Summary
Astrocytes, the most populous glial cell type in the brain, are critical for regulating the brain microenvironment. In various neurodegenerative diseases, astrocytes determine the progression and outcome of the neuropathological process. We have recently revealed the direct involvement of mitochondrial function in human pluripotent stem cell (hiPSC)-derived dopaminergic (DA) neuronal differentiation. Using the astroglial-neuronal co-culture system, we show here that astrocytes effectively rescue defects in neurogenesis of DA neurons with mitochondrial respiratory chain disruption. Co-culture of astrocytes with defective DA neurons completely restored mitochondrial functions and dynamics insulted by mitochondrial toxins. These results suggest the significance of astroglia in maintaining mitochondrial development and bioenergetics during differentiation of hiPSC-derived DA neurons. Our study also provides an active astroglial-neuronal interaction model for future investigation of mitochondrial involvement in neurogenesis and neurodegenerative diseases.
Keywords: astrocytes, dopaminergic neurons, human pluripotent stem cells, mitochondrial dysfunctions
Highlights
-
•
Role of astrocyte on the development of hiPSC-derived dopaminergic neuron
-
•
Astrocyte protects dopaminergic neurogenesis by powering up mitochondrial respiration
-
•
Astrocyte restores mitochondrial function and dynamics in dopaminergic neuron
-
•
Evidence of astroglial and neuronal interaction during dopaminergic neurogenesis
In this article, Shirley ShiDu Yan and colleagues show that human pluripotent stem cell-derived astrocytes effectively rescue defects in neurogenesis of dopaminergic neurons with mitochondrial respiratory chain disruption. Co-culture with astrocytes restored mitochondrial functions and dynamics in dopaminergic neurons insulted by mitochondrial toxins. These results provide evidence of astroglia in maintaining mitochondrial development and bioenergetics during dopaminergic neuronal differentiation.
Introduction
Human pluripotent stem cells (hiPSCs) offer an attractive tool for modeling brain development and disease based on their ability to be directed and differentiated into human cerebral organoids and cells (Lancaster and Knoblich, 2014). Generated from hiPSCs, neural progenitor cells (NPCs) can be used to generate neuronal and glial cells (Maroof et al., 2013, Sofroniew and Vinters, 2010). Direction of these cells into specific cell types allows for identification of the underlying neuropathology and also presents the possibility of replacement therapy for neurodegenerative diseases such as Parkinson's disease (PD), which is caused by selective loss of dopaminergic (DA) neurons in the substantia nigra pars compacta in the midbrain (Kriks et al., 2011).
We have recently revealed (Fang et al., 2016a, Fang et al., 2016b) an active and pivotal role for mitochondria in midbrain DA and cortical neurogenesis. Induced defects in mitochondrial respiratory chains via application of a complex IV inhibitor, KCN (potassium cyanide), or complex I inhibitor, rotenone, restricted neurogenesis of DA neurons. Accumulating studies have demonstrated that astrocytes, the most populous glial cells and important coordinative partners to neighboring neuronal cells, are essential in DA neurogenesis in the subventricular zone of the lateral ventricles, a region for neurogenesis in the midbrain of adult brains, and disruption in astrocytic function promotes neurodegeneration (Lo, 2010). Here, we induced DA neuron differentiation with mature astrocyte co-culture, to mimic the in vivo neuronal-astroglial interaction environment. In the presence of astrocytes, NPCs were resistant to mitochondrial respiratory defects and exhibited normal differentiation into DA neurons. Importantly, using this dynamic astrocyte-neuron interaction model, we clearly display the essential role of mitochondria in DA neuronal differentiation and maturation. Furthermore, astrocytes actively modulate normal neuronal development by rescuing neuronal mitochondrial defects in the process of DA-directed differentiation. This human neuron-glia cross-talk model will facilitate evaluation of the role of astrocytes in modulating mitochondrial function in the pathogenesis of neurodegenerative diseases and identification of therapeutic applications in mitochondrial degeneration.
