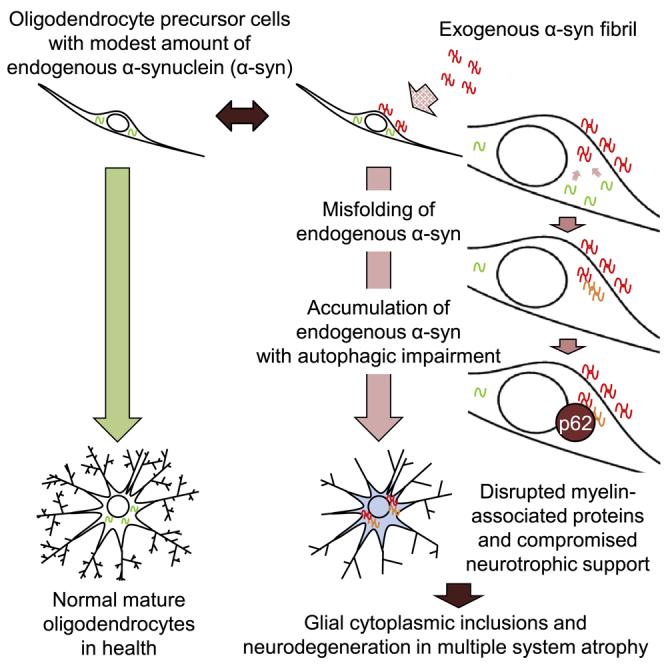
Summary
Glial cytoplasmic inclusions (GCIs), commonly observed as α-synuclein (α-syn)-positive aggregates within oligodendrocytes, are the pathological hallmark of multiple system atrophy. The origin of α-syn in GCIs is uncertain; there is little evidence of endogenous α-syn expression in oligodendrocyte lineage cells, oligodendrocyte precursor cells (OPCs), and mature oligodendrocytes (OLGs). Here, based on in vitro analysis using primary rat cell cultures, we elucidated that preformed fibrils (PFFs) generated from recombinant human α-syn trigger multimerization and an upsurge of endogenous α-syn in OPCs, which is attributable to insufficient autophagic proteolysis. RNA-seq analysis of OPCs revealed that α-syn PFFs interfered with the expression of proteins associated with neuromodulation and myelination. Furthermore, we detected cytoplasmic α-syn inclusions in OLGs through differentiation of OPCs pre-incubated with PFFs. Overall, our findings suggest the possibility of endogenous α-syn accumulation in OPCs that contributes to GCI formation and perturbation of neuronal/glial support in multiple system atrophy brains.
Keywords: α-synuclein, oligodendrocytes, oligodendrocyte precursor cells, primary cell culture, multiple system atrophy, synucleinopathy, glial cytoplasmic inclusion, neurotrophic factor, misfolding, autophagy
Graphical Abstract
Highlights
-
•
Endogenous α-syn in OPCs drastically increases via seeding from exogenous α-syn PFFs
-
•
Exogenous α-syn PFFs do not induce SNCA mRNA overproduction but autophagic impairment
-
•
Exogenous α-syn PFFs compromise gene regulation for neuronal/glial modulation in OPCs
-
•
α-Syn PFF-treated OPCs differentiate into OLGs containing α-syn aggregates
In this article, Maki and Takahashi report that the internalization of exogenous α-synuclein fibrils in oligodendrocyte precursor cells triggers misfolding and accumulation of endogenous α-synuclein via seeding mechanisms, which may eventually lead to neurodegeneration and myelin disruption in multiple system atrophy by compromising the oligodendroglial function of neuronal support and myelination.
Introduction
Multiple system atrophy (MSA) is an α-synucleinopathy characterized by a relentless worsening of motor and non-motor symptoms during a typical time frame of 6–10 years. Glial cytoplasmic inclusions (GCIs) in oligodendrocytes (OLGs), which consist of α-synuclein (α-syn)-positive filamentous components, are the hallmark for a definitive neuropathological diagnosis of MSA. Given that the emergence of GCIs occurs prior to neuronal loss, it is likely that a primary oligodendroglial event is the root of the disease pathology in MSA (Wenning et al., 2008).
Since α-syn is considered to be expressed almost exclusively in neurons, the origin of the α-syn that composes GCIs in oligodendrocytes has been enigmatic. Recent reports have suggested the existence of endogenous α-syn in oligodendrocyte lineage cells, emphasizing the pathological importance of endogenous α-syn as the source of the misfolded α-syn in GCIs (Djelloul et al., 2015). The fibrillary form of α-syn contributes to prion-like propagation of the misfolded structure and disease progression among both in vitro and in vivo models of synucleinopathies (Angot et al., 2010). Considering that exogenous α-syn preformed fibrils (PFFs) seed and recruit endogenous α-syn to form insoluble aggregates in primary neurons, it is of great importance to determine if exogenous α-syn PFFs induce misfolding of endogenous α-syn in primary oligodendrocyte lineage cells (Volpicelli-Daley et al., 2011).
Oligodendrocyte lineage cells support neuronal activity not only by forming a myelin sheath to enable saltatory conduction but also by modulating axonal and neuronal homeostasis through the supply of neurotrophic factors (Wilkins et al., 2003). Myelin-forming mature OLGs are derived from oligodendrocyte precursor cells (OPCs). When activated in response to brain damage, OPCs proliferate and attempt to differentiate into mature OLGs. OPCs, which are immunoreactive to NG2 chondroitin sulfate or platelet-derived growth factor α receptor (PDGFRα), are distributed diffusely within the central nervous system and account for 5%–8% of all cells in adult brains (Levine et al., 2001). Despite the importance of OPCs in brain homeostasis, there are limited numbers of pathological investigations of OPCs in MSA brains.
In the present study, we provide new pathological insight into the interaction between endogenous and exogenous α-syn by using primary rat oligodendrocyte lineage cell cultures, and we propose the possibility of OPC involvement in the pathogenesis of MSA.
Results
Oligodendrocyte Precursor Cells Contain α-Syn Aggregates in MSA Brains
We investigated whether OPCs contain α-syn aggregates in MSA brains. One previous analysis revealed that a small fraction of OPCs in MSA cases showed α-syn immunoreactivity, which was also confirmed by our postmortem investigation (May et al., 2014) (Figure S1A). The α-syn immunoreactivity in OPCs was stained with Thioflavin S, suggesting that the α-syn aggregate was misfolded. These results suggest that not only OLGs but OPCs may also contain α-syn aggregates in MSA brains.
Oligodendrocyte Lineage Cells in Rat Primary Cultures Express Moderate Amounts of α-Syn
To confirm the endogenous α-syn expression in oligodendrocyte lineage cells, primary oligodendrocyte lineage cell cultures were obtained from neonatal rats. Consistent with previous reports, anti-α-syn antibody immunostained endogenous α-syn within OPCs and OLGs with cytoplasmic predominance (Figures S1B and S1C) (Richter-Landsberg et al., 2000). Immunoblot analysis showed that oligodendroglial endogenous α-syn expression at 4–6 days after plating was slightly greater than 20% of the neuronal α-syn expression (Figures S1D and S1E). Consistent with immunoblot analysis, quantitative real-time PCR (qPCR) also suggested that oligodendrocyte lineage cells expressed 10%–20% of the amount of α-syn transcripts expressed in neurons (Figure S1F). Immunoblot analysis, immunocytochemistry, and qPCR analysis using each cell marker validated the high purity of each cell-type culture (Figures S1D and S1G–S1I, and Movies S1 and S2).
Exogenous α-Syn PFFs Are Internalized into OPCs
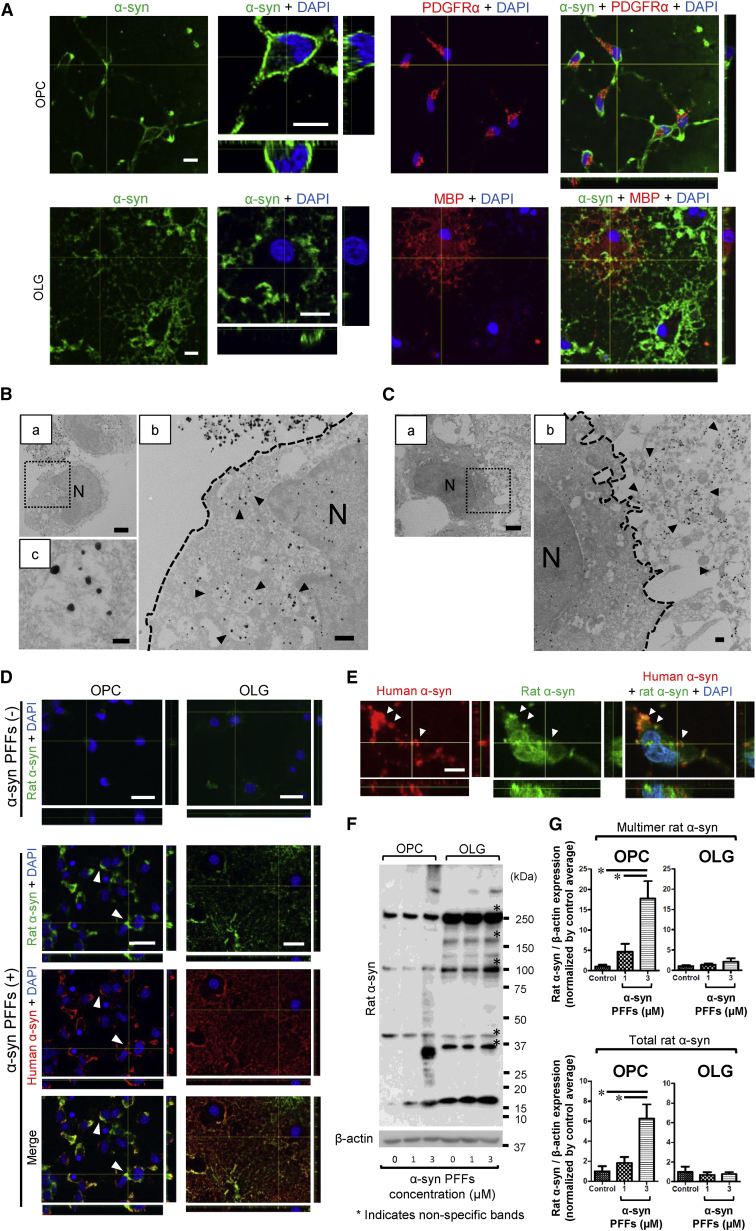
To elucidate the impact of extracellular α-syn PFFs on primary oligodendrocyte lineage cells, these cells were incubated with either recombinant human α-syn PFFs or monomer for 24 hr and immunostained with an anti-α-syn antibody. When OPCs and OLGs were incubated with α-syn PFFs, prominent α-syn immunoreactivity was observed on the cell membranes. Observation of the magnified images obtained by confocal microscopy enabled visualization of internalization of α-syn predominantly in OPCs but not in OLGs (Figure 1A), which was also confirmed by immunoelectron microscopy showing the intracellular localization of α-syn fibrils in OPCs (Figures 1B and 1C). Meanwhile, the enhanced α-syn immunoreactivity was not found either in OPCs or OLGs exposed to an equivalent amount of α-syn monomer (Figure S2A). The cytosolic localization of exogenous α-syn in OPCs was also verified by subcellular fractionation of these cells (Figure S2B).
Figure 1.
Internalization of Recombinant Human α-Syn PFFs Inducing Accumulation and Multimerization of Endogenous Rat α-Syn
(A) Confocal microscopy of OPCs and OLGs incubated with α-syn PFFs (3 μM) shows prominent α-syn accumulation on the cell membranes. The magnified view of an OPC reveals intracellular α-syn immunoreactivity, which is not observed in OLGs. Each scale bar represents 10 μm.
(B) Immunoelectron microscopy of α-syn PFF (1 μM)-treated OPCs reveals intracellular fibril-like structures, which are labeled with anti-α-syn antibody (arrowheads). The antibody recognizes both rat and human α-syn. Each scale bar represents (a) 2 μm, (b) 500 nm, and (c) 100 nm, respectively. (b) Dotted line indicates cell surface. N, nucleus.
(C) Immunoelectron microscopy of α-syn PFF (1 μM)-treated OLGs shows extracellularly distributed layers of fibril-like structures, which are labeled with anti-α-syn antibody (arrowheads). The antibody recognizes both rat and human α-syn. Each scale bar represents (a) 2 μm and (b) 500 nm, respectively. (b) Dotted line indicates cell surface. N, nucleus.
(D) Confocal microscopy with human-specific (exogenous) and rat-specific (endogenous) anti-α-syn antibodies identifies the enhanced expression of endogenous α-syn in α-syn PFF (3 μM)-treated OPCs. The increase in rat-specific α-syn expression is less notable in OLGs treated with α-syn PFFs (3 μM). White arrowheads indicate locations where endogenous rat α-syn accumulation is predominantly observed. Each scale bar represents 20 μm.
(E) The magnified view of α-syn PFF (1 μM)-treated OPCs shows intracellular colocalization of exogenous human and endogenous rat α-syn (white arrowheads). The bar represents 5 μm.
(F) Immunoblot analysis with a rat-specific anti-α-syn antibody reveals that 24-hr incubation with α-syn PFFs induces multimerization of endogenous rat α-syn, with a remarkable increase in the total amount of endogenous rat α-syn in OPCs.
(G) Quantification of endogenous rat α-syn accumulation in α-syn PFF-treated OPCs and OLGs by immunoblot analysis is illustrated. Both total and multimer endogenous rat α-syn are significantly increased in OPCs by α-syn PFF application. Mean ± SEM; n = 5, respectively, independent cultures; one-way ANOVA, ∗p < 0.05.
Endogenous α-Syn Protein Expression in OPCs Dramatically Increases in Response to Exogenous Recombinant Human α-Syn PFFs
To visualize the interaction between exogenous human α-syn and endogenous rat α-syn in OPCs and OLGs, cells were immunostained with an anti-α-syn antibody that specifically recognizes rodent α-syn (endogenous α-syn antibody) (Figure S2C). In response to incubation with exogenous α-syn PFFs, the endogenous α-syn expressions in OPCs were remarkably enhanced (Figures 1D and S2D). The enhanced immunoreactivity of endogenous α-syn colocalized with that of exogenous α-syn in the cytoplasm of OPCs (Figures 1E and S2B). The cytoplasmic inclusions immunostained with the endogenous α-syn antibody were also stained with Thioflavin S, which was exclusively observed in OPCs (Figures S2E and S2F). Immunoblot analysis revealed a drastically increased amount of endogenous α-syn expression in OPCs characterized by the emergence of multimerized α-syn as the result of α-syn PFF application (Figures 1F and 1G). On the other hand, there was minimal change in the total amount of endogenous α-syn expression in OLGs, as shown by both immunostaining and immunoblot analysis. Monomeric α-syn did not alter the protein expression pattern of endogenous α-syn in OPCs (Figure S2G). Despite the striking evidence of inclusion formation in OPCs, the expression of phosphorylated α-syn was not confirmed either in immunostaining or immunoblot analysis (Figures S2H and S2I).
Impairment of Autophagy Contributes to Endogenous α-Syn Accumulation in OPCs
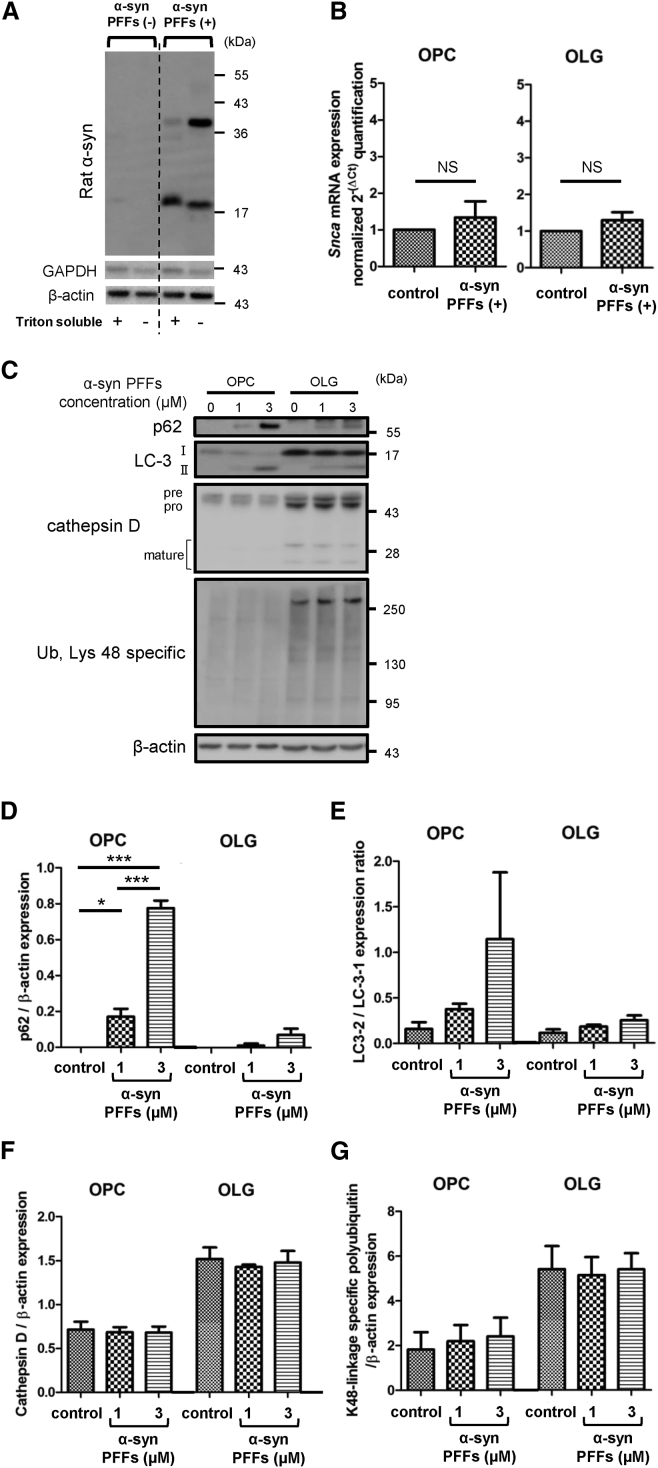
Incubation of OPCs with α-syn PFFs facilitated expression of endogenous α-syn not only in Triton-soluble fractions but also in Triton-insoluble fractions (Figure 2A). Based on the evidence of pathological α-syn expression, we then asked whether the endogenous α-syn protein increase is caused by overproduction, accompanied by increased α-syn transcripts, or by proteolytic dysfunction. qPCR of oligodendrocyte lineage cells 72 hr after application of α-syn PFFs clarified that there was no significant increase in α-syn transcripts (Figure 2B).
Figure 2.
Insufficient Autophagic Degradation in OPCs Triggered by α-Syn PFF Application
(A) Accumulation of Triton-insoluble endogenous rat α-syn in OPCs is triggered by α-syn PFF (3 μM) application.
(B) Quantitative real-time PCR reveals unchanged Snca expression levels after 72-hr incubation of oligodendrocyte lineage cells with 3 μM α-syn PFFs. Mean ± SEM; n = 6, respectively, independent cultures; paired t test. NS, not statistically significant.
(C) Immunoblot analysis with proteolytic markers discloses marked increases in p62 and LC3-II in OPCs, suggesting the induction of an autophagic pathway due to α-syn PFF application.
(D–G) Quantification of each proteolytic marker expression in oligodendrocyte lineage cell is exhibited. (D and E) Autophagic indicators, p62 and LC3-II/LC3-I, show increasing trends in α-syn PFF-treated OPCs. (F) Cathepsin D protein expression is not significantly affected by α-syn PFF application. (G) The expression of lysine-48-linked ubiquitin chains is not affected by α-syn PFF application. Mean ± SEM; n = 4, respectively, independent cultures; one-way ANOVA, ∗p < 0.05, ∗∗∗p < 0.001.
Subsequently, we investigated the effect of 24-hr application of α-syn PFFs on proteolytic systems in oligodendrocyte lineage cells. Immunoblot analysis of α-syn PFF-treated cells revealed p62 accumulation and fraction conversion of LC3-I to LC3-II in OPCs (Figures 2C and 2E), which are correlated with insufficient autophagic clearance and autophagosome accumulation, respectively. These findings, as well as the increase of endogenous α-syn, were also observed when OPCs were incubated for 24 hr with an autophagy inhibitor, chloroquine (Figures S3A–S3C). The interaction of α-syn and autophagy markers (p62, Beclin-1, and LC3) as well as the intra-lysosomal localization of α-syn was verified by immunostaining and LysoTracker probes, implying the possibility of compromised lysosomal degradation (Figures S3D and S3E). Conversely, the protein expression levels of p62 and LC3-II in OLGs treated with α-syn PFFs only slightly increased, which did not reach statistical significance.
Cathepsin D is one of the lysosomal enzymes that are known to regulate cell homeostasis by mediating the degradation of misfolded protein aggregates delivered to lysosomes via autophagy or endocytosis (Bae et al., 2015). Although the α-syn PFF application did not affect the cathepsin D protein expression levels in OPCs or OLGs, the enzymatic activity analysis suggested the reduced cathepsin D activity in α-syn PFF-treated OPCs (Figures 2C, 2F, and S3F).
Lysine-48-linked polyubiquitin chains are well established as the signal for 26S proteasomal degradation (Grice and Nathan, 2016). Both the OPCs and OLGs showed no appreciable increase in lysine-48-linked ubiquitin chains (Figures 2C and 2G). Taken together, these results indicate that α-syn PFFs impair autophagy more severely in OPCs compared with OLGs, leading to the accumulation of endogenous α-syn proteins.
α-Syn PFFs Interfere with mRNA Expression Related to Myelination and Neuronal Support in Oligodendrocyte Precursor Cells
Media lactate dehydrogenase (LDH) and water-soluble tetrazolium (WST) assays revealed that α-syn PFFs did not cause acute cell death after 24-hr exposure (Figures S3G and S3H). Therefore, we assessed the functional influence of α-syn PFF application on OPCs.
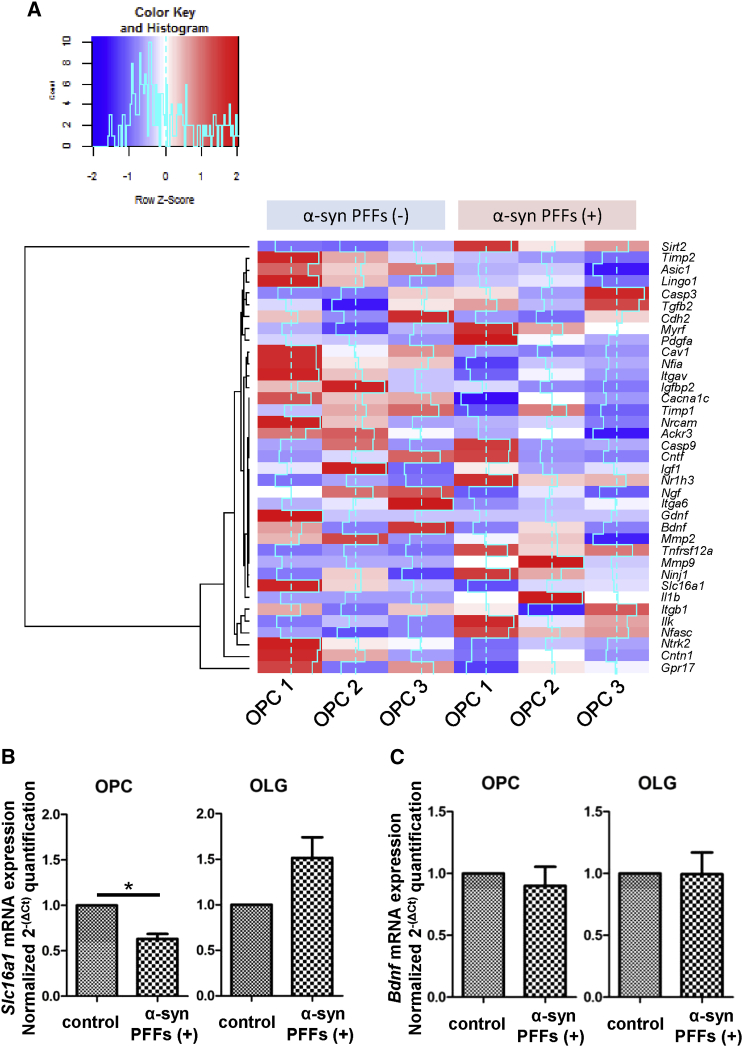
RNA sequencing (RNA-seq) analysis in OPCs revealed remarkable alterations in mRNA profiles associated with OLG maturation and neuromodulation after 72-hr α-syn PFF application (Figure 3A). As for the gene expression involved in oligodendrocyte maturation, α-syn PFF application to OPCs suppressed the gene expressions of myelination-promoting factors such as Ackr3 (encoding CXCR-7) and Cntn1 (encoding Contactin 1), while increasing those of myelination-inhibiting factors such as Sirt2 (encoding sirtuin 2) and Il1b (interleukin 1β). Among the neurotrophic factors that regulate neurodegenerative disease pathology, monocarboxylate transporter 1 (MCT1) encoded by Slc16a1, which mediates neuronal death through the release of lactate, and brain-derived neurotrophic factor (BDNF) showed a tendency to decrease (Lee et al., 2012). Therefore, we verified the alteration of mRNA expression levels of Slc16a1 and Bdnf (Figures 3B and 3C) as well as those of glial cell-derived neurotrophic factor (Gdnf) and insulin-like growth factor-1 (Igf1) by qPCR (Figures S3I and S3J). Interestingly, the mRNA expression level of Slc16a1 was significantly suppressed, whereas those of Bdnf and Gdnf were unchanged. The perturbation of these neurotrophic factors induced by extracellular α-syn PFFs was more severe in OPCs than in OLGs, possibly reflecting the difference of α-syn internalization and susceptibility against seeding. Alterations of mRNA expression levels were observed in various profiles associated with proteolysis and protein trafficking (Figure S3K), phenotypic markers (Figure S3L), and risk genes for familial Parkinson's disease and MSA (Figure S3M). The results and interpretation of RNA-seq analysis regarding possible endocytic players for α-syn PFF uptake in OPCs are described in the Supplemental Information.
Figure 3.
RNA-Seq and qPCR Analysis for mRNA Expression Alteration in α-Syn PFF-treated OPCs
(A) RNA-seq analysis of α-syn PFF-treated OPCs discloses a dramatic shift in the expression of transcripts related to neuromodulation, myelination, and cell survival. Each pair of OPC culture samples (OPC1, OPC2, and OPC3) was allocated for the two groups with and without α-syn PFF (3 μM) application.
(B and C) qPCR elucidates reduced mRNA expression of Slc16a1 in α-syn PFF (3 μM)-treated OPCs, whereas Bdnf mRNA expression is not significantly affected. Mean ± SEM; n = 6, respectively; independent cultures; paired t test, ∗p < 0.05.
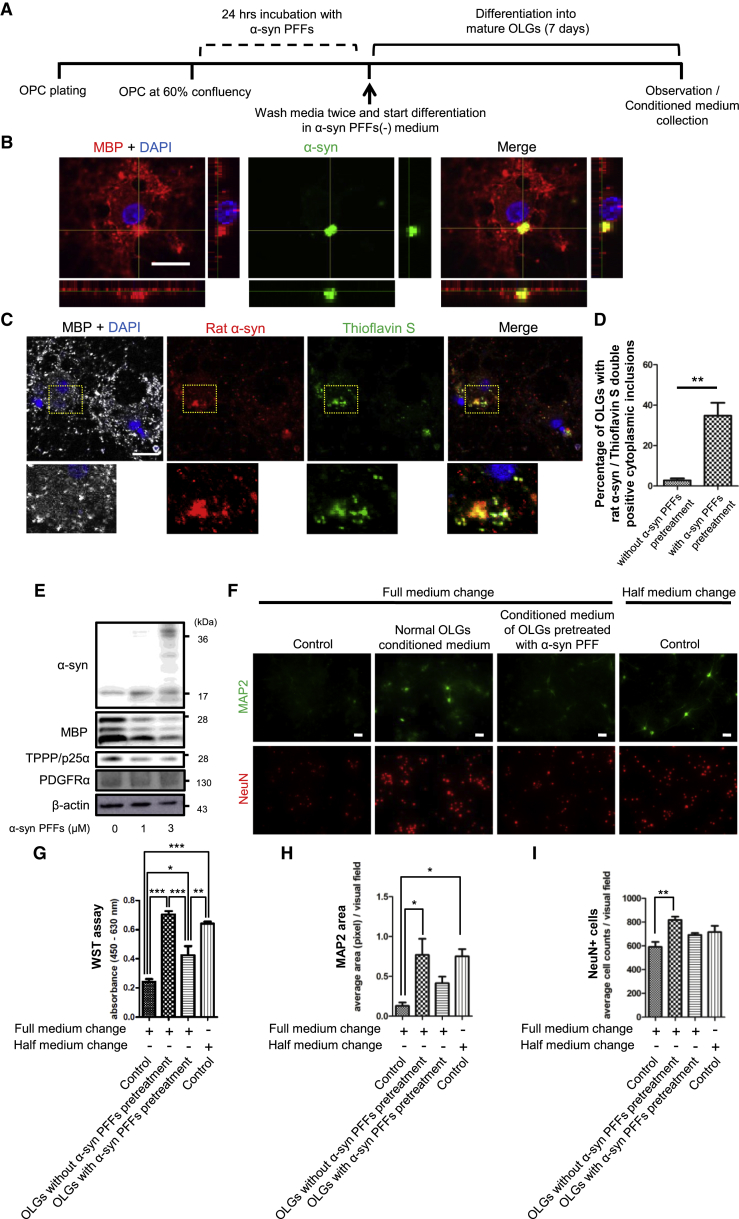
OPCs Pre-incubated with Recombinant Human α-Syn PFFs Differentiate into Mature OLGs with Endogenous α-Syn-Positive Inclusions
To determine if the endogenous α-syn accumulation in OPCs remains even after differentiation into mature OLGs, we tried to differentiate α-syn PFFs-treated OPCs according to the procedure shown in Figure 4A. Immunostaining revealed α-syn aggregates in differentiated OLGs, which were also immunoreactive to an endogenous α-syn antibody (Figures 4B–4D). Immunoelectron microscopy showed the intracellular existence of fibrillar α-syn (Figure S4A). An anti-phosphorylated α-syn antibody detected vague immunoreactivity merged with α-syn aggregates through immunostaining, nevertheless the immunoreactivity was not detectable with immunoblot analysis (Figures S4B and S4C). Pre-incubation with α-syn PFFs also caused a reduction in myelin-associated proteins, such as myelin basic protein (MBP) and tubulin polymerization promoting protein (TPPP/p25α) (Figures 4E, S4D, and S4E). The decrease of these OLG-specific markers was accompanied with an increase in the protein expression levels of PDGFRα and a decreasing trend of Mbp mRNA expression levels, suggesting insufficient differentiation as a result of α-syn PFF application (Figures S4F–S4I).
Figure 4.
Cytoplasmic α-Syn Inclusions and Impaired Neuro-supportive Function in Mature OLGs Derived through Maturation-Induction of α-Syn PFF-Treated OPCs
(A) Time chart of the experimental procedure is displayed. Cells are incubated with α-syn PFFs (1 or 3 μM) for 24 hr followed by complete removal of extracellular α-syn PFFs and initiation of 7 days of maturation.
(B) Cytoplasmic α-syn inclusion is confirmed by confocal microscopy. The scale bar represents 20 μm.
(C) The cytoplasmic inclusions contain endogenous rat α-syn. The inclusions are also labeled with Thioflavin S staining. The scale bar represents 20 μm.
(D) Percentages of OLGs containing cytoplasmic inclusions labeled with both endogenous rat α-syn and Thioflavin S are compared between OLGs with and without α-syn PFF (1 μM) pretreatment before maturation. Mean ± SEM; n = 3, respectively; independent cultures; one-way ANOVA, ∗∗p < 0.01.
(E) Immunoblot analysis reveals reduced myelin-associated proteins, MBP and TPPP/p25α, in OLGs pretreated with α-syn PFFs before maturation.
(F) Immunostaining of primary cortical neurons incubated with conditioned medium from OLGs reveals that reduced neuronal expressions of MAP2 and NeuN are induced by α-syn PFF (3 μM) pretreatment to OPCs before maturation. Each scale bar represents 50 μm.
(G–I) Viability of primary neurons is evaluated by the quantification of (G) WST assay, (H) MAP2-positive areas, and (I) numbers of NeuN-positive cells. Mean ± SEM; n = 4, respectively; independent cultures; one-way ANOVA, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
In order to delineate the functional consequence induced by α-syn PFF application before maturation, we focused on the neuro-supportive function of conditioned medium from OLGs (Figures 4F–4I). The equivalent medium kept under the same conditions in culture flasks without cells was used as control medium. Generally, when primary neurons are incubated with full-medium change, the survival of neurons is impaired compared with half-medium change. The conditioned medium from our OLG culture promoted the survival of primary neurons even with full-medium change. Notably, this neuro-supportive effect was suppressed when OLGs were differentiated from OPCs pretreated with α-syn PFFs.
Discussion
The existence of endogenous α-syn in primary oligodendroglial cell culture has been previously reported by Richter-Landsberg et al. (2000). On the other hand, a previous report of in situ hybridization in GCI-rich regions revealed no increase in α-syn mRNA expression in MSA brains compared with controls (Miller et al., 2005). The in vitro findings of endogenous α-syn accumulation as a reflection of autophagic impairment in oligodendrocyte lineage cells in our study (Figures 1D–1G) are in keeping with both of the previous studies and suggested the possibility that endogenous α-syn in oligodendrocyte lineage cells per se contributes to the formation of α-syn aggregates in MSA.
Macroautophagy is particularly important for pathological α-syn clearance, as it can degrade insoluble or aggregated forms of proteins (Konno et al., 2012). In fact, GCIs in MSA brains are immunoreactive to autophagic markers, such as LC-3 and p62 (Schwarz et al., 2012). In addition, the downregulation of a lysosomal enzyme, cathepsin D, is associated with intracellular α-syn accumulation in SH-SY5Y cells and myelin degeneration in knockout mice (Mutka et al., 2010, Bae et al., 2015). The lysosomal localization of α-syn, as well as the altered cathepsin D activity in OPCs of our study, emphasizes the pathological relevance of insufficient lysosomal degradation for α-syn accumulation and disease progression in MSA patients (Figures S3E and S3F).
Loss of neurotrophic support from oligodendrocytes in MSA has been postulated as the mechanism for neurodegeneration secondary to the primary glial pathology (Fellner et al., 2011). The RNA-seq and qPCR analysis corroborated that OPCs express comparable amounts of transcripts related to neuro-supportive factors, such as MCT1, BDNF, and GDNF (Figures S1H and 3). The lack of metabolic support for neurons by oligodendroglial MCT-1, a key player for the shuttling of lactate, results in neuronal death, which potentially contributes to neuronal degeneration in MSA (Lee et al., 2012). Furthermore, the OLGs containing α-syn aggregates in our experiments showed decreased expression levels of myelin-associated proteins and compromised neuro-supportive function via soluble factors (Figures 4E–4I). These findings are consistent with a previous investigation that detected myelin loss and neurodegeneration in the brains of transgenic MSA mice overexpressing α-syn in OLGs (Shults et al., 2005).
A limitation of the present study is that primary oligodendrocyte lineage cell cultures can only be used in experiments with relatively short incubation times due to the short survival time of these cells. Thus, we conducted the studies with higher concentrations of recombinant human α-syn PFFs compared with the concentrations used in the previous study of primary neurons (Volpicelli-Daley et al., 2011). In consequence, our study could not clearly detect phosphorylated α-syn immunoreactivity in oligodendroglial cells, even with 7 days of incubation after α-syn PFF administration (Figures S2H, S2I, S4B, and S4C). Considering that at least 7–10 days of the incubation period is required for neuronal α-syn to be phosphorylated, a longer observation period is warranted to confirm phosphorylation of α-syn in oligodendrocyte lineage cells with their modest basal α-syn expression compared with neurons (Volpicelli-Daley et al., 2011). As another limitation of the present study, we administered α-syn PFFs to cultured OPCs and OLGs to induce endogenous α-syn aggregation, since this is the standard protocol for α-syn aggregate formation in neurons (Volpicelli-Daley et al., 2011). However, the primary pathogenesis by which oligodendrocytes specifically trigger the production of misfolded α-syn in MSA is yet to be elucidated.
Overall, in vitro α-syn PFF administration in our primary cultures recapitulated a critical aspect of MSA pathogenesis and thus represents a practical model system. We suggest that OPCs potentially play a role in MSA pathology through internalization of extracellular α-syn and accumulation of endogenous α-syn, and that manipulation of α-syn expression in OPCs may serve as a therapeutic strategy against GCI formation.
Experimental Procedures
Study Approval
Autopsied human brains were obtained from Kyoto University Hospital through a process approved by an institutional research committee. All animal procedures were performed according to the guidelines of the Animal Use and Care Committee of Kyoto University and of the Institute of Biomedical Research and Innovation.
Primary Oligodendrocyte Lineage Cell Cultures
Mixed glial cell cultures were obtained from cerebral cortices of 1- to 2-day-old Sprague-Dawley rats and prepared as previously described (Maki et al., 2015). Isolated OPCs were differentiated into mature OLGs by incubation with differentiation medium for 7 days.
Preparation of Recombinant Human α-Syn
Recombinant human α-syn was purified in accordance with a previously established method (Masuda-Suzukake et al., 2013). PFFs were diluted in PBS at 1 μM or 3 μM, sonicated several times (30–60 s in total), filtered with 0.2-μm syringe filters (Life Sciences), and diluted in medium.
Immunostainings Observed with Confocal Laser Microscopy
An Olympus Fluoview FV1000 confocal microscope was used to observe immunostaining with secondary antibodies conjugated to fluorescein isothiocyanate, Texas red, or Cy5 (1:200, Alexa Fluor 488, 594, and 647).
RNA-Seq Analysis of OPCs
Agilent SureSelect Strand Specific RNA prep kit (catalog no. G9691A) was used with 200 ng of total RNA to construct cDNA libraries.
Statistical Analysis
All quantitative data were analyzed by using GraphPad Prism 5.0.
Author Contributions
S.K., study design, data acquisition and analysis, and drafting the manuscript and figures; T.M., study conception and design, supervising the preparation of primary cultures and data acquisition, and critical revision of the manuscript; H.K., N.U., and M.H., supervising the preparation of α-synuclein; T.A. and W.K., histopathological data acquisition and analysis; T.F., supervising electron microscopic data acquisition and analysis; M.U. and K.I., study conception, data interpretation, and critical revision of the manuscript; Y.O. and Y.K., data acquisition by RNA-seq analysis; X.B.M., V.L.D., Q.T., and T.M.D., data acquisition and analysis and critical revision of the manuscript for important intellectual content; R.T., funding, supervising, and critical revision of the manuscript for important intellectual content.
Acknowledgments
We thank all of our colleagues and staff at the Department of Neurology, Graduate School of Medicine, Kyoto University, including H. Yamashita, A. Kuzuya, H. Yamakado, M. Uemura, M. Hishizawa, Y. Taruno, M. Ikuno, E. Nakanishi, M. Sawamura, S. Okuda, K. Yasuda, S. Matsuzawa, Y. Hatanaka, R. Hikawa, and R. Tamano for their expert advice. We thank Dr. M. Takahashi for methodological suggestions on the immunoblot analysis. R.T. is supported by Grants-in-Aid for Scientific Research (A) (15H02540) and Grants-in-Aid for Scientific Research on Innovative Area Brain Environment (23111002) from the Japan Society for the Promotion of Science. T.M. is supported by Grants-in-Aid for Scientific Research (C) (16K07056) from the Ministry of Education, Culture, Sports, Science and Technology in Japan. X.B.M. is supported by NIH/NIA Johns Hopkins ADRC P50 AG05146. X.B.M., T.H.Q., V.L.D., and T.M.D. are supported by JPB and NIH/NINDS grant P50 NS38377. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases. X.B.M., V.L.D., and T.M.D. acknowledge the joint participation by the Adrienne Helis Malvin Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with the Johns Hopkins Hospital and the Johns Hopkins University School of Medicine and the foundation's Parkinson's Disease Program M-2014.
Published: January 11, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, one table, and two movies and can be found with this article online at https://doi.org/10.1016/j.stemcr.2017.12.001.
Contributor Information
Takakuni Maki, Email: harutoma@kuhp.kyoto-u.ac.jp.
Ryosuke Takahashi, Email: ryosuket@kuhp.kyoto-u.ac.jp.
Accession Numbers
The GEO accession number for the full dataset of RNA-seq is GEO: GSE107582; see https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107582 for more information and a full list of supported databases.
Supplemental Information
Primary OPC culture was incubated for 62 hr with proliferation medium containing PDGF-AA and FGF-2. Images were acquired at defined positions every 10 min.
Primary OPC culture was incubated for 62 hr with differentiation medium containing CNTF and T3. Images were acquired at defined positions every 10 min.
References
- Angot E., Steiner J.A., Hansen C., Li J.Y., Brundin P. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010;9:1128–1138. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- Bae E.J., Yang N.Y., Lee C., Kim S., Lee H.J., Lee S.J. Haploinsufficiency of cathepsin D leads to lysosomal dysfunction and promotes cell-to-cell transmission of alpha-synuclein aggregates. Cell Death Dis. 2015;6:e1901. doi: 10.1038/cddis.2015.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djelloul M., Holmqvist S., Boza-Serrano A., Azevedo C., Yeung M.S., Goldwurm S., Frisen J., Deierborg T., Roybon L. Alpha-synuclein expression in the oligodendrocyte lineage: an in vitro and in vivo study using rodent and human models. Stem Cell Reports. 2015;5:174–184. doi: 10.1016/j.stemcr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L., Jellinger K.A., Wenning G.K., Stefanova N. Glial dysfunction in the pathogenesis of alpha-synucleinopathies: emerging concepts. Acta Neuropathol. 2011;121:675–693. doi: 10.1007/s00401-011-0833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice G.L., Nathan J.A. The recognition of ubiquitinated proteins by the proteasome. Cell. Mol. Life Sci. 2016;73:3497–3506. doi: 10.1007/s00018-016-2255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno M., Hasegawa T., Baba T., Miura E., Sugeno N., Kikuchi A., Fiesel F.C., Sasaki T., Aoki M., Itoyama Y., Takeda A. Suppression of dynamin GTPase decreases alpha-synuclein uptake by neuronal and oligodendroglial cells: a potent therapeutic target for synucleinopathy. Mol. Neurodegener. 2012;7:38. doi: 10.1186/1750-1326-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Morrison B.M., Li Y., Lengacher S., Farah M.H., Hoffman P.N., Liu Y., Tsingalia A., Jin L., Zhang P.W. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J.M., Reynolds R., Fawcett J.W. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Maki T., Takahashi Y., Miyamoto N., Liang A.C., Ihara M., Lo E.H., Arai K. Adrenomedullin promotes differentiation of oligodendrocyte precursor cells into myelin-basic-protein expressing oligodendrocytes under pathological conditions in vitro. Stem Cell Res. 2015;15:68–74. doi: 10.1016/j.scr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M., Nonaka T., Hosokawa M., Oikawa T., Arai T., Akiyama H., Mann D.M., Hasegawa M. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V.E., Ettle B., Poehler A.M., Nuber S., Ubhi K., Rockenstein E., Winner B., Wegner M., Masliah E., Winkler J. alpha-Synuclein impairs oligodendrocyte progenitor maturation in multiple system atrophy. Neurobiol. Aging. 2014;35:2357–2368. doi: 10.1016/j.neurobiolaging.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.W., Johnson J.M., Solano S.M., Hollingsworth Z.R., Standaert D.G., Young A.B. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J. Neural Transm. (Vienna) 2005;112:1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- Mutka A.L., Haapanen A., Kakela R., Lindfors M., Wright A.K., Inkinen T., Hermansson M., Rokka A., Corthals G., Jauhiainen M. Murine cathepsin D deficiency is associated with dysmyelination/myelin disruption and accumulation of cholesteryl esters in the brain. J. Neurochem. 2010;112:193–203. doi: 10.1111/j.1471-4159.2009.06440.x. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C., Gorath M., Trojanowski J.Q., Lee V.M. alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J. Neurosci. Res. 2000;62:9–14. doi: 10.1002/1097-4547(20001001)62:1<9::AID-JNR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Schwarz L., Goldbaum O., Bergmann M., Probst-Cousin S., Richter-Landsberg C. Involvement of macroautophagy in multiple system atrophy and protein aggregate formation in oligodendrocytes. J. Mol. Neurosci. 2012;47:256–266. doi: 10.1007/s12031-012-9733-5. [DOI] [PubMed] [Google Scholar]
- Shults C.W., Rockenstein E., Crews L., Adame A., Mante M., Larrea G., Hashimoto M., Song D., Iwatsubo T., Tsuboi K., Masliah E. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J. Neurosci. 2005;25:10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley L.A., Luk K.C., