Abstract
Cytokine signaling is indispensable for regulatory T-cell (Treg) development in the thymus, and also influences the homeostasis, phenotypic diversity, and function of Tregs in the periphery. Because Tregs are required for establishment and maintenance of immunological self-tolerance, investigating the role of cytokines in Treg biology carries therapeutic potential in the context of autoimmune disease. This review discusses the potent and diverse influences of interleukin (IL)-2 signaling on the Treg compartment, an area of knowledge that has led to the use of low-dose IL-2 as a therapy to reregulate autoaggressive immune responses. Evidence suggesting Treg-specific impacts of the cytokines transforming growth factor β (TGF-β), IL-7, thymic stromal lymphopoietin (TSLP), IL-15, and IL-33 is also presented. Finally, we consider the technical challenges and knowledge limitations that must be overcome to bring other cytokine-based, Treg-targeted therapies into clinical use.
CD4+Foxp3+ regulatory T cells (Tregs) are indispensable for the maintenance of immunological self-tolerance because of their ability to suppress autoreactive T cells that escape negative selection in the thymus. Humans and mice lacking the signature Treg transcription factor Foxp3 succumb to fatal lymphoproliferative disease early in life. In keeping with this role, clinical and experimental data have implicated Treg dysfunction in the pathogenesis of many autoimmune diseases (Grant et al. 2015), which collectively account for an enormous burden of morbidity and mortality. Meanwhile, the nonspecific therapies used to control these conditions (e.g., corticosteroids) often have limited efficacy and severe adverse effects.
As with other T-cell lineages, many aspects of Treg biology are regulated by a vast and intricate network of cytokine signals. A detailed understanding of how these cytokines impact Treg differentiation, homeostasis, and suppressive function will facilitate new therapies that disrupt autoimmunity through precise targeting of its underlying molecular pathways. The recent use of low-dose interleukin (IL)-2 immunotherapy has already shown the promise of this cytokine-based approach. Low-dose IL-2, which selectively expands and activates the Treg compartment in vivo (Aoyama et al. 2012; Kosmaczewska 2014; Yu et al. 2015), has proven beneficial to patients with systemic lupus erythematosus (He et al. 2016), hepatitis C virus–induced vasculitis (Saadoun et al. 2011), alopecia areata (Castela et al. 2014), and graft-versus-host disease (Koreth et al. 2011; Matsuoka et al. 2013). Early phase clinical trials have also been conducted in the setting of type 1 diabetes (Hartemann et al. 2013; Todd et al. 2016).
Numerous cytokines besides IL-2 are now known to affect Tregs, and behind each one lies a potential new tool for the immunotherapy arsenal. Nevertheless, preliminary efforts to manipulate these pathways have often been hobbled by poor efficacy or severe off-target effects, a challenge resulting from the highly pleiotropic nature of cytokine signals. Exploiting the full range of opportunity provided by cytokine-based therapies will require more advanced insight into the complexities of cytokine signaling as it relates to the Treg compartment. Accordingly, the purpose of this review is to describe how several extensively studied cytokines, IL-2, IL-7, thymic stromal lymphopoietin (TSLP), IL-15, transforming growth factor β (TGF-β), and IL-33, are known to influence Tregs.
INTERLEUKIN-2
Originally identified as a growth factor for T cells in vitro (Morgan et al. 1976), IL-2 is produced primarily by activated CD4+ and CD8+ T conventional (Tconv) cells (Malek 2008). The high-affinity IL-2 receptor (IL-2R) comprises three subunits, IL-2Rα (CD25), IL-2Rβ (CD122), and the common γ chain (γc or CD132). Tregs and antigen-activated Tconv cells are the predominant populations that express all three simultaneously, positioning them to respond efficiently to IL-2 in vivo. Although Tregs do not produce IL-2, they express CD25 at uniquely high and consistent levels, whereas elevated CD25 expression in Tconv cells is generally a transient event during activation (Malek 2008). During signaling, IL-2 first binds to CD25, forming a dimer that recruits CD122 and γc (Stauber et al. 2006). Following receptor engagement, the Janus kinase (JAK)1 and JAK3 associate with the cytoplasmic tails of IL-2Rβ and γc, leading to phosphorylation of the JAKs along with three key tyrosine residues in the tail of IL-2Rβ. These phosphotyrosines activate three major intracellular signaling pathways: mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K), both mediated by the Shc adaptor protein, as well as the signal transducer and activator of transcription 5 (STAT5—composed of two similar proteins, STAT5A and STAT5B). These downstream signaling pathways govern survival, proliferation, and memory formation among antigen-activated Tconv lineages (Cheng et al. 2011), but are differentially regulated in the Treg compartment to support its suppressive function. Tregs are uniquely reliant on IL-2-dependent STAT5 activation, due in part to high levels of the lipid phosphatase and tensin homolog (PTEN), which acts to suppress IL-2-dependent PI3K and mechanistic target of rapamycin (mTOR) activity (Walsh et al. 2006; Huynh et al. 2015). IL-2 directly up-regulates Foxp3 and CD25 through STAT5 in a positive feedback mechanism to establish and maintain Treg transcriptional identity (Fontenot et al. 2005b; Burchill et al. 2007b; Feng et al. 2014). In conjunction with elevated CD25 expression, molecular adaptations in these pathways allow Treg development and homeostasis to be supported by a uniquely low threshold of IL-2 signaling, explaining the effectiveness of low-dose therapy (Yu et al. 2009). Tregs can also outcompete activated Tconv cells for available IL-2, and this cytokine deprivation has been highlighted as one mechanism of Treg-mediated immunosuppression (Pandiyan et al. 2007).
IL-2 is the predominant cytokine involved in regulating Treg development, stability, function, and peripheral homeostasis. Mouse models with abrogated expression of IL-2, CD25, or CD122 experience aggressive lymphoproliferation and fatal multiorgan autoimmunity (Sadlack et al. 1993, 1995; Suzuki et al. 1995; Willerford et al. 1995), and rare loss-of-function mutations in human CD25 produce a similar clinical syndrome in patients (Roifman 2000). This occurs because IL-2 signaling deficiency leads to a failure of Treg maturation and a consequent breakdown of self-tolerance (Fig. 1). Although CD4+Foxp3+ T cells are still present in IL-2/IL-2R-deficient mice, they show a ∼50% numerical reduction, and Foxp3 expression on a per-cell basis is reduced twofold (Josefowicz et al. 2012a). These Foxp3lo cells have diminished expression of the suppressive mediators CTLA4, CD39, and CD73, suggesting that thymic Tregs generated in the setting of IL-2 deprivation lack adequate functional programming (Cheng et al. 2013). This idea is supported by adoptive transfer studies, as introducing a low number (1–3 × 105) of CD4+CD25+ Tregs into neonatal Il2rb−/− mice provides full protection from autoimmunity, with treated animals retaining a donor-derived Treg compartment throughout life (Malek et al. 2002). Moreover, transgenic thymic rescue of IL-2Rβ signaling in these mice is sufficient to restore a normal Treg compartment and prevent autoimmunity, further demonstrating a nonredundant requirement for the IL-2R in production of functional Tregs (Malek et al. 2000; Malek and Bayer 2004; Bayer et al. 2007).