Results
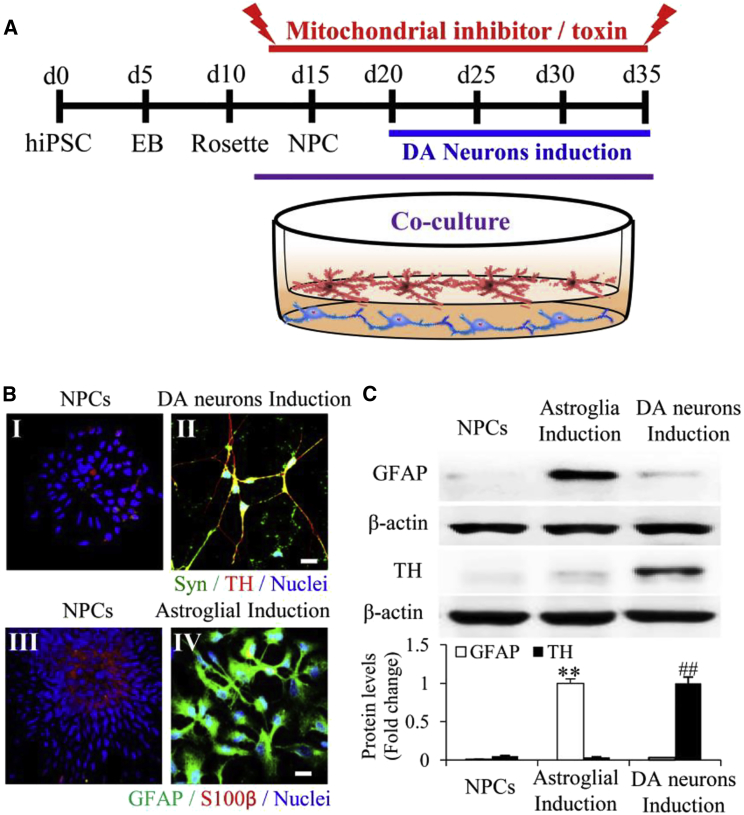
Efficient Differentiation of GFAP+ Mature Human Astrocytes from NPCs
We have previously shown that human DA neurons are derived from NPCs (Fang et al., 2016a). In this study, NPCs derived from BM2-3 hiPSCs were differentiated into astrocytes. Following the protocol shown in Figure S1A, S100β-positive cells were derived from astroglial progenitor cells after about 70 days induction with ciliary neurotrophic factor (CNTF) in astroglial medium (Figures S1B and S1C). Emergence of an astrocyte-like phenotype following 70 days induction was indicated by expression of the astrocyte markers S100β and GFAP (Figure S1A).
Populations of GFAP-positive astrocytes were robustly increased between the 3rd and the 9th month of differentiation by removing growth factors epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF2) (Figures S1B and S1C). Definitive mature astrocytes with expression of GFAP appeared 3 to 9 months following removal of growth factors EGF and FGF2 from astroglial medium (Figures S1B and S1C). Astrocyte maturation was characterized by the presence of the astrocyte marker GFAP (Figures 1B, 1C, and S1C). Quantification of GFAP-positive cells showed that the percentage of GFAP-expressing cells was 27.7% and 83.8% with 3 and 9 months of differentiation, respectively (quantification data not shown). In contrast, GFAP expression in human DA neuronal cultures was barely detected compared with astroglial culture (Figures 1C and S1D). DA neurons were verified by the absence of TH (tyrosine hydroxylase) expression in 9-month astroglial culture (Figures 1B, 1C, and S1D). Therefore, 9-month mature astrocytes were used in our co-culture system.
Figure 1.
Differentiation and Development of hiPSC Line-Induced Human Dopaminergic Neurons and Astrocytes
(A) A schematic representation of the differentiation of mitochondrial inhibitor/toxin (KCN or rotenone)-treated hiPSC line-induced DA neurons with astroglial co-culture. EB, embryoid body.
(B) Immunocytochemistry of synaptophysin (Syn)-positive (green) puncta and TH (red) in neural progenitor cells (NPCs; I) and mature DA neurons (II) and mature astroglial marker (GFAP, green) and astroglial progenitor marker (S100β, red) in NPCs (III) and astrocytes induced for 9 months (IV).
(C) Representative immunoblots for GFAP and TH expression in NPCs, astrocytes induced for 9 months, and DA neurons. β-actin was used as a protein loading control. ∗∗p < 0.01 versus NPCs and DA neurons (open bars, GFAP), and ##p < 0.01 versus NPCs and DA neurons (black bars, TH).
Scale bars, 25 μm in (B). For (C), statistical analysis was performed using StatView software (SAS Institute, v.5.0.1). One-way ANOVA was used for repeated-measure analysis, followed by Fisher's protected least significant difference for post hoc comparisons. Data are presented as means ± SEM. n = 3 independent experiments.
