by simultaneous deficiency of FA and p53. Taken together, these results suggest that although deletion of p53 in Fanca−/− mice expanded the HSPC pool, these two genetic alterations cooperate somehow to induce progressive HSC exhaustion.
The Cell-Cycle Regulatory Activity of p53 Is Required for Preventing Fanca−/− HSC Exhaustion
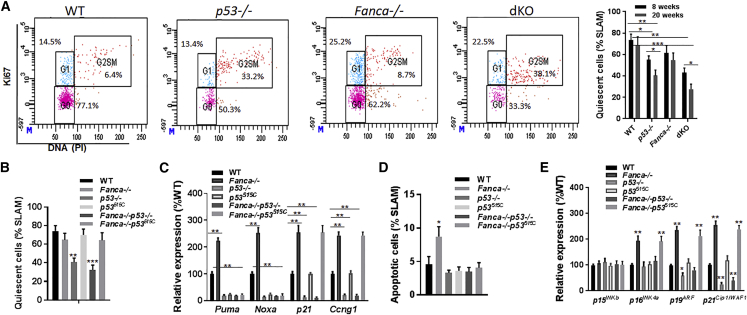
The increase in HSC turnover in dKO mice measured by BrdU incorporation in vivo (Figure 2A) would suggest decreased quiescence, which might be the cause of reduced repopulating potential we observed with the dKO HSCs (Figures 2C and 2D). Cell-cycle analyses using an antibody to Ki-67, a nuclear marker of cell cycling, showed that 30%–40% fewer HSCs were quiescent (in the G0 phase) in p53−/− and dKO mice compared with WT and Fanca−/− mice at 20 weeks of age (Figure 3A). Quiescence was also decreased in p53−/− and dKO mice at 8 weeks of age, albeit less profound than that observed in older (20 weeks) mice (Figure 3A).
Figure 3.
The Cell-Cycle Regulatory Activity of p53 Is Required for Preventing Fanca−/− HSC Exhaustion
(A) Cell-cycle analysis of SLAM cells. BM cells from mice with the indicated genotype at 8 and 20 weeks of age were gated for the SLAM population, and analyzed for cell-cycle phases by flow cytometry. Representative dot plots for 20 weeks (left) and quantification of quiescent (G0) cells within the SLAM population for both 8 and 20 weeks (right) are shown. Results are means ± SD of three independent experiments (n = 9 per group).
(B) Quiescence is not compromised in Fanca−/− SLAM cells expressing the p53515C/515C mutation. BM cells from mice with the indicated genotype at 20 weeks of age were gated for the SLAM population, and analyzed for cell-cycle phases by flow cytometry. Quantification of quiescent (G0) cells within the SLAM population is shown. Results are means ± SD of three independent experiments (n = 9 per group).
(C) qRT-PCR analysis of the expression of apoptosis and cell-cycle genes. SLAM cells isolated from mice with the indicated genotype at 20 weeks of age were subjected to qPCR analysis using primers for the indicated genes. Levels of the expression in each sample were normalized to the level of GAPDH mRNA, and the expression levels of the WT samples were normalized as 100.
(D) The p53515C mutation selectively impairs the p53 function in apoptosis. Cells from mice with the indicated genotype at 20 weeks of age were gated for SLAM population and analyzed for apoptosis by Annexin V and 7AAD. Quantification is shown. Results are means ± SD of three independent experiments (n = 9 per group).
(E) qRT-PCR analysis of the expression of negative cell-cycle regulator genes. SLAM cells isolated from mice with the indicated genotype at 20 weeks of age were subjected to qPCR analysis using primers for the indicated genes. Levels of the expression in each sample were normalized to the level of GAPDH mRNA, and the expression levels of the WT samples were normalized as 100.