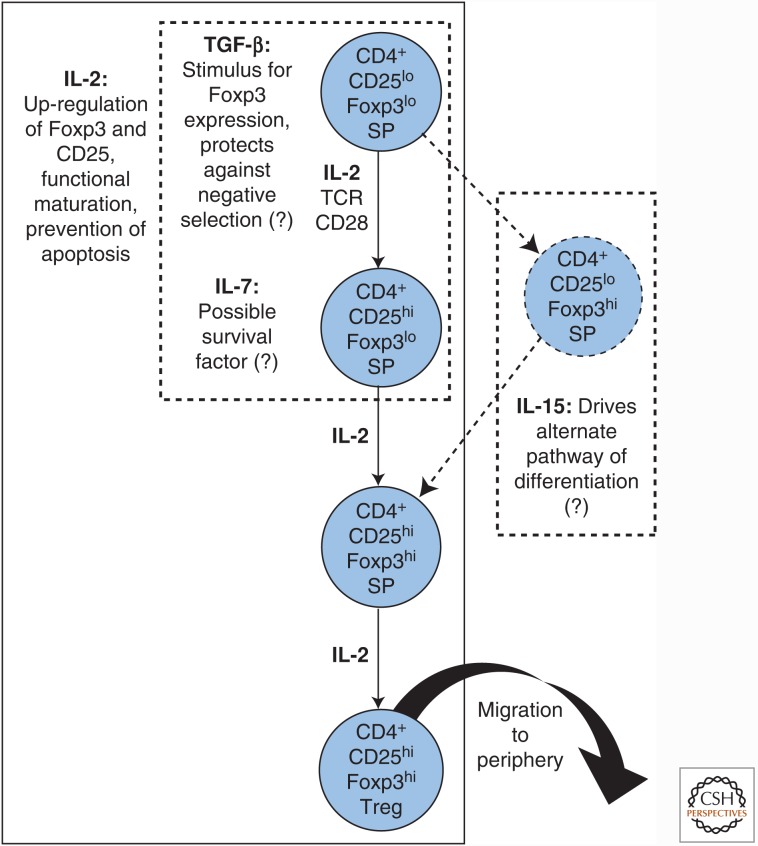
Figure 1.
Cytokine contributions to regulatory T-cell (Treg) maturation in the thymus. Tregs develop from CD4 single-positive (SP) thymocyte precursors. Signaling through a high-affinity, self-reactive T-cell receptor (TCR) combined with CD28 costimulation is thought to trigger up-regulation of CD25. This potentiates the ability of Treg precursors to respond to interleukin (IL)-2, which prevents apoptosis, induces Foxp3 expression, and establishes Treg transcriptional identity and suppressive capacity. Boxes with dotted lines denote developmental stages where other cytokines may act. Dotted arrows denote a possible parallel pathway of Treg development. TGF-β, Transforming growth factor β.
Experiments using CD4 single-positive thymocytes confirm that IL-2 is an essential driver of Treg lineage commitment. Transgenic expression of a constitutively active Stat5 transgene is sufficient to divert thymocyte precursors of naïve Tconv cells into the Treg lineage (Burchill et al. 2008). Mice deficient in the mediator TRAF3, which opposes IL-2R signaling, exhibit a two- to threefold increase in thymic Treg frequency (Yi et al. 2014). Active from the earliest stages of Treg differentiation, IL-2/IL-2R interaction is a critical inducer of Foxp3 expression in nascent Tregs (Bayer et al. 2007; Burchill et al. 2007a; Huehn et al. 2009), and also acts as a survival factor, up-regulating Bcl-2 to prevent their apoptosis (Tai et al. 2013). In vitro and in vivo experiments support a model whereby signals from a high-affinity, self-reactive T-cell receptor (TCR) and CD28 induce CD25 up-regulation in Treg precursors, leading to IL-2-driven induction of Foxp3 and resultant commitment to the Treg cell fate (Fig. 1) (Burchill et al. 2008; Lio and Hsieh 2008).
Although a majority of mature Tregs found in the periphery are of thymic origin, naïve Tconv cells can be instructed to differentiate into Tregs through a combination of molecular signals that includes IL-2. Treating cultured CD4+CD25− T cells from humans or mice with the cytokine TGF-β (described below), in conjunction with TCR stimulation, can elicit Foxp3 expression and generate CD4+CD25+ “induced Tregs” (iTregs) with immunosuppressive function (Chen et al. 2003; Fantini et al. 2004). Although IL-2 on its own cannot convert naïve Tconv cells to a Treg cell fate (Xu et al. 2010), experiments using cytokine deprivation by antibody blockade and germline knockout (KO) show that IL-2 is essential for TGF-β-mediated induction of Foxp3, as well as subsequent iTreg clonal expansion and in vitro suppressor activity (Zheng et al. 2007). Adoptive transfer studies support the idea that iTreg persistence, transcriptional identity, and phenotypic stability are also IL-2-dependent in vivo (Shevach and Thornton 2014). iTregs are transcriptionally distinct from their thymic Treg counterparts on generation in vitro (Haribhai et al. 2009; Feuerer et al. 2010), and functional comparisons in some experimental models suggest that iTregs have lower phenotypic stability and suppressive capacity (Yadav et al. 2013; Shevach and Thornton 2014). These shortcomings have hindered the application of iTregs as a therapeutic tool, although cell-culture protocols for optimizing their potency and durability are a subject of continuing investigation (Kanamori et al. 2016; Schmidt et al. 2016).
Tconv precursors are also capable of differentiating into Tregs in vivo within peripheral tissue sites. Generation of these immunosuppressive cells, termed “peripheral Tregs” (pTregs) to distinguish them from cell culture–derived iTregs, is favored by subimmunogenic doses of peptide antigen in conjunction with TGF-β and IL-2 (Povoleri et al. 2013). Mice that undergo thymectomy at 3 days of age to abolish thymic Treg maturation develop autoimmune disease, demonstrating that pTregs alone are insufficient to maintain self-tolerance (Sakaguchi et al. 1995; Asano et al. 1996). Nevertheless, pTregs possess functional specializations that make them uniquely suited to suppressing inflammation in certain peripheral sites, particularly mucosal surfaces (Bilate and Lafaille 2012; Josefowicz et al. 2012b; Yadav et al. 2013). pTregs are key contributors to immune homeostasis in the intestine, a tissue microenvironment that appears to be especially favorable for their differentiation (Tanoue et al. 2016). pTregs present at this interface between host and external environment are integral for maintaining tolerance to an enormous array of antigens derived from self, ingested material, and microbiota (Littman and Rudensky 2010; Harrison and Powrie 2013). Crucially, gut-localized CD4+ T cells in humans and mice exhibit an unusual degree of phenotypic plasticity between the pTreg and proinflammatory Th17 lineages, with IL-2 serving as an important regulator of this balance (Fig. 2). In vivo, TGF-β may be involved in differentiation toward either the Treg or Th17 cell fate (Konkel and Chen 2011), and additional cytokine signals are required to tip the scales toward the transcriptional program of either Foxp3 or the Th17-defining transcription factor RORγt (Hoechst et al. 2011; Kleinewietfeld and Hafler 2013; Ueno et al. 2015). Exposing Tconv cells to TGF-β in an environment rich in proinflammatory signals (particularly IL-6) favors Th17 differentiation (Kimura and Kishimoto 2010; Fujimoto et al. 2011). Conversely, IL-2-dependent STAT5 signaling promotes TGF-β-mediated differentiation of pTregs in the intestine (Povoleri et al. 2013; Yadav et al. 2013), while impeding the generation of Th17 cells from naïve precursors (Laurence et al. 2007; Zheng et al. 2008). Since the shift of this dynamic equilibrium away from pTregs and toward Th17 is essential to the pathogenesis of inflammatory bowel disease (Ueno et al. 2015), the role of IL-2 in this context has clear therapeutic implications.