Co-culture with Astrocytes Rescues Impaired DA Neuronal Differentiation and Synaptogenesis
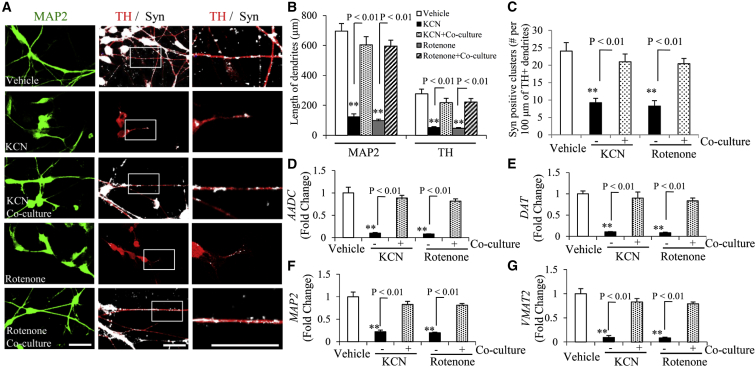
Accumulating evidence indicates the emerging role of astrocytes in the pathogenesis of neurological disorders, such as PD (Maragakis and Rothstein, 2006). Given the importance of mitochondrial respiratory function in DA neuron differentiation and synaptogenesis (Fang et al., 2016a), we investigated the effect of astrocytes on DA neuronal development under respiratory chain impairment using a neuron-astrocyte co-culture system. Impairment of mitochondrial respiratory function was induced by the addition of inhibitor for complex IV (KCN, 500 nM) and complex I (rotenone, 10 nM), as we previously described (Fang et al., 2016a). Consistent with our previous results, KCN- or rotenone-induced inhibition of mitochondrial respiratory function significantly suppressed synapse development and maturation of DA neurons. However, with astrocyte co-culture, the lengths of MAP2 (a marker for neuronal dendrites)- and TH (a marker for DA neurons)-positive processes of neurons were significantly longer compared with those neurons without astrocyte co-culture in the presence of KCN or rotenone (Figures 2A and 2B). The number of synaptophysin-positive puncta in TH dendrites was also significantly increased (Figures 2A and 2C). Accordingly, quantitative real-time PCR showed that levels of mature DA neuronal markers, including aromatic L-amino acid decarboxylase (AADC), dopamine transporter (DAT), and vesicular monoamine transporter 2 (VMAT2), and the marker for mature neurons, microtubule-associated protein 2 (MAP2), were markedly elevated with astroglia co-culture (Figures 2D–2G). These results indicate that co-culture with astrocytes restores DA neuronal differentiation ability and synaptogenesis by improving mitochondrial function. Given that soma size is an important index for neuronal morphology and development, we further analyzed soma size of DA neurons in the aforementioned treatments. No significant changes in soma size of DA neurons were observed between DA neurons with and without astrocyte co-culture exposed to KCN or rotenone (data not shown).
Figure 2.
Astrocyte Co-culture Rescues Differentiation of hiPSC Line-Induced Human DA Neurons under KCN or Rotenone Treatment
(A) Representative images for immunostaining of MAP2 (green), TH (red), and Syn (white, color changed from far red) in induced DA neurons treated with KCN or rotenone with or without astrocyte co-culture. Scale bars, 25 μm.
(B) Quantification of neuronal process length of MAP2-positive neurons and TH-positive DA neurons using NIH ImageJ program.
(C) Quantification of numbers of Syn-positive clusters along the branches of hiPSC-derived DA neurons (TH-positive dendrites).
(D–G) Quantitative real-time PCR results of the indicated gene expression levels of AADC (D), DAT (E), VMAT2 (G), and MAP2 (F) in DA neurons with (+) or without (−) co-culture of astrocytes in the presence of vehicle, KCN, or rotenone.
Scale bars, 25 μm in (A). For (B)–(G), ∗∗p < 0.01 versus the vehicle group. Statistical analysis was performed using StatView software (SAS Institute, v.5.0.1). One-way ANOVA was used for repeated-measure analysis, followed by Fisher's protected least significant difference for post hoc comparisons. Data are presented as means ± SEM. n = 3 independent experiments, with 12–13 cells quantified per experiment in (B) and (C). n = 3 independent experiments in (D)–(G).
Co-culture with Astrocytes Rescues Mitochondrial Defects in hiPSC-Differentiated DA Neurons
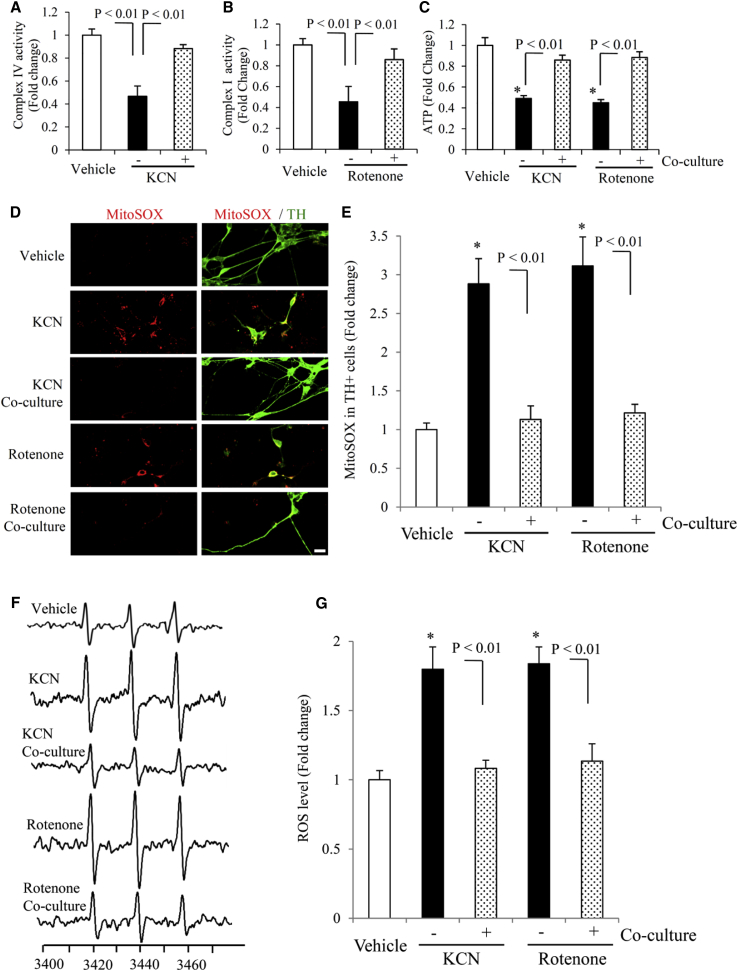
To further determine the effect of astroglial co-culture on neuronal mitochondrial functions, we evaluated the activity of mitochondrial complexes I and IV (key respiratory chain enzymes), ATP levels, and mitochondrial reactive oxygen species (ROS) levels. Co-culture with astrocytes rescued KCN or rotenone-induced mitochondrial dysfunction as shown by increased complex IV and I activities and ATP levels in DA neurons (Figures 3A–3C). Furthermore, in the presence of astrocytes, mitochondrial ROS levels evaluated by MitoSOX staining intensity in TH+ neuronal terminals (Figures 3D and 3E) and intracellular ROS levels measured by highly specific electron paramagnetic resonance (EPR) spectroscopy (Figures 3F and 3G) were both significantly diminished in DA neurons exposed to KCN or rotenone. These results demonstrate that astroglial co-culture rescues mitochondrial respiratory function defects and eliminates ROS overproduction and accumulation during differentiation of hiPSCs into DA neurons.
Figure 3.
Effect of Astrocyte Co-culture on Mitochondrial Functions in hiPSC Line-Induced Human DA Neurons under KCN or Rotenone Treatment
(A–C) Complex IV (A) and I (B) activity and ATP levels (C) were determined in hiPSC-derived DA neurons treated with KCN or rotenone with or without astrocyte co-culture. Data are expressed as fold change relative to the vehicle group in (A)–(C) (n = 3 independent experiments; mean ± SEM).
(D and E) Mitochondrial ROS levels were measured by MitoSOX staining. Representative images for MitoSOX staining for hiPSC-derived DA neurons with the above treatments are shown in (D), and quantification of MitoSOX staining intensity is shown in (E) (n = 3 independent experiments; mean ± SEM, with five cells quantified per experiment). Scale bars, 10 μm in (D).
(F and G) Assessment of intracellular ROS levels measured by EPR spectroscopy. Representative images for EPR are shown in (F), and quantifications are shown in (G) (n = 3 independent experiments; mean ± SEM).
∗p < 0.05 versus the vehicle group (C, E, and G). Statistical analysis was performed using Statview software (SAS Institute, Version 5.0.1). One-way ANOVA was used for repeated measure analysis, followed by Fisher’s protected least significant difference for post hoc comparisons (A–C, E, and G).
Co-culture with Astrocytes Protects against Mitochondria Toxin-Induced Alterations in Mitochondrial Morphology and Mobility in hiPSC-Differentiated DA Neurons
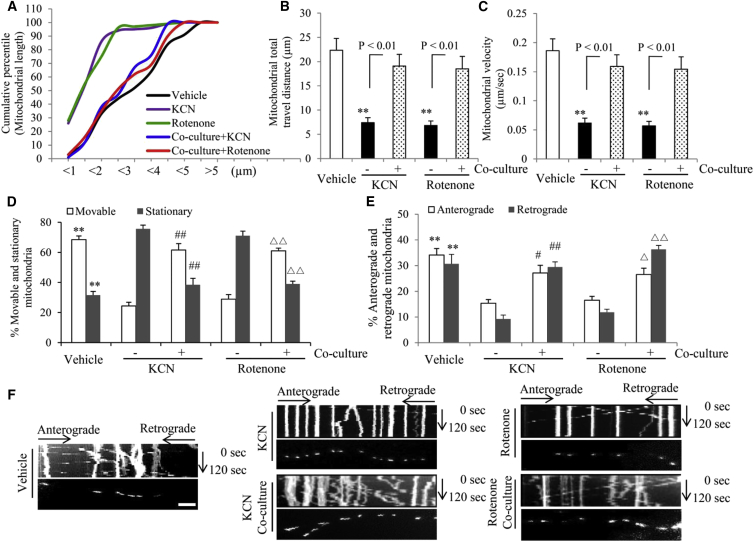
Finally, we investigated mitochondrial morphology and mobility in the processes of hiPSC-differentiated DA neurons. Cumulative distribution data in Figure 4A clearly demonstrate that astroglial co-culture increases the percentage of long mitochondria in hiPSC-differentiated DA neurons under KCN or rotenone treatment. Mitochondrial travel distance and velocity were significantly increased in DA neurons co-cultured with astrocytes compared with DA neurons without astrocytes in the presence of KCN or rotenone (Figures 4B and 4C). Similarly, the percentages of anterograde, retrograde, and movable mitochondria were all increased (Figures 4D and 4E), whereas the percentage of stationary mitochondria was decreased in neurons cultured with astrocytes (Figure 4D). Representative kymograph images revealed more movable mitochondria in the neuronal processes of KCN- and rotenone-treated DA neurons with astroglial co-culture (Figure 4F). Video recorded from living mitochondrial images revealed a significant improvement in mitochondrial movement speed and travel distance with astrocyte co-culture (Movie S1). Collectively, our results indicate that astrocytes reverse mitochondrial morphological and dynamic defects in hiPSC-derived DA neurons insulted by mitochondrial toxins.