Statistical significance compared with WT. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
It is well known that p53 controls the G1/S cell-cycle checkpoint and is required for both apoptosis and senescence in various cellular contexts (Iwakuma and Lozano, 2007). Given that loss of p53 alone reduced quiescence without causing significant HSC exhaustion, we postulated that the FA proteins cooperate with the cell-cycle activity of p53 in HSC maintenance. To test this hypothesis, we crossed our Fanca−/− mice to a mutant p53 mouse strain harboring a separation-of-function mutation in p53, p53515C, in which its apoptotic function is abolished but its cell-cycle checkpoint activities remain intact (Liu et al., 2004). We confirmed the functionality of the p53515C mutation using three independent assays: cell-cycle analysis, gene expression profiling of p53 targets, and apoptosis analysis. First, cell-cycle analyses using an antibody to Ki-67 showed that cell-cycle patterns of BM SLAM cells in p53515C/515C were not significantly different from those in WT or Fanca−/− littermates (Figure 3B). Importantly, p53515C/515C had minimal effect on the quiescence of Fanca−/− HSCs. Second, RT-PCR analysis of expression levels of p53 target genes in cell-cycle control (Ccng1, p21) and apoptosis (Puma, Noxa) in BM SLAM cells showed that the transcription of the cell-cycle genes was similar among WT, p53515C/515C, and p53515C/515C Fanca−/− mice; however, we observed a dramatic difference between p53−/− Fanca−/− and p53515C/515C Fanca−/− mice, in which the expression of the cell-cycle genes was abolished in p53−/− Fanca−/− mice, but remained robust in p53515C/515C Fanca−/− mice (Figure 3C). We found that the expression of the apoptosis genes was abolished in the p53515C/515C background (Figure 3C). We also noticed that the mRNA levels of cell-cycle and apoptosis genes were elevated in Fanca−/− BM SLAM cells compared with WT SLAM cells (Figure 3C). Lastly, we evaluated cell apoptosis by Annexin V staining and found that p53515C significantly reduced the frequency of apoptotic (Annexin V+/PI–) HSCs, similar to the null mice (Figure 3D). Collectively these data demonstrate that the p53515C mutation selectively impairs the p53 function in apoptosis, but leaves cell-cycle checkpoint unaffected.
The observation that Fancc−/− HSCs carrying the p53515C mutation lost apoptosis but remained quiescent prompted us to investigate the underlying molecular mechanism. To this end, we analyzed the p53-related negative cell-cycle regulators p15INKb, p16INK4a, p19ARF, and p21Cip1/WAF1 (Bartek and Lukas, 2001). Interestingly, three of the four G1 arrest-associated cell-cycle regulators, namely p16INK4a, p19Arf, and p21Cip1/WAF1, exhibited significantly higher expression in the SLAM cells isolated from the Fancc−/− littermates in the p53515C background (p53515C/515C Fanca−/−) than those in the p53-null background (p53−/− Fanca−/−; Figure 3E). These results suggest that a pathway regulating the expression of p16INK4a, p19Arf, and p21Cip1/WAF1 may act as molecular checkpoints for cycling and thus expansion of FA HSCs.
Next, we performed limiting dilution CRU assays to determine whether an intact cell-cycle function of p53 could prevent FA HSC exhaustion in vivo, by transplanting grading numbers of donor CD45.2+ bone marrow SLAM cells, along with protector BM cells, into lethally irradiated CD45.1+ congenic mice. At 16 weeks after transplant, the frequency of CRUs in phenotypic BM SLAM cells of p53515C/515C Fanca−/− mice was 1 in 97, significantly higher than the frequency, 1 in 458, in those of p53−/− Fanca−/− mice (Table 1). It should be noted that the frequency of CRUs in SLAM cells from p53−/− (1 in 63) or Fanca−/− (1 in 79) sKO mice, but not from those of p53515C/515C (1 in 21) mice, was significantly lower than the frequency of WT cells (1 in 19) (Table 1). These results indicate that the p53-dependent cell-cycle control is specifically required for avoiding further deterioration of FA HSC function. These data also suggest that total elimination of p53 function may promote FA HSC exhaustion.
Table 1.
