Abstract
Despite continuous deployment of new treatment strategies and agents over many decades, most disseminated cancers remain fatal. Cancer cells, through their access to the vast information of human genome, have a remarkable capacity to deploy adaptive strategies for even the most effective treatments. We note there are two critical steps in the clinical manifestation of treatment resistance. The first, which is widely investigated, requires deployment of a mechanism of resistance that usually involves increased expression of molecular machinery necessary to eliminate the cytotoxic effect of treatment. However, the emergence of a resistant phenotype is not in itself clinically significant. That is, resistant cells affect patient outcomes only when they form a sufficiently large population to allow tumor progression and treatment failure. Importantly, proliferation of the resistant phenotype is by no means certain and, in fact, depends on complex Darwinian dynamics governed by the costs and benefits of the resistance mechanisms in the context of the local environment and competing populations. Attempts to target molecular machinery of resistance have had little clinical success largely because of the diversity within the human genome—therapeutic interruption of one mechanism simply results in its replacement by an alternative. We explore an alternative strategy for overcoming treatment resistance that seeks to understand and exploit the critical evolutionary dynamics that govern proliferation of the resistant phenotypes. In general, this approach has shown that, although emergence of resistance mechanisms in cancer cells to every current therapy is inevitable, proliferation of the resistant phenotypes is not and can be delayed and even prevented with sufficient understanding of the underlying ecoevolutionary dynamics.
The dynamic cancer ecosystem, rich in temporal and spatial diversity in environmental conditions and heritable cell phenotypes, is extraordinarily robust to therapeutic perturbations. In part, this is caused by cellular diversity, as spatial heterogeneity in the genotypic and phenotypic properties of tumor cells inevitably alter their response to applied treatments. In part, this is also caused by temporal and spatial heterogeneity in the tumor environment, largely governed by variations in blood flow, which can alter both the delivery of a systemic agent and local concentrations of factors (e.g., oxygen) that may alter the efficacy of the treating agent. Finally, in part, the response and resistance of cancer cells is governed by their complex interactions with adjacent host cells, which can provide local sanctuaries that permit tumor cells to survive treatment that would ordinarily be lethal.
Despite the many advances in cancer therapy over the past few decades, evolution of resistance continues to limit their efficacy in reducing overall survival. Even after a response to therapy that is clinically complete, residual cancer cells adapt and the tumor almost inevitably returns. Although the evolution of resistance continues to be the central cause of death in most cancer patients, recent analysis found that evolutionary terms were included in fewer than one percent of manuscripts on cancer treatment outcomes; this has remained unchanged for 30 years.
In general, there are two approaches to investigating the dynamics of cancer cell resistance to therapy. The most common avenue of research focuses on identifying the specific molecular mechanisms of resistance. This is perhaps best illustrated in the extensive literature on membrane extrusion pumps—P-glycoproteins (PgP), for example—that use ATP to actively remove cytotoxic agents from the tumor cell cytoplasm and send them back into the environment. This approach has the advantage of identifying molecular mechanisms that can be targeted and, thus defeated. However, despite decades of research in well-recognized adaptive mechanism such as the multidrug resistance (MDR) genes, this approach has not led to clinically significant therapy. In large part, this is the result of the remarkable diversity of adaptive strategies available through the human genome. Thus, successful therapeutic intervention in one resistance mechanism simply selects for a second available strategy.
Importantly, however, we note that the expression of a resistance mechanism does not by any means insure that the resistant population will rapidly proliferate leading to tumor progression. Thus, although the mechanisms of adaptation to the toxic effects of therapy are molecular, the clinical relevance of resistance is entirely dependent on proliferation of resistant populations—a process ultimately governed by Darwinian dynamics based on the phenotypic cost and benefit of the molecular mechanisms of resistance in the context of the local environment and competing cellular populations.
Here, we review treatment strategies that explicitly incorporate evolutionary principles to limit proliferation of resistant and prolong time to progression. Nearly all conventional cancer therapies assume that the maximum patient benefit is achieved through killing the greatest possible number of tumor cells. Thus, cancer treatments are typically administered at the maximum tolerated dose (MTD). Indeed, this principle is so universally accepted that phase 1 trials—necessary for clinical translation of any cancer drug—seeks to define the MTD, which is then used in subsequent investigations. Although killing the maximum number of tumor cells with the greatest possible drug dose is intuitively appealing, we propose that it is usually evolutionarily unwise. This reflects the principle noted above—evolution of resistant molecular machinery is virtually impossible to evade but proliferation of resistant populations is dependent on complex Darwinian dynamics. In fact, by applying intense Darwinian selection for resistant clones and elimination of all competing population, MTD cancer therapy actually “accelerates” proliferation of resistant populations (also well-known as an evolutionary phenomenon termed “competitive release”) (Gatenby 2009; Renton et al. 2014). Thus, evolutionary responses of cancer cells to therapy becomes the proximate cause of death in most cancer patients and will most likely remain as such in want of basic changes in the tumor treatment paradigm.
The majority of conceptual models of tumor evolution during chemotherapy stress resistance acquired in a step-wise fashion through some mutation following the start of therapy (Anderson and Quaranta 2008). If resistance arises stochastically and before treatment there are no resistant cells present, then maximum dose-density therapy will reduce the number of cells that can acquire the mutation with the probability of resistance lessening. However, in most cases, the resistant cells or some level of resistance appears before chemotherapy starts (Easwaran et al. 2014).
The assumption that a “resistance mutation” is necessary for tumor adaptation underestimates the vast information content, including xenobiotic pathways, that is available to cancer cells within the normal human genome (Gatenby 2009). That is, many, and probably most, resistance strategies only require an increased expression of one or more normal genes (PgP; see Schneider et al. 1989; Fletcher et al. 2010; Wind and Holen 2011). Therefore, rather than an “all or none” phenomenon, resistance can be graded among cells in a population and, importantly, change in the same cell over time as it acclimatizes to environmental stresses. For example, PgP is a hypoxia inducible factor (HIF)1α client (Thews et al. 2011) and its expression often increases in hypoxic and acidic environments even without cytotoxins. In addition, numerous mechanisms of de novo therapy resistance have been identified. For example, in environmentally mediated drug resistance (EMDR), components of tumor mesenchyma protect cancer cells from what would otherwise be lethal concentrations of cytotoxic drugs (Meads et al. 2009; Moulder 2010). Similarly, tumor cells in regions of hypoxia may be protected as a result of increased expression of PgP, up-regulation survival pathways, increased mutagenesis, and decreased drug delivery.
EVOLUTIONARY DYNAMICS OF CANCER THERAPY
Cancers can be described as open complex adaptive systems. “Open” because they freely communicate with their surroundings, “complex” because they contain multiple components, and “adaptive” because each element can change over time and interact with other components in complicated, often nonlinear, ways. Based on their nonlinear dynamics, a critical attribute is that their dynamics can be nonintuitive and their response to a perturbation can yield unexpected and unintended consequences.
Traditional application of systemic therapy to cancer is largely based on an intuitively appealing premise because by killing the maximum number of cancer cells, patient benefit is achieved (Norton and Simon 1986; Rodrigues and de Arruda Mancera 2013). This assumption that more is better is so deeply ingrained, that the initial clinical trials (i.e., phase 1 trials), always seeks to establish the maximum tolerated dose for that drug. In other words, there is an implicit assumption that all cancer drugs must be administered at the maximum possible dose to find the MTD in the first phase. Generally, only out of concern for fatal toxicity was the maximum dose density of chemotherapy limited. An alternate approach is the “metronomic” strategy (Gnoni et al. 2015), which administers lower more frequent doses of therapy. This has been shown to be beneficial in lowering toxicity, while permitting higher total drug administration, and inhibiting angiogenesis by increasing tumor cell death. However, the intent of modern therapy remains inducing the greatest possible cell death whether administered through maximum or metronomic dosing.
Significant flaws in conventional assumptions emerge when cancer therapy is viewed as an evolutionary process (Axtell and Arends 1990; Gatenby 2009; Gatenby et al. 2009a; Silva et al. 2012; Renton et al. 2014; Jansen et al. 2015; Kam et al. 2015). To be clear, when it is possible to have curative therapy, then the treatment strategy must be designed with that result in mind. However, in a palliative clinical setting (in which metastatic cancer patients nearly always die of their disease), treatment for cure is not only futile, it is, in fact, evolutionarily unsound. In destroying the entire population of sensitive cells, maximum dose therapy imposes intense selection for resistant phenotypes and, by eliminating all potential competitors, their proliferation is maximized (a well-known evolutionary phenomenon termed “competitive release”) (Axtell and Arends 1990; Renton et al. 2014).
Interestingly, insight into these dynamics can be found in an unlikely source—pest and weed management (Oliveira et al. 2007; Neve et al. 2009). For decades, the application of high-dose pesticides was commonplace, but over time it became clear that this approach virtually never eradicated the pest and, in fact, promoted the rapid emergence of uncontrollable, resistant strains. Since 1968, the policy toward pest management by the U.S. Department of Agriculture (USDA) has been much more nuanced placing greater emphasis on the controlled application of pesticides. The goal is to prevent the emergence of pesticide resistance while maintaining a low and acceptable level of crop damage (Oliveira et al. 2007; Neve et al. 2009). Incorporation of temporal data sampling and Darwinian dynamics into the management of invasive species is now mandated by USDA policy. To assist agriculturalists in creating the best treatment strategies, computational models guide pest management, similar to those that we propose for adaptively managing advanced, incurable metastatic cancers.
APPLICATION OF EVOLUTIONARY PRINCIPLES TO CANCER THERAPY RESISTANCE
Evolutionary therapy usually zeroes in on the competition for space and resources among cancer populations, and in particular, Darwinian interactions between resistant and nonresistant populations. When a cancer therapy results in cell death, this imposes strong selection forces for adaptive strategies. The size and complexity of the human genome practically assures the presence of numerous potentially adaptive pathways in which to avoid cell death. Thus, although targeting combinations of pathways can control human immunodeficiency virus (HIV) with only nine protein-encoding genes, this strategy has not as yet proved successful in preventing the emergence of resistance in human cells.
If resistant populations cannot be prevented, then tumor control requires therapy designed to slow or stop the proliferation of resistance. Here, two basic fundamentals must be emphasized: first, the growth of the resistant population is subject to evolutionary forces and, therefore, can be controlled by altering its fitness or that of competing populations. Second, evolving populations can only adapt to local and current environmental selection forces; they can never anticipate the future. Importantly, because cancer therapists can anticipate the future and use the knowledge of these temporal dynamics to a key advantage by using evolution to inhibit the proliferation of resistant populations, they can allow treatment to change over time.
In evolutionary cancer treatment, a major component of Darwinian dynamics is the cost of resistance. To become resistant, typically through up-regulation of established molecular defense mechanisms, cancer cells must alter their observable properties. Expression, maintenance, and utilization of these molecular pathways require resources, which, in an environment of limited substrate, must be diverted from proliferation and invasion. These dynamics are perhaps most clear in up-regulation of xenobiotic pathways such as increased expression of PgP, also known as multidrug resistance 1 (MDR1) (Schneider et al. 1989; Fletcher et al. 2010; Wind and Holen 2011). PgP is a membrane transporter that effectively extrudes a large number of intracellular substrates, including chemotherapies and, consequently, minimizes the efficacy of these compounds. PgP and most other membrane pumps hydrolyze two ATP for every transported molecule. Indeed, in experimental studies this operation cost (as well as “capital cost” for synthesis and maintenance of the pumps) can approach 50% of the cell’s energy budget (Silva et al. 2012). In cell culture conditions with abundant resources, this may have minimum effect on cellular proliferation. However, under resource limitation (e.g., in vivo), cancer cells must trade-off resistance costs that permit survival with other essential functions such as proliferation, movement, and cell maintenance. This fitness cost can be inferred by the simple observation that the MDR phenotype is rare in pretherapy tumors and becomes common only following treatment (Wind and Holen 2011). Furthermore, the drug resistant phenotype is typically evolved through regular exposure to a cytotoxic drug and quickly lost when the drug is removed (Kam et al. 2015).
Thus, in the presence of a cytotoxic drug, survival benefits of resistance exceed the fixed and operating costs of the resistance mechanisms. But, in the absence of therapy, the cost of resistance becomes an ecological burden that can be exploited (Silva et al. 2015).
The ecological cost of resistance is most apparent in traditional chemotherapy in which the mechanisms of resistance and their associated “construction and operation” costs are fairly clear. This is not as apparent in targeted therapies. However, Chmielecki et al. (2011) have shown a cost of resistance to tyrosine kinase inhibitors (TKIs).
EXPLOITING THE COST OF RESISTANCE
Theodosius Dobzhansky famously stated “Nothing in biology makes sense except in the light of evolution” (Ayala and Dobzhansky 1977). Yet, the typical cancer therapy is administered without regard to the evolutionary consequences. Drugs, doses, and schedules follow a fixed protocol that changes only in the event of unacceptable toxicity or unequivocal evidence of cancer progression.
There are three crucial elements that make cancers and their treatment highly dynamic. First, the distribution and abundance of cancer clones. These can be viewed as coexisting cytotypes, with perhaps distinctive niches (Lloyd et al. 2016) that can change rapidly. Second, through feedbacks between cancer cell activities, the host and intratumoral properties, the tumor ecosystem can rapidly change in size, character, and spatial heterogeneity. Third, the heritable traits of some or all of the cancer cell types may change directly in response to therapy or in response to changes in the tumor environment. The first two dynamics describe the ecological dynamics of cancers and the tumor environment; the third describes the evolutionary dynamics of changing frequencies and heritable phenotypes of the cancer cytotypes themselves. For these reasons, cancers are highly dynamic systems with tremendous spatial and temporal heterogeneity that can rapidly change with perturbations such as applied therapy. Almost certainly, the tumor and its resident cancer-cell populations treated in the second cycle of chemotherapy are quite different that those treated in the first cycle. Indeed, any tumor that persists or recurs after therapy will differ radically from the tumor at diagnosis. The persistent or recurrent cancer cells will possess new phenotypes and genotypic profiles.
We propose that cancer therapy become as dynamic as the tumor system being treated. Ideally, treatment strategies should anticipate, corral, and stay ahead of the intratumoral evolution through strategic application of different drugs and drug doses that move beyond the traditional goal of maximal cell death. Maximum control of the tumor becomes the goal, in which the objective can include quality and longevity of life, cumulative dosing of therapies (radiation, chemo-agents, and/or immunotherapy), and the patient’s sense of well-being both during and in the absence of therapy.
ADAPTIVE THERAPY
One such approach is adaptive therapy (AT) (Gatenby 2009; Gatenby et al. 2009a). The premise of AT is similar to current pest management in that the time to progression of extant anticancer drugs can be revised if their administration is guided by Darwinian principles. This approach has a number of features that differ from conventional chemotherapy strategies. First, the goal of AT is maximizing progression-free survival and not abatement in cancer burden. Second, the amount of drug administered is the minimum necessary to maintain patient quality of life and tumor stability. Third, the drugs and dose schedules are not fixed but instead constantly adjusted to steer and exploit evolutionary dynamics and maintain a stable tumor.
Although there are a number of Darwinian dynamics that may be exploited during cancer therapy, the theory behind AT focuses on the phenotypic costs of the molecular mechanism(s) of resistance. Extant or potential cancer phenotypes within the ecological context of the tumor reside on an adaptive landscape describing fitness as a function of a cancer cell’s phenotype. It is dependent on the fitness of that phenotype compared with that of other existent populations. Note that the fitness of any phenotype depends on context. A phenotype that, for example, expressing the PgP membrane pump is fitter than nonexpressing cells in the presence of chemotherapy. However, in the absence of therapy, owing to the cost of the resistance mechanism, the fitness of the resistant cells will be lower than that of sensitive cells (Fig. 1). In this case, the success of a cancer cell depends on its ability to compete successfully with other cancer cells within the context of the presence or absence of therapy. To exploit this, AT administers limited therapy with the explicit goal of maintaining a stable population of treatment-sensitive cells (Fig. 2). Therapy is reduced or withheld once the tumor size is stabilized. Although this may allow for some tumor cell proliferation, at the expense of the resistant cells, the fitness advantage of sensitive cells will allow their population to grow. Repeated administrations of small doses of drugs are then used to reduce the tumor volume. The goal is to administer “much less than maximum” dose while shrinking or maintaining at an acceptable level the overall tumor burden and total population size of cancer cells.
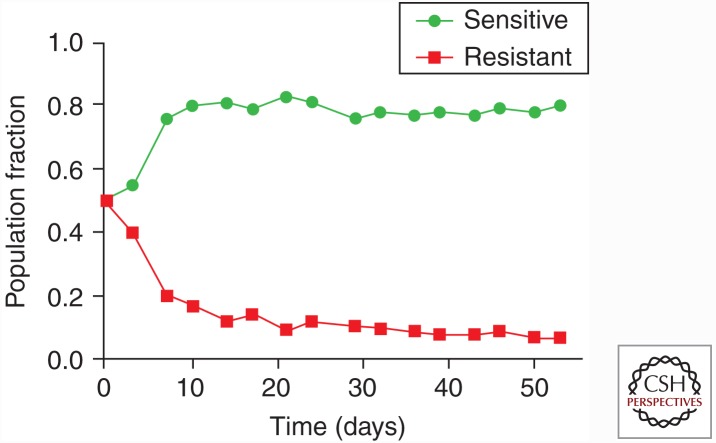
Figure 1.
Illustrating the evolutionary consequence of the cost of resistance. To mimic growth dynamics of resistant and sensitive cell populations, labeled MCF7 (sensitive) and MCF7/Dox (resistant) cells were cocultured in the absence or doxorubicin with physiological levels of glucose. The phenotypic cost of resistance decreased fitness of the resistant cells and allowed the sensitive population to proliferate at the expense of the resistant phenotype.
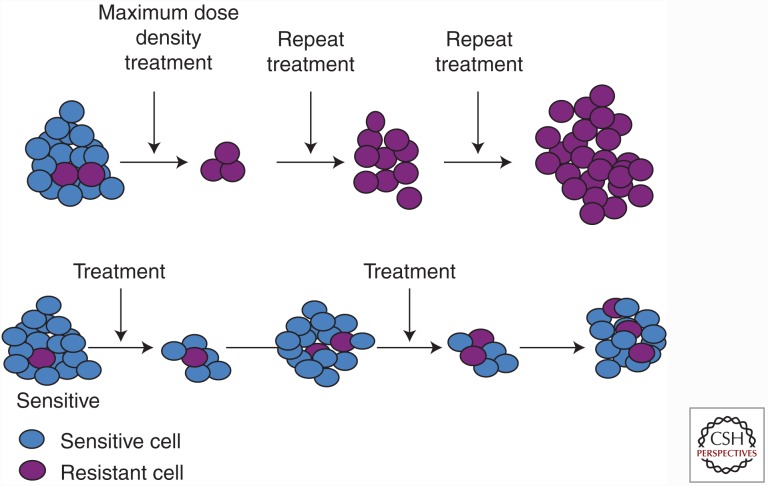
Figure 2.
Conventional, high dose-density treatment compared to an evolution-based strategy. (Top row) Conventional high dose-density therapy explicitly aims to eliminate all cancer cells that are sensitive to therapy. However, this maximally selects for resistant phenotypes and eliminates competitors permitting rapid progression—an evolutionary dynamic termed “competitive release.” (Bottom row) Adaptive therapy (AT) explicitly aims to maintain a small population of cells that are sensitive to treatment. Although the resistant cells survive, the metabolic cost of the molecular machinery of resistance (Fig. 1) renders them less fit in the absence of therapy. Thus, when therapy is withdrawn, the tumor will regrow but the fitness advantage of the sensitive cells allows them to proliferate at the expense of the resistant population. At the end of each cycle, the tumor remains sensitive to therapy.
The success of AT is dependent on the cost of resistance and the competitive interaction between sensitive and resistant cancer cells. AT can be expected to be superior to maximum dose if three conditions are met. First, the therapy is highly effective against sensitive cancer cells. Second, sensitive cells outcompete resistant cells in the absence of therapy. Third, sensitive cells in the absence of therapy can proliferate faster than resistant cells can in the presence of therapy. This leads to either persistent or declining cycles of total tumor burden. Initial therapy rapidly drops the population size of sensitive cells. This results in the evolution and increase of resistant populations of cancer cells. Therapy is then stopped before the resistant cells completely replacing the sensitive cells. At this point, the resistant cells by virtue of their faster proliferation rates and greater competitive ability will both increase and suppress the resistant cells. Before the sensitive cells returning to threatening population sizes, therapy must be resumed. This sequence represents a complete cycle. Such cycling works because of the therapy can decisively crash the sensitive cells, and the recovery of the sensitive cells provide the therapy for the resistant cells. In practice, effective AT will require tailoring the therapy protocol to the nature of the interventions, the characteristics of the particular cancer, the nature and cost of resistance, useful metrics of tumor burden and composition, and associated mathematical model to integrate tumor metrics to determine and manage optimal timing of therapy dosing.
The AT hypothesis was initially framed using catastrophe theory (Gatenby and Frieden 2008). For any drug or drug combination, it can be shown that the probability of eradicating all of the tumor cells is maximized when phenotypes and environments are homogeneous. In this mathematical model, intratumoral heterogeneity results in phase differences between therapy and tumor that permits survival. The biological interpretation of “phase difference” is therapy that is unsuccessful because of the pretreatment presence of resistant phenotypes or environments that are relatively sheltered from the toxic effects of therapy and permit rapid evolution and proliferation of cancer cells with resistant strategies. It is not unsuccessful because of the evolution of resistance during therapy. Showing the feasibility of this theoretical model, a preclinical model of ovarian cancer (OVCAR-3 cells) was developed and treated with carboplatin. Control, standard therapy (60 mg/kg q4 days x 3), and AT groups were established. The AT algorithm was based solely on tumor volume. Following the initial carboplatin dose of 50 mg/kg, tumor volumes were measured every three days. Subsequent doses of carboplatin were then adjusted to maintain stable tumor volume. For example, if the tumor volume increased in size for two consecutive measurements, the dose of carboplatin would be increased. Similarly, doses would be decreased when tumor size declined. Compared with control and standard therapy, this AT resulted in prolonged tumor control and improved survival of mice (Gatenby et al. 2009a).
In these and in more recent studies using breast cancer cell lines, an unexpected observation is a biphasic pattern in tumor response to AT was noted. That is, when treatment is initially applied, the tumors are typically growing exponentially. Forcing the growth curve to plateau required the full treatment dose. However, once the tumor volume stabilized the amount of drug necessary to maintain stability diminished rapid. In the above experiment, prolonged tumor control was often maintained using 5 mg/kg carboplatin. Of note, the computational models did not predict this phenomenon and it remains under investigation, but may be caused by tumor vascularity. Over time, it appeared that the tumor’s blood system equilibrated on a more normal vascularity than the greatly dysregulated angiogenesis seen in the tumors of the control and standard of care mice. This observation points to the need to model and appreciate all three dynamics: cancer cell population size and evolution, tumor size and heterogeneity, and cancer cell–tumor ecosystem feedbacks.
INCREASING THE COST OF RESISTANCE—ERSATZDROGES
As mentioned previously, membrane extrusion pumps are a common mechanism of cancer cell resistance to chemotherapy. Targeting these pumps with treatments that reduces their effectiveness has been explored extensively but has not resulted in any clinically effective strategies to prolong treatment response (Gatenby et al. 2009b). As noted above, an alternative approach exploits the evolutionary consequences of the metabolic capital and operation costs for maintenance of the pumps. For example, Broxterman et al. (1988) showed that the extrusion activity of PgP pumps activated by verapamil could consume cell’s ATP budget by 50%. Importantly, in the intratumoral environment of limited resources, this increased ATP demand requires diversion of substrate from other activities including proliferation and invasion. Thus, an alternative treatment strategy to blocking pump function, seeks to exhaust the cancer cell’s resources by maximizing pump activity through administration of nontoxic (or minimally toxic) substrate (Kam et al. 2015). Thus, by forcing cells to expend energy to extrude a “fake drug” (hence the name ersatzdroges) in the absence of chemotherapy, this strategy increases the phenotypic cost of the cells’ resistance strategy.
Currently available ersatzdroges, including antibiotics and verapamil (a calcium channel blocker prescribed to treat arrhythmia), are used in the treatment of other diseases. Thus, when the tumor cell detects the presence of these “fake” drugs, it uses its resistant mechanisms such as the MDR1 system to pump the drug from the cytosol.
Exposure to verapamil in a preclinical model of breast cancer dramatically altered the energy dynamics with cancer cells and reduced both proliferation and invasion. In vivo and in vitro, administration of various ersatzdroges significantly increased glucose flux in tumors and reduced tumor growth (Kam et al. 2015). The use of the esatzdroges as chaff exploits ecological and evolutionary dynamics. The resistant cells waste energy bailing the ersatzdroges. The cell becomes less competitive relative to other cells with fewer pumps. And, evolution should now disfavor the membrane pumps or select for fewer pumps thus maintaining original drug efficacy. This represents a form of evolutionary therapy known as a “double bind therapy” (Gatenby et al. 2009b). A double bind therapy uses the cancer’s adaptive response to one therapy (in this case a cytotoxin) to make another more effective (forced bailing of the ersatzdroges) and vice-versa.
TURNING THE TABLES: TARGETING THE ADAPTIVE STRATEGIES
In general, the application of evolutionary strategies to optimize tumor therapy requires “temporal” thinking. As shown in AT, the therapist must look beyond the immediate effects of treatment (i.e., tumor cell reduction). It requires anticipating and exploiting the longer-term ecological and evolutionary dynamics as new phenotypic properties arise and increase among the cancer cells. Thinking ahead may identify the opportunities for double bind therapies (Fig. 1) (Gatenby et al. 2009b) or “sucker’s gambits” (Maley et al. 2004). How often can one find a first line treatment to induce a phenotypic adaptation that is then more highly susceptible to an especially chosen second line therapy?
A double-bind strategy was successfully applied to antibiotic treatment of Heliobacter pylori (Fuentes-Hernandez et al. 2015). In cancer treatment, Antonia et al. (2006) examined the efficacy of a p53 vaccine in patients (n = 29) with small cell lung cancer. Most patients elicited immune responses but only one partial response was observed. However, when the patients subsequently underwent chemotherapy, a response rate of 67% was observed (compared with the historic response rate of <5%); efficacy increased in those patients that had the greatest immune activation (Chiappori et al. 2010). We interpret this as an example of an evolutionary double bind in which the tumor cells’ adaptive strategy to the immunotherapy rendered them more vulnerable to cytotoxic drugs.
Finally, the ideal double bind therapy creates an evolutionarily futile cycle in which cyclical application of the treatments matches precisely the pattern of evolution of resistance in the underlying cancer populations. In our clinical example, it is possible that this evolutionary cycle could have been completed by revaccinating the patients after chemotherapy, which may have selected for increased p53 expression.
EXPLOITING PROPERTIES OF COMPLEX DYNAMIC SYSTEMS FOR CANCER CONTROL
All current cancer therapies act as biocides with various mechanisms for killing cancer cells. This is certainly reasonable but also inevitably produces Darwinian forces that promote resistance. An alternative approach targets environmental selection forces to alter the underlying evolutionary dynamics with the explicit goal of promoting a less proliferative or invasive tumor phenotype. In general, cancers can be viewed as open complex dynamical systems: “complex” because the tumor has many components, “dynamic” because the components interact and change over time (often nonlinearly), and “open” because it must freely interact with the host (for instance, all cancer cells derive their nutrients from the host). Traditional analysis of such systems emphasizes the challenge in predicting outcomes because of their nonlinear dynamics and sensitivity to initial conditions and/or slight perturbations of current conditions (weather is probably the most familiar complex dynamic system). For example, the famous “butterfly effect” posits an insect flapping its wings in Asia can cause a tornado in North America.
Importantly, however, it has been noted that this established pattern of complex systems to magnify some small perturbations can be exploited to steer the system along a desired course with minimal application of force (Wang et al. 2012). This, of course, requires a modest understanding of the underlying intratumoral dynamics, but it does not require a full understanding. One has a sufficient level of understanding when interventions suggest themselves that perturb the tumor environment in ways that dissipate the cancer system or at least coax it along a less clinically aggressive path. What small, yet decisive, biological forces are available? Perturbing pH may prove useful. Most tumors are net-producers of acid and intratumoral acidosis has been shown to select for phenotypes that are highly motile and invasive. However, small perturbations of the extracellular pH (an increase of ∼0.2 pH units) can alter these Darwinian dynamics and select for less aggressive, more indolent phenotypes (Ibrahim-Hashim et al. 2012).
CLINICAL APPLICATIONS
Mastering complex systems (as in weather forecasting) (Wang et al. 2012) require three components: (1) defining and mathematically framing first principles; (2) necessary and sufficient data to parameterize mathematical models; and (3) sophisticated computational methods.
We propose that evolutionary and ecological dynamics serve as first principles in cancer therapy and that computational models can be readily constructed (e.g., computer models for pest management are widely available). The absence of usable data is the greatest stumbling block to the clinical application of evolutionary principles to cancer therapy. Although concerns for dealing with “big data” from molecular analysis are often expressed in oncology, these data lack spatial and temporal resolution and therefore have limited value in evolutionary models. In fact, good time-series data in oncology is largely limited to serum markers and repeated cross-sectional imaging. There is an urgent need to develop methods that can use sparse data for computational models and extract more information from available clinical data (e.g., radiomic analysis of computed tomography [CT] and magnetic resonance imaging [MRI] images [Gatenby et al. 2013]).
Limitations withstanding, some clinical trials that illustrate successful application of evolutionary dynamics have been and are being performed (Schweizer et al. 2015). We suggest that the applications of evolutionary concepts and therapies can allow us make quantum strides by simply changing our use of existing therapies, patient and tumor metrics, and data analysis and modeling. Such results may be possible at a fraction of the cost of novel drug discovery and development.
SUMMARY AND FUTURE DIRECTIONS
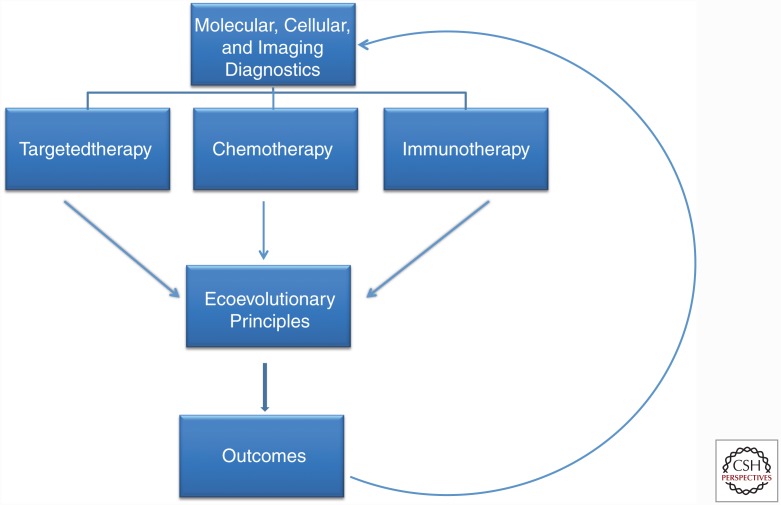
Cancers are complex, dynamic systems that begin to evolve resistance strategies immediately on the application of any therapy. Current cancer treatment is typically applied in a static fashion: the same drugs, doses, and schedules are administered until the protocol ends or the tumor progresses. When the cancer cells of a tumor respond to treatment, the typical strategy is to simply give more of the same. In contrast, evolution-based therapy seeks to become as adaptive and flexible as the tumor populations being treated. This will require a new paradigm in cancer therapy in which Darwinian dynamics are explicitly incorporated into trial design and execution (Fig. 3). In AT, for example, once the tumor response to a specific treatment is observed, the best course might be to switch to a new strategy or withdraw therapy because ongoing treatment with the successful drug will only result in greater selection for resistance.
Figure 3.
Current “personalized medicine” paradigms almost exclusive focus on defining predictive biomarkers that can identify effective treatments. This approach, however, fails to recognize that even highly effective therapies are almost always defeated by evolution of resistance. Here, we propose that “precision medicine” in cancer care requires both identification of optimal treatment modality and understanding of the Darwinian dynamics that govern response and resistance to therapy and, thus, ultimate patient outcomes.
In preclinical experiments (Gatenby et al. 2009a; Silva et al. 2012; Kam et al. 2015), we have shown that applying evolutionary principles to conventional chemotherapy agents can substantially prolong progression-free survival in both breast and ovarian cancer. Schweizer et al. (2015) recently showed that evolutionary principles could be used to prolong response to anti-androgen therapy in a cohort of men with castrate-resistant prostate cancer. A clinical trial using an adaptive-therapy algorithm for abiraterone therapy in men with castrate-resistant prostate cancer has recently opened and appears superior to continuous therapy.
There are difficulties in clinical application. These include the requirement to collect reliable time-series data allowing the internal evolutionary dynamics of the cancer to be observed, measured, and estimated. In fact, repeated measures of changes in tumors over time and space are typically quite sparse, largely limited to serum markers and clinical imaging. Future directions must focus on converting clinical data into a dynamical understanding of the complex environmental and phenotypic changes that drives tumor evolution in response to therapy. Ultimately, this understanding will likely require sophisticated patient-specific computational models to provide treating physicians with decision support tools to optimize cancer therapy.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health (NIH) Grants U54CA143970-1 and RO1CA170595 and a grant from the James S. McDonnell Foundation.
Footnotes
Editors: Charles Swanton, Alberto Bardelli, Kornelia Polyak, Sohrab Shah, and Trevor A. Graham
Additional Perspectives on Cancer Evolution available at www.perspectivesinmedicine.org
REFERENCES
- Anderson AR, Quaranta V. 2008. Integrative mathematical oncology. Nat Rev Cancer 8: 227–234. [DOI] [PubMed] [Google Scholar]
- Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, et al. 2006. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 12: 878–887. [DOI] [PubMed] [Google Scholar]
- Axtell RC, Arends JJ. 1990. Ecology and management of arthropod pests of poultry. Annu Rev Entomol 35: 101–126. [DOI] [PubMed] [Google Scholar]
- Ayala FJ, Dobzhansky T. 1977. “Nothing in biology makes sense except in the light of evolution”: Theodosius Dobzhansky: 1900–1975. J Hered 68: 3–10. [DOI] [PubMed] [Google Scholar]
- Broxterman HJ, Pinedo HM, Kuiper CM, Kaptein LC, Schuurhuis GJ, Lankelma J. 1988. Induction by verapamil of a rapid increase in ATP consumption in multidrug-resistant tumor cells. FASEB J 2: 2278–2282. [DOI] [PubMed] [Google Scholar]
- Chiappori AA, Soliman H, Janssen WE, Antonia SJ, Gabrilovich DI. 2010. INGN-225: A dendritic cell-based p53 vaccine (Ad.p53-DC) in small cell lung cancer: Observed association between immune response and enhanced chemotherapy effect. Expert Opin Biol Ther 10: 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, Wang L, Amato KR, Arcila M, Sos ML, et al. 2011. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med 3: 90ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwaran H, Tsai HC, Baylin SB. 2014. Cancer epigenetics: Tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell 54: 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JI, Haber M, Henderson MJ, Norris MD. 2010. ABC transporters in cancer: More than just drug efflux pumps. Nat Rev Cancer 10: 147–156. [DOI] [PubMed] [Google Scholar]
- Fuentes-Hernandez A, Plucain J, Gori F, Pena-Miller R, Reding C, Jansen G, Schulenburg H, Gudelj I, Beardmore R. 2015. Using a sequential regimen to eliminate bacteria at sublethal antibiotic dosages. PLoS Biol 13: e1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA. 2009. A change of strategy in the war on cancer. Nature 459: 508–509. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Frieden BR. 2008. Inducing catastrophe in malignant growth. Math Med Biol 25: 267–283. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Silva AS, Gillies RJ, Frieden BR. 2009a. Adaptive therapy. Cancer Res 69: 4894–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Brown J, Vincent T. 2009b. Lessons from applied ecology: Cancer control using an evolutionary double bind. Cancer Res 69: 7499–7502. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Grove O, Gillies RJ. 2013. Quantitative imaging in cancer evolution and ecology. Radiology 269: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnoni A, Silvestris N, Licchetta A, Santini D, Scartozzi M, Ria R, Pisconti S, Petrelli F, Vacca A, Lorusso V. 2015. Metronomic chemotherapy from rationale to clinical studies: A dream or reality? Crit Rev Oncol Hematol 95: 46–61. [DOI] [PubMed] [Google Scholar]
- Ibrahim-Hashim A, Cornnell HH, Abrahams D, Lloyd M, Bui M, Gillies RJ, Gatenby RA. 2012. Systemic buffers inhibit carcinogenesis in TRAMP mice. J Urol 188: 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G, Gatenby R, Aktipis CA. 2015. Opinion: Control vs. eradication: Applying infectious disease treatment strategies to cancer. Proc Natl Acad Sci 112: 937–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam Y, Das T, Tian H, Foroutan P, Ruiz E, Martinez G, Minton S, Gillies RJ, Gatenby RA. 2015. Sweat but no gain: Inhibiting proliferation of multidrug resistant cancer cells with “ersatzdroges.” Int J Cancer 136: E188–E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd MC, Cunningham JJ, Bui MM, Gillies RJ, Brown JS, Gatenby RA. 2016. Darwinian dynamics of intratumoral heterogeneity: Not solely random mutations but also variable environmental selection forces. Cancer Res 76: 3136–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley CC, Reid BJ, Forrest S. 2004. Cancer prevention strategies that address the evolutionary dynamics of neoplastic cells: Simulating benign cell boosters and selection for chemosensitivity. Cancer Epidemiol Biomarkers Prev 13: 1375–1384. [PubMed] [Google Scholar]
- Meads MB, Gatenby RA, Dalton WS. 2009. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat Rev Cancer 9: 665–674. [DOI] [PubMed] [Google Scholar]
- Moulder S. 2010. Intrinsic resistance to chemotherapy in breast cancer. Womens Health 6: 821–830. [DOI] [PubMed] [Google Scholar]
- Neve P, Vila-Aiub M, Roux F. 2009. Evolutionary-thinking in agricultural weed management. New Phytologist 184: 783–793. [DOI] [PubMed] [Google Scholar]
- Norton L, Simon R. 1986. The Norton-Simon hypothesis revisited. Cancer Treat Rep 70: 163–169. [PubMed] [Google Scholar]
- Oliveira EE, Guedes RNC, Totola MR, De Marco P Jr. 2007. Competition between insecticide-susceptible and -resistant populations of the maize weevil, Sitophilus zeamais. Chemosphere 69: 17–24. [DOI] [PubMed] [Google Scholar]
- Renton M, Busi R, Neve P, Thornby D, Vila-Aiub M. 2014. Herbicide resistance modelling: Past, present and future. Pest Manag Sci 70: 1394–1404. [DOI] [PubMed] [Google Scholar]
- Rodrigues DS, de Arruda Mancera PF. 2013. Mathematical analysis and simulations involving chemotherapy and surgery on large human tumours under a suitable cell-kill functional response. Math Biosci Eng 10: 221–234. [DOI] [PubMed] [Google Scholar]
- Schneider J, Bak M, Efferth T, Kaufmann M, Mattern J, Volm M. 1989. P-glycoprotein expression in treated and untreated human breast cancer. Br J Cancer 60: 815–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer MT, Antonarakis ES, Wang H, Ajiboye AS, Spitz A, Cao H, Luo J, Haffner MC, Yegnasubramanian S, Carducci MA, et al. 2015. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: Results from a pilot clinical study. Sci Transl Med 7: 269ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AS, Kam Y, Khin ZP, Minton SE, Gillies RJ, Gatenby RA. 2012. Evolutionary approaches to prolong progression-free survival in breast cancer. Cancer Res 72: 6362–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R, Vilas-Boas V, Carmo H, Dinis-Oliveira RJ, Carvalho F, de Lourdes Bastos M, Remião F. 2015. Modulation of P-glycoprotein efflux pump: Induction and activation as a therapeutic strategy. Pharmacol Ther 149: 1–123. [DOI] [PubMed] [Google Scholar]
- Thews O, Nowak M, Sauvant C, Gekle M. 2011. Hypoxia-induced extracellular acidosis increases p-glycoprotein activity and chemoresistance in tumors in vivo via p38 signaling pathway. Adv Exp Med Biol 701: 115–122. [DOI] [PubMed] [Google Scholar]
- Wang WX, Ni X, Lai YC, Grebogi C. 2012. Optimizing controllability of complex networks by minimum structural perturbations. Phys Rev E 85: 026115. [DOI] [PubMed] [Google Scholar]
- Wind NS, Holen I. 2011. Multidrug resistance in breast cancer: From in vitro models to clinical studies. Int J Breast Cancer 2011: 967419. [DOI] [PMC free article] [PubMed] [Google Scholar]