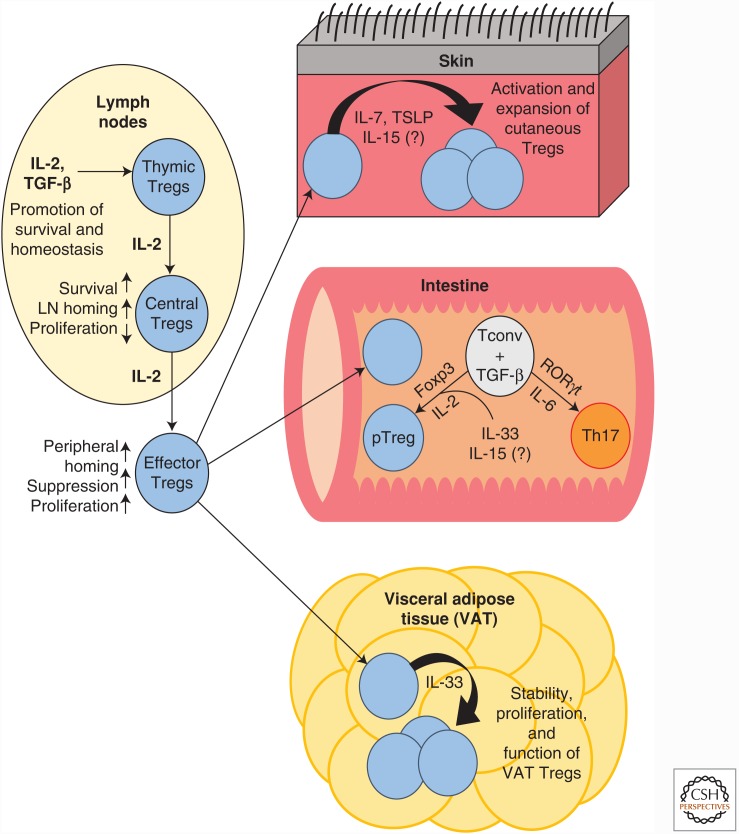
Figure 2.
Effects of cytokine signaling on regulatory T cells (Tregs) in peripheral tissue sites. IL, Interleukin; TGF-β, transforming growth factor β; TSLP, thymic stromal lymphopoietin; LN, lymph node; Tconv, T conventional cell.
Treg homeostasis does not require ongoing output from the thymus but is instead based on continuous self-renewal of thymic Tregs, which seed peripheral lymphoid tissues and undergo IL-2 driven expansion during early life (Bayer et al. 2005, 2007). There is substantial evidence that IL-2R signaling contributes to homeostatic maintenance of the peripheral Treg pool (Fig. 2), although the precise mechanisms have not been fully clarified. Many studies have positively correlated CD25 with Foxp3 expression and phenotypic stability among Tregs in the periphery (Fontenot et al. 2005a; Barbi et al. 2014). IL-2 has also been found to participate in the epigenetic regulation that maintains Treg identity. Sustained heritability of Foxp3 expression depends on the Foxp3 promoter as well as a cis-regulatory element within the Foxp3 locus known as conserved noncoding sequence 2 (CNS2) or the Treg-specific demethylated region (TSDR). Both the Foxp3 promoter and CNS2 contain STAT5-binding motifs, and CNS2 appears to function as an IL-2 sensor when this cytokine is present in limiting amounts. IL-2-dependent STAT5 signaling in Tregs enables CNS2 to interact with the Foxp3 promoter, which prevents loss of Foxp3 expression during cell-cycle progression or exposure to proinflammatory cytokines (Zheng et al. 2010; Feng et al. 2014; Li et al. 2014). Cellular adoptive transfer and bone marrow chimera studies, using IL-2/IL-2R-deficient mouse models, show an IL-2-mediated contribution to the survival, engraftment, and proliferation of Tregs in lymphoid tissues (Bayer et al. 2005, 2007; Cheng et al. 2013). Notably, administering exogenous IL-2 to Il2−/− mice not only rescues the numerical deficit in Tregs, but also corrects many of their transcriptional abnormalities, including low expression of suppression mediators such as CTLA4, CD39, and CD73 (Fontenot et al. 2005b; Barron et al. 2010). One report identified the antiapoptotic protein Mcl-1, which is known to be regulated by IL-2 signaling, as an essential survival factor for Tregs in the periphery (Pierson et al. 2013). However, it should be noted that interpretation of findings from IL-2/IL-2R-deficient models is problematic because it is often unclear whether Treg defects are attributable to impaired survival in the periphery, defective maturation in the thymus, or a combination of both. One study attempted to isolate peripheral signaling effects using antibody blockade of IL-2 in thymectomized adult mice. This treatment led to a selective reduction in Treg numbers and induction of autoimmunity, establishing an unequivocal role for IL-2 in peripheral homeostasis (Setoguchi et al. 2005). Emerging genetic tools, which permit fate mapping of Foxp3+ cells and inducible deletion of signaling components in mice, have provided new opportunities to clarify the nature and importance of the IL-2R for Tregs in the periphery, although a clear consensus has yet to emerge (Rubtsov et al. 2010; Chinen et al. 2016).
These ambiguities surrounding the contribution of peripheral IL-2 signaling may be partially explained by heterogeneity within the Treg compartment itself. Current evidence indicates that mature Tregs can be divided into phenotypically and functionally distinct subpopulations. “Central” Tregs (cTregs) express high levels of the lymphoid homing marker CD62L, and exhibit a phenotype geared toward long-term survival and diminished functional activation. CD62Llo “effector” Tregs (eTregs), which originate from cTregs, show comparatively reduced survival, elevated expression of activation markers, and efficient trafficking to nonlymphoid tissues (Fig. 2) (Yuan et al. 2014). Preliminary evidence indicates that Treg subsets vary in their degree of dependence on IL-2R-mediated signals, although the exact nature of this variation is still uncertain. In a mouse model with attenuated IL-2Rβ signaling, which possessed a grossly normal Treg compartment, a highly activated subset of tissue-resident eTregs bearing the surface marker Klrg1 failed to develop (Cheng et al. 2012). However, the functionally immature Tregs from Il2−/− or Il2ra−/− mice are skewed toward highly activated phenotypes, a fact that may suggest that cTreg survival is more reliant on IL-2 than that of eTregs (Campbell and Koch 2011). Resolving these multiple sites of action will require an understanding of Treg heterogeneity on a scale more refined than the cTreg/eTreg dichotomy. Accordingly, the search for cell-surface molecules capable of delineating functionally specialized cTreg and eTreg subsets is ongoing (Toomer et al. 2016).
TRANSFORMING GROWTH FACTOR β
The TGF-β signaling pathway provides a versatile mechanism to coordinate essential cellular functions, including proliferation, differentiation, and morphogenesis (Massague 2012), and is key to shaping immune responses and carcinogenesis. The three mammalian isoforms (TGF-β1, TGF-β2, and TGF-β3) are constitutively expressed across many tissues, with cells of the immune system predominantly expressing TGF-β1 (Prud’homme 2007). Among the major downstream targets of TGF-β signal transduction are the Smad family of transcription factors, which interact with each other and additional cofactors to form a diverse array of DNA-binding complexes (Massague et al. 2005).
Consistent with this intrinsic complexity, TGF-β can exert a vast range of proinflammatory and anti-inflammatory effects on many types of immune cells (Prud’homme 2007; Li and Flavell 2008; Travis and Sheppard 2014). Yet remarkably, the Tgfb1−/− mouse was found to experience aggressive, multiorgan autoimmunity leading to premature death at 3–4 weeks of age (Kulkarni and Karlsson 1993), a phenotype strikingly similar to that of the Foxp3 KO mouse. This observation suggested that TGF-β might exert its influence, in part, through the Treg compartment. In keeping with this idea, CD4+CD25+ T cells were found to express high levels of surface-bound TGF-β, and secreted this cytokine when activated (Nakamura et al. 2001; Green et al. 2003). Application of TGF-β-blocking antibodies or chemicals led to impairment of Treg-suppressive function (Aoki et al. 2005). Subsequently, a mouse model harboring a T-cell-specific deletion of TGF-β receptor II reproduced the lethal autoimmune phenotype, confirming that the central pathologic insult in this model is uncontrolled activation of self-reactive Tconv cells (Marie et al. 2006). This autoimmunity may be partially explained by the fact that TGF-β secreted from Tregs acts as a suppressive mediator in some models of inflammation (Schmidt et al. 2012). However, one study indicated that Tregs in the Tgfb1−/− mouse were diminished in number and had reduced expression of Foxp3. Following adoptive transfer into lymphopenic mice, both wild-type and Tgfb1−/− Tregs showed lower Foxp3 expression in hosts treated with TGF-β blocking antibody. Given that no phenotypic deficits were observed in CD4+CD25+ thymocytes, these findings suggest that TGF-β also makes a direct contribution to Treg maintenance and lineage commitment in the periphery (Fig. 2) (Marie et al. 2005). Notably, other studies do support a role for TGF-β in Treg thymic development. A T-cell-specific deletion, targeting the murine TGF-β receptor I, was found to temporarily block the appearance of CD4+CD25+Foxp3+ thymocytes during the first week of postnatal life (Liu et al. 2008). Another study found evidence that TGF-β supports Treg development indirectly by curtailing negative selection of thymocyte precursors (Fig. 1) (Ouyang et al. 2010). The existence of new, more precise models of targeted genetic deletion may help clarify the nature and extent of these TGF-β-mediated developmental effects (Chen and Konkel 2015).