Competitive Repopulating Units
| Genotype | WT | Fanca−/− | p53−/− 20 | p53515C | Fanca−/− p53−/− | Fanca−/− p53515C |
|---|---|---|---|---|---|---|
| CRU frequency | 1/19 | 1/79 | 1/63 | 1/21 | 1/458 | 1/97 |
Discussion
In this study, we used several mouse models of FA and p53 to study the in vivo effect of overactive p53 on FA hematopoiesis. Our results suggest that overactive p53 may play a compensatory role in preventing murine FA HSCs from replicative exhaustion. In this context, our study thus cautions that targeting p53 in FA, while potentially eliciting temporary growth improvement, might have the unanticipated detrimental consequence of increasing the long-term risk of HSC defect leading to BMF, which is the hematopoietic hallmark of FA.
One interesting observation of our study is that p53 deficiency in Fanca−/− mice increases the HSC pool in young mice (Figure 1). These results are consistent with previous reports that HSCs expressing lower levels of p53 protein can outcompete WT HSCs independent of DNA damage (Bondar and Medzhitov, 2010, Marusyk et al., 2010). Although the underlying mechanism remains unclear, we show that, in murine HSCs, some of the p53-related negative cell-cycle regulators, such as p16INK4a, p19ARF, and p21Cip1/WAF1, may be responsible for limiting the expansion of HSCs with normal (WT) or higher (FA) levels of p53. In addition, other mechanisms may also be in play in the context of defects in FA HSCs. For instance, there is evidence that FA HSPCs show abnormalities in reactive oxygen species detoxification (Du et al., 2008) and cytokine signaling (Anur et al., 2012, Li et al., 2007). Another related mechanism is the aberrant activation of the TLR4/8 and MAP kinase pathways that release myelosuppressive cytokines, such as tumor necrosis factor alpha, to which FA HSCs are hypersensitive (Svahn et al., 2015, Vanderwerf et al., 2009). It is also reported that mice deficient for FA DNA repair pathway (Fancd2) and acetaldehyde detoxification (aldehyde dehydrogenase 2; Aldh2) suffer dramatic reduction in the HSC pool (Garaycoechea et al., 2012). More recently, it has been shown that FA deficiency exacerbates the transforming growth factor β signaling pathway, which is detrimental to FA HSCs (Zhang et al., 2016).
HSC exhaustion, defined as a decrease in the number of HSCs caused by their enhanced cell cycling, is considered one cellular mechanism of BMF in FA. Under steady state, FA mice, including Fanca−/−, Fancb−/−, Fancc−/−, Fancd2−/−, and Fancg−/− mice, fail to recapitulate the anemia phenotype of FA patients (Carreau et al., 1998, Cheng et al., 2000, Du et al., 2015, Yang et al., 2001, Zhang et al., 2010). Our current study suggests that overactive p53 may be a causal factor that prevents or delays the loss of HSCs in Fanca−/− mice. Several studies, including ours, have shown that the p53 pathway is upregulated in several FA mouse models (Du et al., 2016, Ceccaldi et al., 2012, Freie et al., 2004). In two well-established assays designed to evaluate self-renewal (competitive limiting dilution assay) and hematopoietic repopulating (competitive BM transplantation assay) ability of an HSC, we observed a significant decrease in both CRU and long-term repopulation by Fanca−/− p53−/− dKO HSCs compared with Fanca−/− cells (Figure 2). Thus, it is tempting to speculate that a p53 function is required for the maintenance Fanca−/− HSCs. Furthermore, we demonstrated that replicative exhaustion, as demonstrated by an initial expansion followed by a progressive decline of both phenotypic HSCs and their long-term repopulating activity, requires simultaneous inactivation of Fanca and p53. This suggests that the HSC exhaustion phenotype observed in Fanca−/− p53−/− dKO mice may result from a synergistic effect of the double deficiencies. In this context, we speculate a coordinate regulation of HSC functions by the p53 and FA pathways.