Figure 4.
Effect of Astrocyte Co-culture on Mitochondrial Dynamic Parameters in hiPSC-Derived DA Neurons under KCN or Rotenone Treatment
(A) Cumulative distribution data showed increases in the numbers of long mitochondria in hiPSC-derived DA neurons treated with KCN or rotenone with or without astrocyte co-culture.
(B and C) Average mitochondrial travel distance (B) and mitochondrial travel velocity (C) were calculated. Longer mitochondrial travel distance and higher mitochondrial travel velocity were obtained from DA neurons with astrocyte co-culture. ∗∗p < 0.01 versus the vehicle group.
(D and E) Higher percentage of movable and lower percentage of stationary (D) and more anterograde and retrograde movable mitochondria (E) in DA neurons with astrocyte co-culture. n = 3 independent experiments; mean ± SEM, with 10 mitochondrial movements quantified per experiment in (B)–(E). ∗∗p < 0.01 versus the open and gray bars in the KCN- and rotenone-treated groups without astrocyte (−), respectively. #p < 0.05 and ##p < 0.01 versus the open and gray bars in the KCN-treated groups (−), respectively. ▵p < 0.05 and ▵▵p < 0.01 versus the open and gray bars in the rotenone-treated groups (−), respectively. Statistical analysis was performed using Statview software (SAS Institute, Version 5.0.1). One-way ANOVA was used for repeated measure analysis, followed by Fisher’s protected least significant difference for post hoc comparisons (B–E).
(F) Kymographs generated from live-imaging movies represent hiPSC-derived DA neurons cultured with above treatments. In the kymographs, the x axis is mitochondrial position and the y axis represents the time lapse of 0–120 s. Vertical white lines represent stationary mitochondria and diagonal lines represent moving mitochondria. Anterograde movements are from left to right and retrograde movements are from right to left. Scale bar, 10 μm.
Discussion
Accumulating evidence now suggests that astrocytes are key neuromodulators that actively communicate/cross talk with neurons during different phases of neurodevelopment (Kettenmann and Verkhratsky, 2008, Sofroniew and Vinters, 2010). Astroglial cells are involved in many cellular functions, including providing trophic support for neurons, neurotransmission, metabolite and electrolyte homeostasis, cell signaling, inflammation, and synapse modulation in the CNS (Rouach et al., 2008). Loss of normal homeostatic function, toxic stimulation, or a combination of both activates astrocytes, contributing to the neuropathology of neurodegenerative diseases (Allaman et al., 2011, Booth et al., 2017).
Recently, several studies have demonstrated that astrocytes generated from hiPSCs mimic normal development and functions in vivo (Ruiz et al., 2010, Shaltouki et al., 2013). Exposure to FGF was sufficient to induce a mature quiescent spinal cord astrocyte phenotype (Roybon et al., 2013). CNTF has been shown to direct differentiation of precursor cells into astrocytes while inhibiting neuronal differentiation (Bonni et al., 1997). By application of FGF at the astroglial progenitor stage and CNTF at the late stage, we directed hiPSCs into mature GFAP-positive astrocytes 9 months after astroglial induction.
In the CNS, astrocytes have been widely shown to protect neurons from oxidative insults (Shih et al., 2003, Sofroniew and Vinters, 2010). The co-culture system offers a model for mimicking the in vivo environment for detailed study of the astrocyte-neuron coordination relationship. In this study, using transwell inserts plated with mature astrocytes, mitochondrial development and differential ability were restored in DA neurons with mitochondrial respiratory inhibitor insult. Similarly, incubation of astrocyte conditioned medium protects against mitochondrial toxin-induced defects in mitochondrial function, differentiation, and synaptogenesis in hiPSC line-induced human DA neurons (Figure S2). These findings indicate that astrocytes are of key importance in modulating neuronal mitochondrial dysfunction induced by mitochondrial insults in vivo.
Mitochondria, highly dynamic organelles and the primary energy-generating system, contain complex structures involved in multiple processes, including energy metabolism, ROS generation, and mitochondrial dynamics and distribution. Mitochondrial defects occur in a wide variety of degenerative diseases such as aging, mal-neurodevelopment, and neurodegenerative diseases (Mattson et al., 2008). Excess mitochondrial ROS in NPCs could lead to mitochondrial dysfunctions, resulting in failure of DA neurons to differentiate (Fang et al., 2016a). Since astrocytes are potent scavengers of ROS, neurons are damaged by oxidative stress triggered by the impaired redox scavenging ability of dysfunctional astrocytes (Drukarch et al., 1998). Indeed, astroglial-neuronal co-culture reduced intracellular mitochondrial ROS levels and restored mitochondrial function along with differentiation of DA neurons insulted by mitochondrial toxin, suggesting the protective effect of astrocytes on DA neuronal function and differentiation is, at least in part, via suppressing ROS to stabilize mitochondrial functions.
Mitochondrial ROS is a strong stimulus, which activates many signal pathways and transcription factors, including nuclear factor κB and p38 or ERK mitogen-activated protein (MAP) kinases. Oxidative stress-mediated activation of p38 MAP kinase contributes to aberrant axonal mitochondrial transport and aberrant mitochondrial dynamics and function (Yu et al., 2016, Yu et al., 2017). Disruption of ERK MAP kinase signaling perturbs the balance of mitochondrial dynamics via increased mitochondrial fission protein Dlp1 expression in Alzheimer's disease trans-mitochondrial “cybrid” (cytoplasmic hybrid) neuronal cells (Gan et al., 2014, Gan et al., 2015). Our most recent study demonstrated the link of mitochondrial ROS to mitochondrial clearance through the PINK1-mediated autophagy pathway to maintain homeostasis of mitochondria (Du et al., 2017). Thus, mitochondrial ROS-initiated signals may contribute importantly to the astrocyte-involved DA neuronal maturation and synaptogenesis.
Mitochondria continuously undergo fission and fusion cycles. During DA neuronal differentiation, mitochondria favor the fusion process (Fang et al., 2016a). The fission and fusion balance is maintained via quality control of mitochondrial proteins, such as Drp1, a key mitochondrial fission protein found to be indispensable in somatic cell reprogramming to pluripotency (Vazquez-Martin et al., 2012), and fusion proteins. Importantly, mitochondrial trafficking regulator Miro1 (Mitochondrial Rho GTPase 1), a key factor in normal embryonic development, is also involved in the differentiation of iPSCs (Chen et al., 2003, Yamaoka et al., 2011).
In addition, multiple trophic factors as bFGF, BDNF, GDNF, and NGF, derived from astrocytes, have been implicated in regulating mesencephalic DA neuronal survival, differentiation, and synaptic plasticity in vitro and in vivo (Liu et al., 2015, Yang et al., 2014). These tropic factors are potentially important for the astrocyte-involved mitochondrial improvement, synaptogenesis, and development of DA.
Endoplasmic reticulum (ER) stress results in accumulation of unfolded or misfolded proteins in the ER lumen. ER and mitochondria form networks essential to maintain cellular homeostasis; therefore, ER stress perturbs mitochondrial function (Malhotra and Kaufman, 2011). Under chronic KCN and rotenone treatments, the protein disulfide isomerase, a stress protein abundant in ER, and the ER chaperone glucose-regulated protein 78 were elevated in the DA neurons (Figure S3). Notably, co-culture with astrocytes almost abolished KCN- or rotenone-mediated upregulation of these ER stress markers. These results suggest a possible link of mitochondrial dysfunction to ER stress via astrocytes, contributing to the normal differentiation and synaptogenesis in DA neurons.
Mitochondrial dysfunction and oxidative stress play a major role in the pathogenesis of PD. Deficiency of mitochondrial complex I of the respiratory chain has long been implicated in the cause of the degeneration of PD-affected neurons (Mizuno et al., 1998). In addition, combined decreased complex I and IV activity was seen in the platelet mitochondria of PD (Benecke et al., 1993) and 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine (MPTP)- or 6-hydroxydopamine-induced animal models of PD (Desai et al., 1996, Glinka and Youdim, 1995). Here, we induced DA neuron differentiation by co-culture with mature astrocytes to mimic the in vivo neuronal-astroglial interaction environment. Therefore, evaluation of the consequences of mitochondrial toxin-mediated inhibition of mitochondrial respiratory function via complex I or IV on dopamine neurons holds a potential significance for the pathogenesis of DA degeneration relevant to the PD pathology.
In summary, we have clearly demonstrated the direct role of mitochondria in DA neuron maturation and an active astrocyte-neuron coordination relationship. The present model provides an approach for the study of abnormal mitochondrial structure, function, and dynamics in the pathogenesis of neurodegenerative diseases and, importantly, an approach for therapeutic discovery in neurodegenerative diseases such as PD.
Experimental Procedures
hiPSC Culture
Bone marrow 2-3 (BM2-3) from hiPSCs passaged 16–20 times were obtained from Dr. Sunita L. D'Souza. This BM2-3 iPSC line was derived from the bone marrow of a clinically normal human subject and verified by fluorescence in situ hybridization test showing 46, XX. Cytogenetic analysis of cultured human stem cells revealed a normal female karyotype. This analysis did not show any evidence of a clinically significant numerical or structural chromosomal abnormality (Fang et al., 2016a). Cells were maintained under feeder-free conditions using Matrigel (BD Biosciences)-coated six-well tissue culture plates in Essential 8 Medium (Life Technologies), supplemented with 10 μM ROCK inhibitor Y27632 (Life Technologies). Cells were routinely passaged as small clumps using a previously described EDTA method (Fang et al., 2016a).
Neuron and Astrocyte Co-culture System
For co-culture experiments, 2.5 × 104 astrocytes per well were first seeded on the 12-well transwell cell culture insert system (pore size 0.4 μm; Falcon, USA) and allowed to grow for 5 days. On day 6, astroglial medium was changed to DA neuronal differentiation medium. After 2 days, the transwell inserts were placed into wells of NPCs during induction, and future experiments were conducted 2 days after the insertion of the astroglial transwells (Figure 1A).
Author Contributions
S.S.Y. initiated, directed, designed, and supervised research and wrote the paper. F.D. and Q.Y. developed the concept and designed research. F.D., Q.Y., A.C., and D.C. conducted experiments, analyzed data, and wrote the paper.
Acknowledgments
We thank Dr. Sunita L. D'Souza (Department of Gene and Cell Medicine and Black Family Stem Cell Institute and Department of Developmental and Regenerative Biology, Icahn School of Medicine at Mount Sinai, NY, USA) for providing iPSCs. This study was supported by grants from the NIH (R37AG037319, R01AG044793, R01AG05341, and R01NS089116). We thank Dr. Justin T. Douglas for assistance using the EPR instrument. The EPR instrumentation was provided by NSF Chemical Instrumentation Grant (no. 0946883).
Published: January 25, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and one movie and can be found with this article online at https://doi.org/10.1016/j.stemcr.2017.12.021.
Supplemental Information
References
- Allaman I., Belanger M., Magistretti P.J. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Benecke R., Strumper P., Weiss H. Electron transfer complexes I and IV of platelets are abnormal in Parkinson's disease but normal in Parkinson-plus syndromes. Brain. 1993;116(Pt 6):1451–1463. doi: 10.1093/brain/116.6.1451. [DOI] [PubMed] [Google Scholar]
- Bonni A., Sun Y., Nadal-Vicens M., Bhatt A., Frank D.A., Rozovsky I., Stahl N., Yancopoulos G.D., Greenberg M.E. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- Booth H.D.E., Hirst W.D., Wade-Martins R. The role of astrocyte dysfunction in Parkinson's Disease pathogenesis. Trends Neurosci. 2017;40:358–370. doi: 10.1016/j.tins.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Detmer S.A., Ewald A.J., Griffin E.E., Fraser S.E., Chan D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai V.G., Feuers R.J., Hart R.W., Ali S.F. MPP(+)-induced neurotoxicity in mouse is age-dependent: evidenced by the selective inhibition of complexes of electron transport. Brain Res. 1996;715:1–8. doi: 10.1016/0006-8993(95)01255-9. [DOI] [PubMed] [Google Scholar]
- Drukarch B., Schepens E., Stoof J.C., Langeveld C.H., Van Muiswinkel F.L. Astrocyte-enhanced neuronal survival is mediated by scavenging of extracellular reactive oxygen species. Free Radic. Biol. Med. 1998;25:217–220. doi: 10.1016/s0891-5849(98)00050-1. [DOI] [PubMed] [Google Scholar]
- Du F., Yu Q., Yan S., Hu G., Lue L.F., Walker D.G., Wu L., Yan S.F., Tieu K., Yan S.S. PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer's disease. Brain. 2017;140:3233–3251. doi: 10.1093/brain/awx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D., Qing Y., Yan S., Chen D., Yan S.S. Development and dynamic regulation of mitochondrial network in human midbrain dopaminergic neurons differentiated from iPSCs. Stem Cell Reports. 2016;7:678–692. doi: 10.1016/j.stemcr.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D., Yan S., Yu Q., Chen D., Yan S.S. Mfn2 is required for mitochondrial development and synapse formation in human induced pluripotent stem Cells/hiPSC derived cortical neurons. Sci. Rep. 2016;6:31462. doi: 10.1038/srep31462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X., Huang S., Wu L., Wang Y., Hu G., Li G., Zhang H., Yu H., Swerdlow R.H., Chen J.X. Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer's disease cybrid cell. Biochim. Biophys. Acta. 2014;1842:220–231. doi: 10.1016/j.bbadis.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X., Huang S., Yu Q., Yu H., Yan S.S. Blockade of Drp1 rescues oxidative stress-induced osteoblast dysfunction. Biochem. Biophys. Res. Commun. 2015;468:719–725. doi: 10.1016/j.bbrc.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Y.Y., Youdim M.B. Inhibition of mitochondrial complexes I and IV by 6-hydroxydopamine. Eur. J. Pharmacol. 1995;292:329–332. doi: 10.1016/0926-6917(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Verkhratsky A. Neuroglia: the 150 years after. Trends Neurosci. 2008;31:653–659. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- Liu H., Tang X., Gong L. Mesencephalic astrocyte-derived neurotrophic factor and cerebral dopamine neurotrophic factor: new endoplasmic reticulum stress response proteins. Eur. J. Pharmacol. 2015;750:118–122. doi: 10.1016/j.ejphar.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Lo E.H. Degeneration and repair in central nervous system disease. Nat. Med. 2010;16:1205–1209. doi: 10.1038/nm.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra J.D., Kaufman R.J. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb. Perspect. Biol. 2011;3:a004424. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis N.J., Rothstein J.D. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Maroof A.M., Keros S., Tyson J.A., Ying S.W., Ganat Y.M., Merkle F.T., Liu B., Goulburn A., Stanley E.G., Elefanty A.G. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P., Gleichmann M., Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y., Yoshino H., Ikebe S., Hattori N., Kobayashi T., Shimoda-Matsubayashi S., Matsumine H., Kondo T. Mitochondrial dysfunction in Parkinson's disease. Ann. Neurol. 1998;44:S99–S109. doi: 10.1002/ana.410440715. [DOI] [PubMed] [Google Scholar]
- Rouach N., Koulakoff A., Abudara V., Willecke K., Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Roybon L., Lamas N.J., Garcia A.D., Yang E.J., Sattler R., Lewis V.J., Kim Y.A., Kachel C.A., Rothstein J.D., Przedborski S. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell Rep. 2013;4:1035–1048. doi: 10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S., Brennand K., Panopoulos A.D., Herrerias A., Gage F.H., Izpisua-Belmonte J.C. High-efficient generation of induced pluripotent stem cells from human astrocytes. PLoS One. 2010;5:e15526. doi: 10.1371/journal.pone.0015526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltouki A., Peng J., Liu Q., Rao M.S., Zeng X. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells. 2013;31:941–952. doi: 10.1002/stem.1334. [DOI] [PubMed] [Google Scholar]
- Shih A.Y., Johnson D.A., Wong G., Kraft A.D., Jiang L., Erb H., Johnson J.A., Murphy T.H. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Martin A., Cufi S., Corominas-Faja B., Oliveras-Ferraros C., Vellon L., Menendez J.A. Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging. 2012;4:393–401. doi: 10.18632/aging.100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka S., Nakajima M., Fujimoto M., Tsutsumi N. MIRO1 influences the morphology and intracellular distribution of mitochondria during embryonic cell division in Arabidopsis. Plant Cell Rep. 2011;30:239–244. doi: 10.1007/s00299-010-0926-5. [DOI] [PubMed] [Google Scholar]
- Yang F., Liu Y., Tu J., Wan J., Zhang J., Wu B., Chen S., Zhou J., Mu Y., Wang L. Activated astrocytes enhance the dopaminergic differentiation of stem cells and promote brain repair through bFGF. Nat. Commun. 2014;5:5627. doi: 10.1038/ncomms6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Du F., Douglas J.T., Yu H., Yan S.S., Yan S.F. Mitochondrial dysfunction triggers synaptic deficits via activation of p38 MAP kinase signaling in differentiated Alzheimer's Disease trans-mitochondrial cybrid cells. J. Alzheimers Dis. 2017;59:223–239. doi: 10.3233/JAD-170283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Fang D., Swerdlow R.H., Yu H., Chen J.X., Yan S.S. Antioxidants rescue mitochondrial transport in differentiated Alzheimer's Disease trans-mitochondrial cybrid cells. J. Alzheimers Dis. 2016;54:679–690. doi: 10.3233/JAD-160532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.