As outlined previously, TGF-β is integral to the generation of Tregs from naïve Tconv precursors in vitro (iTregs) and in vivo (pTregs), an activity that has major physiological relevance within the intestinal microenvironment. The dual ability of this cytokine to provoke Th17 differentiation may be partly concentration-dependent, with larger amounts of TGF-β favoring Foxp3 induction (Omenetti and Pizarro 2015). Even at high levels, however, TGF-β alone cannot program the complete Treg transcriptional and functional profile. In addition to other cytokines (e.g., IL-2), the signals influencing Treg differentiation include retinoic acid, TCR affinity, dose and administration route of peptide antigen, and interactions with antigen-presenting cells (Povoleri et al. 2013; Yadav et al. 2013). As compared to thymic Tregs, iTregs boast a distinct pattern of epigenetic modifications at the Foxp3 locus. In particular, the regulatory region known as conserved noncoding sequence 1 (CNS1) contains binding sites for the TGF-β downstream mediator Smad3, which acts cooperatively with other transcriptional regulators to induce Foxp3 expression (Tone et al. 2008). Deletion of CNS1 severely and selectively impairs iTreg differentiation, although it is dispensable for Foxp3 induction and Treg maturation in the thymus (Zheng et al. 2010; Josefowicz et al. 2012b).
INTERLEUKIN-7/THYMIC STROMAL LYMPHOPOIETIN
A critical factor for survival and maturation of T and B lymphocytes, IL-7 signals through a dimeric receptor consisting of the IL-7Rα chain (CD127) and the γc (Fry and Mackall 2005; El Kassar and Gress 2010). IL-7Rα also forms one component of the dimeric receptor for the cytokine TSLP, a distant paralog of IL-7, which also exerts wide-ranging effects on the immune system (Ziegler et al. 2013). Unlike most cytokines, which are secreted by immune cells, IL-7 is produced mainly by nonlymphoid cells within lymphoid organs such as bone marrow stroma and thymic epithelium (Jiang et al. 2005). Il7−/− (von Freeden-Jeffry et al. 1995) and Il7r−/− (Peschon et al. 1994) mice exhibit lymphoid hypoplasia with deficiencies in T- and B-cell compartments, γδ T-cell and natural killer T (NKT)-cell lineages (Maki et al. 1996; Boesteanu et al. 1997), and mutational loss of either IL-7Rα or the γc causes severe combined immunodeficiency (SCID) in man (Puel et al. 1998). IL-7R regulates survival and expansion of double-negative thymocytes, and is down-regulated during the double-positive stage (Alpdogan and van den Brink 2005; Fry and Mackall 2005; Xiong et al. 2013). Re-expressed in the single-positive stage, it is retained on most mature Tconv cells and serves as an important signal governing peripheral homeostasis (Alpdogan and van den Brink 2005; Bradley et al. 2005; Fry and Mackall 2005; Mackall et al. 2011).
Despite its general importance for T-cell maturation in the thymus, evidence suggests that IL-7 does not play a fundamental role in the differentiation of Tregs. In vitro, IL-7 triggers pSTAT5 phosphorylation (albeit to a lesser extent than IL-2) and supports maturation of CD4+CD25+Foxp3− thymic Treg precursors (Vang et al. 2008), but this contribution is less clear in vivo. Although a block in thymic Treg development has been reported in Il7r−/− mice, interpretation of this finding is complicated by generalized defects in T-cell production (Bayer et al. 2008; Mazzucchelli et al. 2008). CD4+Foxp3+ cells are virtually absent in mice doubly deficient in IL-2Rβ and IL-7Rα, indicating a developmental impairment more severe than that produced by IL-2/IL-2R deficiency alone (Bayer et al. 2008). Despite these findings, selective thymic reconstitution in Il2rb/Il7r double KOs showed that restoration of IL-2Rβ signaling alone can rescue Treg production, completely compensating for a lack of IL-7Rα (Bayer et al. 2008). Thus, IL-7Rα is dispensable for the generation of a functionally mature Treg compartment. It is possible that IL-7 provides a contributory signal to support survival of thymic Tregs during the CD4 single-positive precursor stage, but discerning where and how this effect might operate will require further investigation (Fig. 1).
A number of contradictory results have been obtained regarding the effects of IL-7 on Tregs in the periphery. Mature Tregs have traditionally been considered to express uniformly low levels of CD127, which should render them largely insensitive to IL-7-mediated effects (Liu et al. 2006; Seddiki et al. 2006). In keeping with this idea, mouse studies have found IL-7 signaling to be dispensable for survival, proliferation, phenotypic stability, and suppressive capacity in the peripheral Treg compartment (Peffault de Latour et al. 2006; Mazzucchelli et al. 2008). In vivo experiments using IL-7-transgenic mice and exogenous cytokine administration show that Tregs in the periphery can proliferate in response to IL-7 when it is present at elevated levels (Simonetta et al. 2012). However, this pathway does not appear to play a major role in overall Treg homeostasis under normal physiological conditions. One study examining lymphopenia-driven proliferation of CD4+ T cells found that Tconv expansion was closely tied to IL-7 levels, whereas expansion of Tregs was instead governed by IL-2 (Le Campion et al. 2012). There is some evidence to suggest an IL-7-mediated contribution to Treg suppression. One study evaluating a specialized population of immunosuppressive dendritic cells in the context of diabetes onset showed that dendritic cell (DC)-produced IL-7 acted as a survival factor for CD4+CD25+ Tregs in vitro, as well as in vivo following adoptive transfer into nonobese diabetic mice (Harnaha et al. 2006). A possible mechanism behind this effect is suggested by another study, which reported that IL-7 enabled Tregs in culture to maintain CD25 expression, potentiating their ability to respond efficiently to IL-2. Adoptive transfers into IL-7-deficient or IL-7-overexpressing mice showed that this effect was maintained in vivo as well (Simonetta et al. 2014).
It appears that IL-7 may exert a more pronounced regulatory effect on specialized subpopulations of Tregs than on the cell lineage as a whole. For example, this cytokine has been reported as a positive regulator of Treg survival and function in the setting of cutaneous immunosuppression (Fig. 2). Using a mouse model of inducible self-antigen expression in skin, one study reported that IL-7, rather than IL-2, was indispensable for the maintenance of highly activated cutaneous Foxp3+ Tregs after self-antigen expression was turned off (Gratz et al. 2013). Another study examining Tregs in murine lymph nodes revealed that expression of the activation markers ICOS and CD103 defined a population of Tregs that express high levels of IL-7Rα (Simonetta et al. 2010). Tregs were found to up-regulate IL-7Rα during activation both in vitro and in vivo, suggesting an augmented responsiveness to IL-7-mediated signals (Simonetta et al. 2010). Both studies noted a CD127hi phenotype in skin-resident Tregs characterized ex vivo, strengthening the idea that low expression of this receptor is not an inherent aspect of the Treg functional program (Simonetta et al. 2010; Gratz et al. 2013).
Emerging evidence suggests that IL-7Rα signaling through epithelium-derived TSLP is another important contributor to cutaneous Treg maintenance and function (Fig. 2). In one mouse model, cutaneous inflammation was initiated by a tamoxifen-inducible, tissue-specific deletion of the chromatin remodeler Mi-2β in the basal epidermis. This disruption of epigenetic regulation caused production of numerous inflammatory mediators and progression to a systemic autoimmune response, although the animals recovered completely (Kashiwagi et al. 2017). In this proinflammatory context, TSLP drove the proliferation of cutaneous Tregs as well as their differentiation into eTreg phenotypes, with corresponding up-regulation of transcripts related to activation, epithelial localization, and immunosuppression. Bone marrow chimera experiments showed that adoptively transferred Tregs with a deletion of Crlf2 (which encodes the second “TSLPR” component of the heterodimeric TSLP receptor) were impaired in their ability to expand in skin following Mi-2β loss. Notably, in mice lacking TSLP-responsive Tregs, the progression to systemic autoimmunity went unchecked and resulted in a lethal phenotype (Kashiwagi et al. 2017). In another experiment, topical administration of a vitamin D3 analog to mice caused cutaneous Tregs to proliferate in a TSLP-dependent manner. This treatment reduced the incidence of autoimmune diabetes in nonobese diabetic mice, and ameliorated clinical symptoms in experimental autoimmune encephalomyelitis-induced mice (Leichner et al. 2017). These results suggest that IL-7Rα-mediated activation and expansion of cutaneous Tregs can influence systemic immune homeostasis, an intriguing possibility that may be exploited by future therapeutic approaches.
INTERLEUKIN-15
The IL-15 receptor (IL-15R) shares two of its three subunits in common with the IL-2R, comprising a high-affinity IL-15Rα chain, CD122, and the γc (Olsen et al. 2007). However, this structural similarity belies a vast divergence of effects in vivo, as IL-15 is highly pleiotropic and influences a much broader array of immune cell types than IL-2. Il15−/− and Il15ra−/− mice exhibit numerical reductions in various innate and adaptive cell populations, including CD8+ T cells, natural killer (NK) cells, NKT cells, and γδ intraepithelial lymphocytes. In addition to acting as a prominent lymphocyte survival factor (Lodolce et al. 2002; Alpdogan and van den Brink 2005), IL-15 provides a specific and potent stimulus for memory-phenotype (CD44hi) CD8+ T cells in vivo (Zhang et al. 1998), and appears to regulate homeostasis and acquisition of memory functions in naïve CD4+ T cells (Chen et al. 2014). IL-15 signaling also promotes antigen presentation and production of type I cytokines (IL-12 and interferon γ [IFN-γ]) by dendritic cells and macrophages (Ohteki et al. 2001), and potentiates NK cell proliferation and cytotoxicity (Becknell and Caligiuri 2005).
As for IL-7, IL-15 signaling promotes STAT5 phosphorylation, Foxp3 expression, and maturation in CD4+CD25hi single-positive thymocytes cultured in vitro, albeit to a lesser extent than IL-2 (Lio and Hsieh 2008; Vang et al. 2008). Nevertheless, an IL-15-mediated contribution to thymic Treg development has been challenging to identify in vivo. The similarity in clinical phenotype between CD25-deficient and CD122-deficient mice shows that IL-15 cannot independently support development of a functional Treg compartment (Sakaguchi et al. 2008). IL-15-deficient mice have nearly normal numbers of Tregs, suggesting that this pathway is dispensable for their generation (Burchill et al. 2007b). Nevertheless, mice deficient in both IL-2 and IL-15 signaling pathways have a more profound deficit in Treg numbers than those lacking IL-2 alone (Burchill et al. 2007b; Sakaguchi et al. 2008). This finding suggests that IL-15 can contribute to Treg survival through IL-2Rβ/γc signaling when IL-2 is absent, although it evidently cannot rescue Treg function because the clinical course of lethal autoimmunity is unchanged. New mouse models for mapping the fates of thymocyte lineages in vivo suggest that IL-15 may make an ancillary contribution to Treg development. One such model featured deletion of the TCR signaling mediator Zap70, which abrogated thymic production of T cells. Reconstitution of T-cell development using a tetracycline-inducible Zap70 transgene allowed de novo–generated Tregs to be selectively analyzed by eliminating any mature Foxp3+ cells of extrathymic origin (Marshall et al. 2014). This approach corroborated the generally accepted model that Tregs originate from CD4+CD25hi single-positive thymocytes that up-regulate Foxp3 expression in response to IL-2. However, among CD4 single-positive cells, a second lineage of CD25−Foxp3+ precursors were found to be capable of developing efficiently into Tregs. Survival and maturation of these precursors was dependent on IL-15 produced by the thymic stroma, indicating that this cytokine may support an alternative pathway of Treg differentiation (Fig. 1) (Marshall et al. 2014).
Although the possible influence of IL-15 on mature Tregs in the periphery is only beginning to be explored, emerging evidence suggests that this cytokine may potentiate Treg-suppressive function in certain specialized tissue environments. Using an explant culture system, it was reported that resident Tregs in human skin can be selectively expanded by treatment with IL-15 (Fig. 2) (Clark and Kupper 2007). One recent experiment suggests that IL-15 may contribute to immune homeostasis in the gut by regulating the equilibrium between Treg and Th17 differentiation. Purified Tregs that were adoptively transferred into an IL-15-deficient RAGKO mouse model showed skewing toward a Th17 phenotype, with accelerated loss of Foxp3 expression and up-regulation of RORγt. This led to acquisition of Th17 effector functions by the transferred cells, fueling aggressive colitis in the recipient hosts (Fig. 2) (Tosiek et al. 2016). These findings highlight the possibility of targeting the IL-15 signaling axis therapeutically, to drive expansion or differentiation of tissue-resident Tregs.
INTERLEUKIN-33
IL-33 is constitutively expressed at high levels in epithelial cells, lymphoid cells, and stromal cells of both humans and mice, and is also up-regulated in response to inflammatory stimuli (Cayrol and Girard 2014). A member of the IL-1 family of cytokines, IL-33, signals by binding ST2, a member of the Toll-like/IL-1-receptor superfamily. The resulting dimer then forms a complex with the ubiquitously expressed receptor component IL-1R accessory protein, whose cytosolic domain activates MyD88 signaling to trigger production of inflammatory mediators (Miller 2011). Given its abundance in epithelium, IL-33 is believed to function as an “alarmin,” rapidly activating the innate immune system when tissue is damaged by trauma or infectious insults (Chan et al. 2012). IL-33 expressed under homeostatic conditions is retained in the cell nucleus, which may serve to increase the propagation speed of this “danger” signal by allowing the cytokine to be released preformed from necrotic epithelial cells (Cayrol and Girard 2014). IL-33 is unique among IL-1 family cytokines in promoting Th2-polarized immune responses, suggesting a conserved evolutionary function in stimulating antiparasite immunity at mucosal surfaces (Miller 2011). Current evidence suggests that IL-33 is an important contributor to Th2-driven allergic diseases such as asthma (Borish and Steinke 2011; Cayrol and Girard 2014; Saluja et al. 2015) and atopic dermatitis (Cevikbas and Steinhoff 2012), as well as rheumatoid arthritis, psoriatic arthritis, and systemic lupus erythematosus (Miller 2011; Duan et al. 2013; Macedo et al. 2016).
As discussed previously, Tregs are known to acquire functional adaptations and unique transcriptional profiles that support efficient suppressive activity in nonlymphoid tissue sites (Feuerer et al. 2010; Campbell and Koch 2011). IL-33 has recently been identified as a candidate cytokine that appears to support these tissue-specific adaptations. Despite its established role as a mediator of Th2-mediated inflammation at environmental interfaces, IL-33 seems to play a dichotomous role in inflammatory bowel disease. Although levels of IL-33 and ST2 are increased in the intestines and serum of inflammatory bowel disease (IBD) patients, experimental findings suggest that this signaling axis also supports epithelial repair and resolution of inflammatory injury (Pastorelli et al. 2011; Duan et al. 2012; Grobeta et al. 2012; Sedhom et al. 2013). The recently discovered capacity of IL-33 to promote Treg activity in the gut may clarify this apparent contradiction. ST2 is preferentially expressed on colonic Tregs from mice, and signaling through this receptor promotes survival, proliferation, and suppressive activity of these Tregs both in vitro and in vivo. In addition, IL-33 was found to enhance TGF-β-mediated conversion of naïve T cells into pTregs (Fig. 2) (Schiering et al. 2014).
A similar role for IL-33 has also been characterized in visceral adipose tissue (VAT). VAT-resident Tregs are a specialized population of considerable clinical importance, mediating the link between obesity-induced inflammation and its associated disease outcomes, particularly insulin resistance leading to type 2 diabetes (Feuerer et al. 2009). ST2 was found to be very highly expressed in murine Tregs from visceral adipose tissue compared to those from lymphoid organs. Moreover, this expression was specifically enriched among Tregs with a highly activated phenotype (Vasanthakumar et al. 2015). ST2-deficient mice exhibited clinical evidence of insulin resistance, drastically reduced Treg frequency in the VAT, and impaired maintenance of Treg functional and transcriptional identity. Notably, in vivo administration of IL-33 was sufficient to drive proliferation of VAT Tregs in normal mice, and this treatment also rescued the numerical deficit of VAT Tregs in obese mice with corresponding improvements in glucose tolerance (Fig. 2) (Vasanthakumar et al. 2015).
CONCLUDING REMARKS
As illustrated by the preceding examples, we have a reasonable understanding of how several cytokines shape the Treg compartment. IL-2 is a critical cytokine for Treg development while also importantly influencing Treg homeostasis, stability, and function. TGF-β provides critical signaling for the development of pTregs. Emerging data also support the notion that IL-7, TSLP, IL-15, and IL-33 help to shape the activity of Tregs in various nonlymphoid tissue sites. However, in some other instances, the search for causal links between a particular cytokine signal and certain aspects of Treg development and function has yielded contradictory results. In particular, the roles of IL-7, IL-15, and TGF-β in the development and peripheral lymphoid homeostasis of thymic Tregs are less clear. Factors that contribute to these inconsistencies include functional redundancy among cytokines and their receptor subunits, the capacity of many cytokines to exert pleiotropic effects, and experimental variables such as choice of genetic model, inflammatory milieu, or tissue site.
Therapeutic applications of these cytokines to boost Tregs and promote immune tolerance must take into consideration such redundancy and pleiotropy. The promising clinical results using low-dose IL-2 to enhance the Treg compartment are largely because of our current understanding that many critical aspects of Tregs are supported by low levels of IL-2R signaling, whereas T effector and memory cells require more extensive IL-2R signaling. As such, low-dose IL-2 decreases the pleotropic activity and minimizes nonspecific toxicity of IL-2. Nevertheless, many essential parameters such as dose–response relationships, optimal administration schedule, and duration of treatment remain undefined for low-dose IL-2 (Klatzmann and Abbas 2015).
Therapies that exploit the Treg-promoting activities of IL-7, TSLP, IL-15, and IL-33 must also be devised to minimize their pleiotropic effects and render them selective toward Tregs. Unlike IL-2, we do not know whether signal thresholds for these cytokines might be favorably directed toward Tregs. Thus, a critical investigative frontier for this group of cytokines may be finding methods to deliver them with precision to minimize off-target effects. The evidence presented here suggests that even highly pleiotropic cytokines can exert defined and predictable influences on a Treg subpopulation specialized for a particular tissue compartment. Thus, rather than taking aim at the Treg pool in its entirety, it may be far more tractable to work toward precision delivery of an immunomodulatory agent to a peripheral anatomical site to engage one or more specialized tissue-resident Treg subsets with well-characterized signaling requirements. Accordingly, it appears that future progress in cytokine-based therapy will occur on a much finer scale.
ACKNOWLEDGMENTS
Our research is supported by grants from the National Institutes of Health (R01 DK093866, R01 AI055815, F31 AI124629), the American Diabetes Association (1-15-BS-125), and the Diabetes Institute Research Foundation. The authors declare no competing interests.
Footnotes
Editors: Warren J. Leonard and Robert D. Schreiber
Additional Perspectives on Cytokines available at www.cshperspectives.org
REFERENCES
- Alpdogan O, van den Brink MR. 2005. IL-7 and IL-15: Therapeutic cytokines for immunodeficiency. Trends Immunol 26: 56–64. [DOI] [PubMed] [Google Scholar]
- Aoki CA, Borchers AT, Li M, Flavell RA, Bowlus CL, Ansari AA, Gershwin ME. 2005. Transforming growth factor β (TGF-β) and autoimmunity. Autoimmun Rev 4: 450–459. [DOI] [PubMed] [Google Scholar]
- Aoyama A, Klarin D, Yamada Y, Boskovic S, Nadazdin O, Kawai K, Schoenfeld D, Madsen JC, Cosimi AB, Benichou G, et al. 2012. Low-dose IL-2 for In vivo expansion of CD4+ and CD8+ regulatory T cells in nonhuman primates. Am J Transplant 12: 2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M, Toda M, Sakaguchi N, Sakaguchi S. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med 184: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbi J, Pardoll D, Pan F. 2014. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev 259: 115–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O’Gorman WE, Abbas AK. 2010. Cutting edge: Mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol 185: 6426–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AL, Yu A, Adeegbe D, Malek TR. 2005. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J Exp Med 201: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AL, Yu A, Malek TR. 2007. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol 178: 4062–4071. [DOI] [PubMed] [Google Scholar]
- Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. 2008. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol 181: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becknell B, Caligiuri MA. 2005. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol 86: 209–239. [DOI] [PubMed] [Google Scholar]
- Bilate AM, Lafaille JJ. 2012. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol 30: 733–758. [DOI] [PubMed] [Google Scholar]
- Boesteanu A, Silva AD, Nakajima H, Leonard WJ, Peschon JJ, Joyce S. 1997. Distinct roles for signals relayed through the common cytokine receptor γ chain and interleukin 7 receptor α chain in natural T cell development. J Exp Med 186: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borish L, Steinke JW. 2011. Interleukin-33 in asthma: How big of a role does it play? Curr Allergy Asthma Rep 11: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley LM, Haynes L, Swain SL. 2005. IL-7: Maintaining T-cell memory and achieving homeostasis. Trends Immunol 26: 172–176. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vang KB, Farrar MA. 2007a. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett 114: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. 2007b. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 178: 280–290. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. 2008. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 28: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ, Koch MA. 2011. Phenotypic and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol 11: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P, Lacour JP, Passeron T. 2014. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol 150: 748–751. [DOI] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. 2014. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol 31: 31–37. [DOI] [PubMed] [Google Scholar]
- Cevikbas F, Steinhoff M. 2012. IL-33: A novel danger signal system in atopic dermatitis. J Invest Dermatol 132: 1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J. 2012. Alarmins: Awaiting a clinical response. J Clin Invest 122: 2711–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Konkel JE. 2015. Development of thymic Foxp3+ regulatory T cells: TGF-β matters. Eur J Immunol 45: 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Bobbala D, Cepero Donates Y, Mayhue M, Ilangumaran S, Ramanathan S. 2014. IL-15 trans-presentation regulates homeostasis of CD4+ T lymphocytes. Cell Mol Immunol 11: 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Yu A, Malek TR. 2011. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev 241: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Yuan X, Tsai MS, Podack ER, Yu A, Malek TR. 2012. IL-2 receptor signaling is essential for the development of Klrg1+ terminally differentiated T regulatory cells. J Immunol 189: 1780–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Yu A, Dee MJ, Malek TR. 2013. IL-2R signaling is essential for functional maturation of regulatory T cells during thymic development. J Immunol 190: 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, Gasteiger G, Feng Y, Fontenot JD, Rudensky AY. 2016. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol 17: 1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Kupper TS. 2007. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood 109: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Chen J, Zhang H, Yang H, Zhu P, Xiong A, Xia Q, Zheng F, Tan Z, Gong F, et al. 2012. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3+ regulatory T-cell responses in mice. Mol Med 18: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Chen J, Gong F, Shi G. 2013. The role of IL-33 in rheumatic diseases. Clin Dev Immunol 2013: 924363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kassar N, Gress RE. 2010. An overview of IL-7 biology and its use in immunotherapy. J Immunotoxicol 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. 2004. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 172: 5149–5153. [DOI] [PubMed] [Google Scholar]
- Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. 2014. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell 158: 749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. 2010. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci 107: 5919–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Dooley JL, Farr AG, Rudensky AY. 2005a. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med 202: 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. 2005b. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 6: 1142–1151. [DOI] [PubMed] [Google Scholar]
- Fry TJ, Mackall CL. 2005. The many faces of IL-7: From lymphopoiesis to peripheral T cell maintenance. J Immunol 174: 6571–6576. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Nakano M, Terabe F, Kawahata H, Ohkawara T, Han Y, Ripley B, Serada S, Nishikawa T, Kimura A, et al. 2011. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol 186: 32–40. [DOI] [PubMed] [Google Scholar]
- Grant CR, Liberal R, Mieli-Vergani G, Vergani D, Longhi MS. 2015. Regulatory T-cells in autoimmune diseases: Challenges, controversies and—yet—unanswered questions. Autoimmun Rev 14: 105–116. [DOI] [PubMed] [Google Scholar]
- Gratz IK, Truong HA, Yang SH, Maurano MM, Lee K, Abbas AK, Rosenblum MD. 2013. Cutting edge: Memory regulatory T cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol 190: 4483–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. 2003. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-β-TGF-β receptor interactions in type 1 diabetes. Proc Natl Acad Sci 100: 10878–10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobeta P, Doser K, Falk W, Obermeier F, Hofmann C. 2012. IL-33 attenuates development and perpetuation of chronic intestinal inflammation. Inflamm Bowel Dis 18: 1900–1909. [DOI] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li SH, Simpson PM, Chatila TA, Williams CB. 2009. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol 182: 3461–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnaha J, Machen J, Wright M, Lakomy R, Styche A, Trucco M, Makaroun S, Giannoukakis N. 2006. Interleukin-7 is a survival factor for CD4+ CD25+ T-cells and is expressed by diabetes-suppressive dendritic cells. Diabetes 55: 158–170. [PubMed] [Google Scholar]
- Harrison OJ, Powrie FM. 2013. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb Perspect Biol 5: a018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, Fonfrede M, Rosenzwajg M, Bernard C, Klatzmann D. 2013. Low-dose interleukin 2 in patients with type 1 diabetes: A phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 1: 295–305. [DOI] [PubMed] [Google Scholar]
- He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, et al. 2016. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat Med 22: 991–993. [DOI] [PubMed] [Google Scholar]
- Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. 2011. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood 117: 6532–6541. [DOI] [PubMed] [Google Scholar]
- Huehn J, Polansky JK, Hamann A. 2009. Epigenetic control of FOXP3 expression: The key to a stable regulatory T-cell lineage? Nat Rev Immunol 9: 83–89. [DOI] [PubMed] [Google Scholar]
- Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, et al. 2015. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol 16: 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. 2005. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev 16: 513–533. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. 2012a. Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. 2012b. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori M, Nakatsukasa H, Okada M, Lu Q, Yoshimura A. 2016. Induced regulatory T cells: Their development, stability, and applications. Trends Immunol 37: 803–811. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Hosoi J, Lai JF, Brissette J, Ziegler SF, Morgan BA, Georgopoulos K. 2017. Direct control of regulatory T cells by keratinocytes. Nat Immunol 18: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Kishimoto T. 2010. IL-6: Regulator of Treg/Th17 balance. Eur J Immunol 40: 1830–1835. [DOI] [PubMed] [Google Scholar]
- Klatzmann D, Abbas AK. 2015. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 15: 283–294. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Hafler DA. 2013. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol 25: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel JE, Chen W. 2011. Balancing acts: The role of TGF-β in the mucosal immune system. Trends Mol Med 17: 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP III, Armand P, Cutler C, Ho VT, Treister NS, et al. 2011. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Eng J Med 365: 2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmaczewska A. 2014. Low-dose interleukin-2 therapy: A driver of an imbalance between immune tolerance and autoimmunity. Int J Mol Sci 15: 18574–18592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AB, Karlsson S. 1993. Transforming growth factor-β1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol 143: 3–9. [PMC free article] [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. 2007. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26: 371–381. [DOI] [PubMed] [Google Scholar]
- Le Campion A, Pommier A, Delpoux A, Stouvenel L, Auffray C, Martin B, Lucas B. 2012. IL-2 and IL-7 determine the homeostatic balance between the regulatory and conventional CD4+ T cell compartments during peripheral T cell reconstitution. J Immunol 189: 3339–3346. [DOI] [PubMed] [Google Scholar]
- Leichner TM, Satake A, Harrison VS, Tanaka Y, Archambault AS, Kim BS, Siracusa MC, Leonard WJ, Naji A, Wu GF, et al. 2017. Skin-derived TSLP systemically expands regulatory T cells. J Autoimmun 10.1016/jjaut.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Flavell RA. 2008. TGF-β: A master of all T cell trades. Cell 134: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liang Y, LeBlanc M, Benner C, Zheng Y. 2014. Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell 158: 734–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio CW, Hsieh CS. 2008. A two-step process for thymic regulatory T cell development. Immunity 28: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. 2010. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140: 845–858. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, et al. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203: 1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. 2008. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 9: 632–640. [DOI] [PubMed] [Google Scholar]
- Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A. 2002. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor Rev 13: 429–439. [DOI] [PubMed] [Google Scholar]
- Macedo RB, Kakehasi AM, Melo de Andrade MV. 2016. IL33 in rheumatoid arthritis: Potential contribution to pathogenesis. Rev Bras Reumatol Engl Ed 56: 451–457. [DOI] [PubMed] [Google Scholar]
- Mackall CL, Fry TJ, Gress RE. 2011. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 11: 330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki JI, Ikuta K. 1996. Interleukin 7 receptor-deficient mice lack γδ T cells. Proc Natl Acad Sci 93: 7172–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek TR. 2008. The biology of interleukin-2. Annu Rev Immunol 26: 453–479. [DOI] [PubMed] [Google Scholar]
- Malek TR, Bayer AL. 2004. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol 4: 665–674. [DOI] [PubMed] [Google Scholar]
- Malek TR, Porter BO, Codias EK, Scibelli P, Yu A. 2000. Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J Immunol 164: 2905–2914. [DOI] [PubMed] [Google Scholar]
- Malek TR, Yu A, Vincek V, Scibelli P, Kong L. 2002. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17: 167–178. [DOI] [PubMed] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M, Rudensky AY. 2005. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med 201: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Liggitt D, Rudensky AY. 2006. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity 25: 441–454. [DOI] [PubMed] [Google Scholar]
- Marshall D, Sinclair C, Tung S, Seddon B. 2014. Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. J Immunol 193: 5525–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. 2012. TGFβ signalling in context. Nat Rev Mol Cell Biol 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. 2005. Smad transcription factors. Genes Dev 19: 2783–2810. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, Murase K, Cutler C, Ho VT, Alyea EP, et al. 2013. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 5: 179ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, Willette-Brown J, Hurwitz AA, Leonard WJ, Durum SK. 2008. Development of regulatory T cells requires IL-7Rα stimulation by IL-7 or TSLP. Blood 112: 3283–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM. 2011. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DA, Ruscetti FW, Gallo R. 1976. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 193: 1007–1008. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kitani A, Strober W. 2001. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J Exp Med 194: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. 2001. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat Immunol 2: 1138–1143. [DOI] [PubMed] [Google Scholar]
- Olsen SK, Ota N, Kishishita S, Kukimoto-Niino M, Murayama K, Uchiyama H, Toyama M, Terada T, Shirouzu M, Kanagawa O, et al. 2007. Crystal structure of the interleukin-15·interleukin-15 receptor α complex: Insights into trans and cis presentation. J Biol Chem 282: 37191–37204. [DOI] [PubMed] [Google Scholar]
- Omenetti S, Pizarro TT. 2015. The Treg/Th17 axis: A dynamic balance regulated by the gut microbiome. Front Immunol 6: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Ma Q, Li MO. 2010. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32: 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. 2007. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 8: 1353–1362. [DOI] [PubMed] [Google Scholar]
- Pastorelli L, De Salvo C, Cominelli MA, Vecchi M, Pizarro TT. 2011. Novel cytokine signaling pathways in inflammatory bowel disease: Insight into the dichotomous functions of IL-33 during chronic intestinal inflammation. Therap Adv Gastroenterol 4: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peffault de Latour R, Dujardin HC, Mishellany F, Burlen-Defranoux O, Zuber J, Marques R, Di Santo J, Cumano A, Vieira P, Bandeira A. 2006. Ontogeny, function, and peripheral homeostasis of regulatory T cells in the absence of interleukin-7. Blood 108: 2300–2306. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med 180: 1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, Humblet-Baron S, Schonefeldt S, Herold MJ, Hildeman D, et al. 2013. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nat Immunol 14: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povoleri GA, Scotta C, Nova-Lamperti EA, John S, Lombardi G, Afzali B. 2013. Thymic versus induced regulatory T cells—Who regulates the regulators? Front Immunol 4: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud’homme GJ. 2007. Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest 87: 1077–1091. [DOI] [PubMed] [Google Scholar]
- Puel A, Ziegler SF, Buckley RH, Leonard WJ. 1998. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nat Genet 20: 394–397. [DOI] [PubMed] [Google Scholar]
- Roifman CM. 2000. Human IL-2 receptor α chain deficiency. Pediatr Res 48: 6–11. [DOI] [PubMed] [Google Scholar]
- Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. 2010. Stability of the regulatory T cell lineage in vivo. Science 329: 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D. 2011. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 365: 2067–2077. [DOI] [PubMed] [Google Scholar]
- Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75: 253–261. [DOI] [PubMed] [Google Scholar]
- Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I. 1995. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol 25: 3053–3059. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155: 1151–1164. [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133: 775–787. [DOI] [PubMed] [Google Scholar]
- Saluja R, Khan M, Church MK, Maurer M. 2015. The role of IL-33 and mast cells in allergy and inflammation. Clin Transl Allergy 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, et al. 2014. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513: 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Oberle N, Krammer PH. 2012. Molecular mechanisms of Treg-mediated T cell suppression. Front Immunol 3: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Eriksson M, Shang MM, Weyd H, Tegner J. 2016. Comparative analysis of protocols to induce human CD4+Foxp3+ regulatory T cells by combinations of IL-2, TGF-β, retinoic acid, rapamycin and butyrate. PLoS ONE 11: e0148474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, et al. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedhom MA, Pichery M, Murdoch JR, Foligne B, Ortega N, Normand S, Mertz K, Sanmugalingam D, Brault L, Grandjean T, et al. 2013. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut 62: 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. 2005. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med 201: 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM, Thornton AM. 2014. tTregs, pTregs, and iTregs: Similarities and differences. Immunol Rev 259: 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetta F, Chiali A, Cordier C, Urrutia A, Girault I, Bloquet S, Tanchot C, Bourgeois C. 2010. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol 40: 2528–2538. [DOI] [PubMed] [Google Scholar]
- Simonetta F, Gestermann N, Martinet KZ, Boniotto M, Tissieres P, Seddon B, Bourgeois C. 2012. Interleukin-7 influences FOXP3+CD4+ regulatory T cells peripheral homeostasis. PLoS ONE 7: e36596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetta F, Gestermann N, Bloquet S, Bourgeois C. 2014. Interleukin-7 optimizes FOXP3+CD4+ regulatory T cells reactivity to interleukin-2 by modulating CD25 expression. PLoS ONE 9: e113314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber DJ, Debler EW, Horton PA, Smith KA, Wilson IA. 2006. Crystal structure of the IL-2 signaling complex: Paradigm for a heterotrimeric cytokine receptor. Proc Natl Acad Sci 103: 2788–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. 1995. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268: 1472–1476. [DOI] [PubMed] [Google Scholar]
- Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, Guinter T, McCaughtry T, Etzensperger R, Feigenbaum L, et al. 2013. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity 38: 1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T, Atarashi K, Honda K. 2016. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol 16: 295–309. [DOI] [PubMed] [Google Scholar]
- Todd JA, Evangelou M, Cutler AJ, Pekalski ML, Walker NM, Stevens HE, Porter L, Smyth DJ, Rainbow DB, Ferreira RC, et al. 2016. Regulatory T cell responses in participants with Type 1 diabetes after a single dose of interleukin-2: A non-randomised, open label, adaptive dose-finding trial. PLoS Med 13: e1002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. 2008. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 9: 194–202. [DOI] [PubMed] [Google Scholar]
- Toomer KH, Yuan X, Yang J, Dee MJ, Yu A, Malek TR. 2016. Developmental progression and interrelationship of central and effector regulatory T cell subsets. J Immunol 196: 3665–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosiek MJ, Fiette L, El Daker S, Eberl G, Freitas AA. 2016. IL-15-dependent balance between Foxp3 and RORγt expression impacts inflammatory bowel disease. Nat Commun 7: 10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Sheppard D. 2014. TGF-β activation and function in immunity. Annu Rev Immunol 32: 51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno A, Ghosh A, Hung D, Li J, Jijon H. 2015. Th17 plasticity and its changes associated with inflammatory bowel disease. World J Gastroenterol 21: 12283–12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. 2008. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol 181: 3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, et al. 2015. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol 16: 276–285. [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med 181: 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, Bensinger SJ, Hancock WW, Turka LA. 2006. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest 116: 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. 1995. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3: 521–530. [DOI] [PubMed] [Google Scholar]
- Xiong J, Parker BL, Dalheimer SL, Yankee TM. 2013. Interleukin-7 supports survival of T-cell receptor-β-expressing CD4− CD8− double-negative thymocytes. Immunology 138: 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Kitani A, Strober W. 2010. Molecular mechanisms regulating TGF-β-induced Foxp3 expression. Mucosal Immunol 3: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Stephan S, Bluestone JA. 2013. Peripherally induced Tregs—Role in immune homeostasis and autoimmunity. Front Immunol 4: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Lin WW, Stunz LL, Bishop GA. 2014. The adaptor TRAF3 restrains the lineage determination of thymic regulatory T cells by modulating signaling via the receptor for IL-2. Nat Immunol 15: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Zhu L, Altman NH, Malek TR. 2009. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 30: 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, Malek TR. 2015. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in Type 1 diabetes. Diabetes 64: 2172–2183. [DOI] [PubMed] [Google Scholar]
- Yuan X, Cheng G, Malek TR. 2014. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol Rev 259: 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8: 591–599. [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. 2007. IL-2 is essential for TGF-β to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 178: 2018–2027. [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Horwitz DA. 2008. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-β are resistant to Th17 conversion by IL-6. J Immunol 180: 7112–7116. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. 2010. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463: 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. 2013. The biology of thymic stromal lymphopoietin (TSLP). Adv Pharmacol 66: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]