The roles of p53 in both apoptosis and cell-cycle arrest are well understood. However, recent studies suggest that the most dramatic cellular outcomes mediated by p53 function can occur independently of, or separately from, these roles (Iwakuma and Lozano, 2007, Liu et al., 2004). In the current study, we have utilized FA HSCs deleted for the entire p53 gene or expressing the p53515C transgene, which selectively impair the p53 function in apoptosis, to demonstrate that the p53 cell-cycle function is specifically required for the regulation of FA HSC proliferation. This finding suggests enticing hypotheses to explain the compensatory role of overactive p53 in preventing FA HSCs from replicative exhaustion and leukemic transformation. Future studies will elucidate the interplay between the cell-cycle function of p53 and FA HSC maintenance.
Experimental Procedures
Detailed methods are included in the Supplemental Information.
Mice and Treatment
Fanca+/− and p53R172P/R172P mice (C57BL/6, CD45.2+) were provided by Dr. Madeleine Carreau (Laval University) and Dr. Guillermina Lozano (University of Texas MD Anderson Cancer Center), respectively (Liu et al., 2004, Wong et al., 2003). p53−/− Fanca−/− mice were generated by interbreeding the heterozygous Fanca+/− with p53+/− mice (C57BL/6: B6, CD45.2+; Jackson Laboratories) (Jacks et al., 1994). p53515C/515C Fanca−/− mice were generated by interbreeding the Fanca+/− with p53515C/+ mice. All the animals including the BoyJ (C57BL/6: B6, CD45.1+) mice were maintained in the animal barrier facility at Cincinnati Children's Hospital Medical Center. All the animals used for the experiments were 8–20 weeks old. All experimental procedures conducted in this study were approved by the Institutional Animal Care and Use Committee of Cincinnati Children's Hospital Medical Center.
Statistical Analysis
Student's t test was performed using GraphPad Prism v6 (GraphPad software). Comparison of more than two groups was analyzed by one-way ANOVA test. p values <0.05 were considered statistically significant. Results are presented as means ± SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Author Contributions
X.L. and W.D. designed and performed the research, analyzed the data, and wrote the paper. A.F.W. performed the research. Q.P. designed the research, analyzed the data, and wrote the paper.
Acknowledgments
We thank Dr. Madeleine Carreau (Laval University) for Fanca+/− mice, Dr. Guillermina Lozano (University of Texas MD Anderson Cancer Center) for p53515C/515C mice, and the Comprehensive Mouse and Cancer Core of the Cincinnati Children's Research Foundation (Cincinnati Children's Hospital Medical Center) for bone marrow transplantation service. We also thank members of the Pang lab for helpful discussions and Ms. Samantha Losekamp for editing the manuscript. This investigation was partially supported by NIH grants R01 HL076712, R01 HD089932, and T32 HL091805. Q.P. was supported by a Leukemia and Lymphoma Scholar award.
Published: January 4, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and can be found with this article online at https://doi.org/10.1016/j.stemcr.2017.12.006.
Contributor Information
Wei Du, Email: wei.du@hsc.wvu.edu.
Qishen Pang, Email: qishen.pang@cchmc.org.
