This Outlook by Murre discusses two new studies in this issue of Genes & Development by Miyai et al. and Li et al. These studies provide new and unprecedented insights into the genetic and epigenetic mechanisms that establish B-cell identity.
Keywords: B-cell programming, B-cell differentiation, epigenetics, chromatin, transcription factor, EBF1, Pax5, IRF4
Abstract
Earlier studies have identified transcription factors that specify B-cell fate, but the underlying mechanisms remain to be revealed. Two new studies by Miyai and colleagues (pp. 112–126) and Li and colleagues (pp. 96–111) in this issue of Genes & Development provide new and unprecedented insights into the genetic and epigenetic mechanisms that establish B-cell identity.
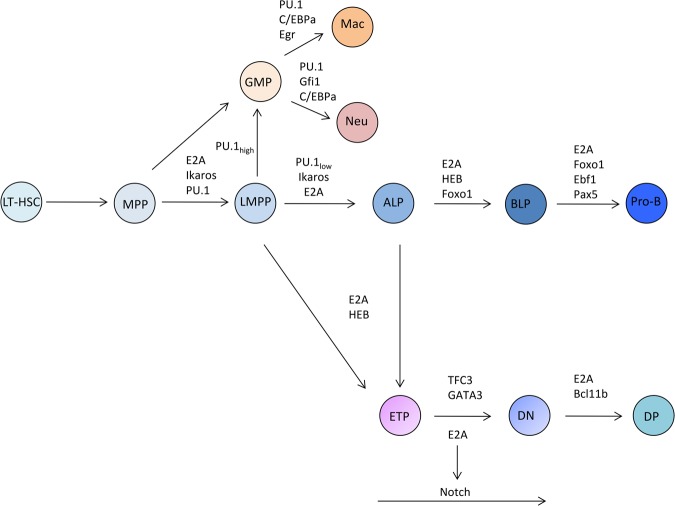
Immune cells originate from self-renewing long-term hematopoietic stem cells (LT-HSCs) through many intermediate steps. Specifically, LT-HSCs give rise to multipotent progenitors (MPPs) and lymphoid-primed multipotent progenitors (LMPPs) that lack self-renewal capacity (Fig. 1). LMPPs have the ability to differentiate into either common lymphoid progenitors (CLPs), granulocyte/macrophage progenitors (GMPs), or early T-cell progenitors (ETPs). The CLP compartment consists of all lymphoid progenitors (ALPs) and B-cell-biased lymphoid progenitors (BLPs). ALPs have the ability to give rise to B- and T-lineage cells, natural killer cells, and lymphoid dendritic cells. BLPs differentiate predominantly into B-lineage cells (Fig. 1).
Figure 1.
Schematic diagram depicting the roles of transcriptional regulators during hematopoiesis. Distinct developmental stages are indicated. Transcription factors associated with developmental checkpoints are shown. (Mac) Macrophages; (Neu) neutrophils; (DN) CD4−CD8− thymocytes; (DP) CD4+CD8+ thymocytes. LT-HSCs, MPPs, LMPPs, ALPs, and BLPs are described in the text.
The developmental progression of hematopoietic progenitor cells is initiated by priming enhancer repertoires to facilitate transcription factor occupancy, dictate enhancer–promoter communication, activate lineage-specific programs of gene expression, and suppress the expression of genes associated with alternative cell lineages. Each of these processes is controlled by a series of now well-characterized transcription factors. In B-cell progenitors, the prominent factors are the E proteins, EBF1, FOXO1, Ikaros, and PAX5 (Fig. 1; Bain and Murre 1998; Singh et al. 2007; Georgopoulos 2017). They act in a hierarchical as well as combinatorial manner. In BLPs, E2A and HEB activate the expression of FOXO1. Once expressed, FOXO1 acts in concert with E2A to induce the expression of EBF1 (Zandi et al. 2008; Mercer et al. 2011). Through a positive intergenic feedback circuitry, EBF1 and FOXO1 activate a B-lineage-specific program of gene expression. Finally, PAX5 acts at multiple levels to commit cells to the B-cell fate (Medvedovic et al. 2011).
These earlier studies provided a molecular framework that underpins early B-cell development. However, since the progenitor populations are rare and quite heterogeneous, a detailed understanding of how gene expression/repression patterns and epigenetic marking are established and change in developing B-cell progenitors is mostly lacking. Miyai et al. (2018) have addressed this issue by engineering an elegant novel culture system. Specifically, the investigators established a long-term culture of B-cell progenitors by conditionally inducing the expression of the antagonist helix–loop–helix protein Id3. This was accomplished by transducing progenitor cells with a retroviral vector expressing an Id3-ER fusion protein in the presence of tamoxifen. Notably, repression of E-protein activity in B-cell progenitor cells readily established a culture of cells that displayed long-term self-renewal potential but, upon withdrawal of tamoxifen, exhibited normal lineage developmental progression and restriction that allowed for a detailed temporal description of the key events that orchestrate the onset of B-cell development. The investigators found that the specifying B-cell fate is closely associated with three waves of distinct transcription signatures. Why does the activation of lineage-specific patterns of gene expression occur in three phases? The data suggest that the programming of B-cell development is ordered in terms of transcription signatures. Moreover, the sequential activation of gene expression is directly related to cell physiology. Specifically, the first wave of gene expression involves the suppression of enhancer repertoires associated with multipotency and the repression of enhancers or promoter regions associated with alternative cell lineages. The second wave involves the activation of genes associated with cell cycle progression and cellular metabolism. Finally, the third wave involves the establishment of a now well-established regulatory circuitry of factors that specify B-cell fate, such as E2A, FOXO1, EBF1, and PAX5. This distinct pattern of waves of transcription signatures in lymphoid progenitors is not unique to the B-cell lineage. The onset of Th17 cell differentiation is also associated with waves of transcription signatures that are characterized by unique physiological activities (Yosef et al. 2013). Thus, it may very well be that multiple phases of gene expression patterns associated with early B-cell development reflect a general feature of differentiating immune cells.
Li et al. (2018) also apply an inducible model of early B-cell development but targeting EBF1 rather than the E proteins. This study aimed to define the hierarchy of transcriptional and epigenetic events that underpin EBF1-mediated B-cell programming. Time-resolved and global analysis of EBF1-bound sites, DNA methylation, chromatin accessibility, and transcription signatures revealed distinct phases of gene expression during EBF1-mediated B-cell programming that correlate well with alterations in the epigenetic landscape analogous to that described by Miyai et al. (2018). The data provide strong support for a “pioneering activity” of EBF1, since most of the EBF1-bound sites were not associated with nucleosome eviction or active epigenetic marks. The pioneering DNA-binding activities of EBF1, however, were restricted to a subset of cognate binding sites, since EBF1 was not able to associate with potential binding sites across the genomes of nonhematopoietic cells. Thus, transcription factors can perform a pioneering function only when expressed in the appropriate cell context. This raises the question of how cells enforce such rules. One possibility is that subsets of transcription factor-binding sites are not accessible in distinct cell lineages, since they are sequestered away in transcriptionally repressive or inert environments such as heterochromatic regions or nuclear structures such as polycomb bodies. Our insights into mechanisms that mediate transcriptional repression remain rudimentary and need to be addressed in much more detail. The data also bring into question how EBF1 activity leads to changes in chromatin accessibility. As pointed out by the investigators, this most likely involves members of the SWI–SNF chromatin remodeling complex (Maier et al. 2004; Li et al. 2018). Initially EBF1 as a pioneer would find its cognate binding sites across a B-lineage-specific enhancer repertoire and promote a change in histone modifications, possibly involving CBP/p300. This would be followed by recruitment of BRG1-containing chromatin remodelers, leading to the depletion of nucleosomes in nearby regions to permit binding of additional regulators, ultimately allowing for full activation of nearby gene expression. Another interesting feature of the study by Li et al. (2018) is the notion that EBF1 transiently binds to a subset of sites associated with genes whose expression declines in maturing B cells. What is the role of transient EBF1 occupancy as it relates to the suppression of programs of gene expression associated with alternative cell lineages? The data suggest that EBF1 acts selectively at an ensemble of sites to facilitate occupancy of transcriptional repressors such as PAX5 to suppress gene expression (Medvedovic et al. 2011). Alternatively, EBF1 may act to modulate nuclear positioning, plausibly involving the activation of noncoding transcription.
In summary, these elegant studies provide new insights into the mechanisms that orchestrate sequential patterns of gene expression to establish B-cell identity. As always, the findings raise many new questions and directions. For example, how do the various transcription factors compete and cooperate in time to generate lineage-restricted lymphocytes? How is transient transcription factor occupancy associated with the suppression of transcription associated with alternative cell lineages? How rigid is the program as it relates to temporal and quantitative levels of expression? The developmental systems engineered and described in these two studies will help to reveal how a complex interplay of transcriptional regulators activates sequential programs of gene expression and may serve as a paradigm for the developmental progression of cells across the animal and plant kingdoms.
Acknowledgments
Research in the Murre laboratory is supported by the National Institutes of Health 1P01AI102853-01 and 1RO1 AI109599-27.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.311357.118.
References
- Bain G, Murre C. 1998. The role of E-proteins in B and T-lymphocyte development. Semin Immunol 10: 143–153. [DOI] [PubMed] [Google Scholar]
- Georgoulos K. 2017. The making of a lymphocyte: the choice among disparate cell fates and the Ikaros enigma. Genes Dev 13: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Cauchy P, Ramamoorthy S, Boller S, Chavez L, Grosschedl R. 2018. Dynamic EBF1 occupancy directs sequential epigenetic and transcriptional events in B-cell programming. Genes Dev (this issue) 10.1101/gad.309583.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier H, Ostraat R, Gao H, Fields S, Shinton SA, Medina KL, Ikawa T, Murre C, Singh H, Hardy RR, et al. 2004. Early B cell factor cooperates with Runx1 and mediates epigenetic changes associated with mb-1 transcription. Nat Immunol 5: 1069–1077. [DOI] [PubMed] [Google Scholar]
- Medvedovic J, Ebert A, Tagoh H, Busslinger M. 2011. Pax5: a master regulator of B cell development and leukemogenesis. Adv Immunol 111: 179–206. [DOI] [PubMed] [Google Scholar]
- Mercer EM, Lin YC, Jhunjhunwala S, Dutkowski J, Flores M, Sigvardsson M, Ideker T, Glass CK, Murre C. 2011. Multilineage priming of enhancer repertoires precedes commitment to the B and myeloid cell lineage in hematopoietic progenitors. Immunity 35: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai T, Takano J, Endo TA, Kawakami E, Agata Y, Motomura Y, Kubo M, Kashima Y, Suzuki Y, Kawamoto H, et al. 2018. Three-step transcriptional priming that drives the commitment of multipotent progenitors towards B cells. Genes Dev (this issue) 10.1101/gad.309575.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Pongubala JM, Medina KL. 2007. Gene regulatory networks that orchestrate the development of B lymphocyte precursors. Adv Exp Med Biol 596: 57–62. [DOI] [PubMed] [Google Scholar]
- Yosef N, Shalek AK, Gaublomme JT, Jin H, Lee Y, Awasthi A, Wu C, Karwacz K, Xiao S, Jorgolli M, et al. 2013. Dynamic regulatory network controlling TH17 cell differentiation. Nature 496: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi S, Mansson R, Tsapogas P, Zetterblad J, Bryder D, Sigvardsson M. 2008. EBF1 is essential for B-lineage priming and establishment of a transcription factor network in common lymphoid progenitors. J Immunol 181: 3364–3372. [DOI] [PubMed] [Google Scholar]