Supplemental Information
References
- Abbas H.A., Pant V., Lozano G. The ups and downs of p53 regulation in hematopoietic stem cells. Cell Cycle. 2011;10:3257–3262. doi: 10.4161/cc.10.19.17721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anur P., Yates J., Garbati M.R., Vanderwerf S., Keeble W., Rathbun K. p38 MAPK inhibition suppresses the TLR-hypersensitive phenotype in FANCC- and FANCA-deficient mononuclear phagocytes. Blood. 2012;119:1992–2002. doi: 10.1182/blood-2011-06-354647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G.C., Jr. Genetic basis of Fanconi anemia. Curr. Opin. Hematol. 2003;10:68–76. doi: 10.1097/00062752-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Bartek J., Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 2001;13:738–747. doi: 10.1016/s0955-0674(00)00280-5. [DOI] [PubMed] [Google Scholar]
- Bondar T., Medzhitov R. p53-Mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6:309–322. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau M., Gan O.I., Liu L., Doedens M., McKerlie C., Dick J.E., Buchwald M. Bone marrow failure in the Fanconi anemia group C mouse model after DNA damage. Blood. 1998;91:2737–2744. [PubMed] [Google Scholar]
- Ceccaldi R., Briot D., Larghero J., Vasquez N., Dubois d'Enghien C., Chamousset D., Noguera M.E., Waisfisz Q., Hermine O., Pondarre C. Spontaneous abrogation of the G₂ DNA damage checkpoint has clinical benefits but promotes leukemogenesis in Fanconi anemia patients. J. Clin. Invest. 2011;121:184–194. doi: 10.1172/JCI43836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi R., Parmar K., Mouly E., Delord M., Kim J.M., Regairaz M., Pla M., Vasquez N., Zhang Q.S., Pondarre C. .Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell. 2012;11:36–49. doi: 10.1016/j.stem.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N.C., van de Vrugt H.J., van der Valk M.A., Oostra A.B., Krimpenfort P., de Vries Y. Mice with a targeted disruption of the Fanconi anemia homolog Fanca. Hum. Mol. Genet. 2000;9:1805–1811. doi: 10.1093/hmg/9.12.1805. [DOI] [PubMed] [Google Scholar]
- Du W., Adam Z., Rani R., Zhang X., Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid. Redox Signal. 2008;10:1909–1921. doi: 10.1089/ars.2008.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Amarachintha S., Erden O., Wilson A., Meetei A.R., Andreassen P.R., Namekawa S.H., Pang Q. Fancb deficiency impairs hematopoietic stem cell function. Sci. Rep. 2015;5:18127. doi: 10.1038/srep18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Amarachintha S., Wilson A.F., Pang Q. SCO2 mediates oxidative stress-induced glycolysis to oxidative phosphorylation switch in hematopoietic stem cells. Stem Cells. 2016;34:960–971. doi: 10.1002/stem.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freie B.W., Ciccone S.L., Li X., Plett P.A., Orschell C.M., Srour E.F., Hanenberg H., Schindler D., Lee S.H., Clapp D.W. A role for the Fanconi anemia C protein in maintaining the DNA damage-induced G2 checkpoint. J. Biol. Chem. 2004;279:50986–50993. doi: 10.1074/jbc.M407160200. [DOI] [PubMed] [Google Scholar]
- Garaycoechea J.I., Crossan G.P., Langevin F., Daly M., Arends M.J., Patel K.J. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489:571–575. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- Houghtaling S., Granville L., Akkari Y., Torimaru Y., Olson S., Finegold M., Grompe M. Heterozygosity for p53 (Trp53+/-) accelerates epithelial tumor formation in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Cancer Res. 2005;65:85–91. [PubMed] [Google Scholar]
- Iwakuma T., Lozano G. Crippling p53 activities via knock-in mutations in mouse models. Oncogene. 2007;26:2177–2184. doi: 10.1038/sj.onc.1210278. [DOI] [PubMed] [Google Scholar]
- Jacks T., Remington L., Williams B.O. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Kiel M.J., Yilmaz O.H., Iwashita T., Yilmaz O.H., Terhorst C., Morrison S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kottemann M.C., Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013;493:356–363. doi: 10.1038/nature11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug U., Ganser A., Koeffler H.P. Tumor suppressor genes in normal and malignant hematopoiesis. Oncogene. 2002;21:3475–3495. doi: 10.1038/sj.onc.1205322. [DOI] [PubMed] [Google Scholar]
- Levine A.J., Oren M. The first 30 years of p53: growing ever more complex. Nat. Rev. Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Sejas D.P., Zhang X., Qiu Y., Nattamai K.J., Rani R., Rathbun K.R., Geiger H., Williams D.A., Bagby G.C., Pang Q. TNF-alpha induces leukemic clonal evolution ex vivo in Fanconi anemia group C murine stem cells. J. Clin. Invest. 2007;117:3283–3295. doi: 10.1172/JCI31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Parant J.M., Lang G., Chau P., Chavez-Reyes A., El-Naggar A.K., Multani A., Chang S., Lozano G. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat. Genet. 2004;36:63–68. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- Liu Y., Elf S.E., Miyata Y., Sashida G., Liu Y., Huang G., Di Giandomenico S., Lee J.M., Deblasio A., Menendez S. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamrak N.E., Shimamura A., Howlett N.G. Recent discoveries in the molecular pathogenesis of the inherited bone marrow failure syndrome Fanconi anemia. Blood Rev. 2017;31:93–99. doi: 10.1016/j.blre.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A., Porter C.C., Zaberezhnyy V., DeGregori J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 2010;8:e1000324. doi: 10.1371/journal.pbio.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray-Zmijewski F., Slee E.A., Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat. Rev. Mol. Cell Biol. 2008;9:702–712. doi: 10.1038/nrm2451. [DOI] [PubMed] [Google Scholar]
- Pant V., Quintás-Cardama A., Lozano G. The p53 pathway in hematopoiesis: lessons from mouse models, implications for humans. Blood. 2012;120:5118–5127. doi: 10.1182/blood-2012-05-356014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokocimer M., Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood. 1994;84:2391–2411. [PubMed] [Google Scholar]
- Svahn J., Lanza T., Rathbun K., Bagby G., Ravera S., Corsolini F., Pistorio A., Longoni D., Farruggia P., Dufour C., Cappelli E. p38 mitogen-activated protein kinase inhibition enhances in vitro erythropoiesis of Fanconi anemia, complementation group A-deficient bone marrow cells. Exp. Hematol. 2015;43:295–299. doi: 10.1016/j.exphem.2014.11.010. [DOI] [PubMed] [Google Scholar]
- TeKippe M., Harrison D.E., Chen J. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp. Hematol. 2003;31:521–527. doi: 10.1016/s0301-472x(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Vanderwerf S.M., Svahn J., Olson S., Rathbun R.K., Harrington C., Yates J., Keeble W., Anderson D.C., Anur P., Pereira N.F. TLR8-dependent TNF-(alpha) overexpression in Fanconi anemia group C cells. Blood. 2009;114:5290–5298. doi: 10.1182/blood-2009-05-222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.V., Leblanc M., Fox N., Mao J.H., Tinkum K.L., Krummel K., Engle D., Piwnica-Worms D., Piwnica-Worms H., Balmain A. Fine-tuning p53 activity through C-terminal modification significantly contributes to HSC homeostasis and mouse radiosensitivity. Genes Dev. 2011;25:1426–1438. doi: 10.1101/gad.2024411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.C., Alon N., Mckerlie C., Huang J.R., Meyn M.S., Buchwald M. Targeted disruption of exons 1 to 6 of the Fanconi Anemia group A gene leads to growth retardation, strain-specific microphthalmia, meiotic defects and primordial germ cell hypoplasia. Hum. Mol. Genet. 2003;12:2063–2076. doi: 10.1093/hmg/ddg219. [DOI] [PubMed] [Google Scholar]
- Yang Y., Kuang Y., Montes De Oca R., Hays T., Moreau L., Lu N. Targeted disruption of the murine Fanconi anemia gene, Fancg/Xrcc9. Blood. 2001;98:3435–3440. doi: 10.1182/blood.v98.12.3435. [DOI] [PubMed] [Google Scholar]
- Zhang H., Kozono D.E., O’Connor K.W., Vidal-Cardenas S., Rousseau A., Hamilton A., Moreau L., Gaudiano E.F., Greenberger J., Bagby G. TGF-β inhibition rescues hematopoietic stem cell defects and bone marrow failure in Fanconi Anemia. Cell Stem Cell. 2016;18:1–14. doi: 10.1016/j.stem.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.S., Marquez-Loza L., Eaton L., Duncan A.W., Goldman D.C., Anur P., Watanabe-Smith K., Rathbun R.K., Fleming W.H., Bagby G.C., Grompe M. Fancd2-/- mice have hematopoietic defects that can be partially corrected by resveratrol. Blood. 2010;116:5140–5148. doi: 10.1182/blood-2010-04-278226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.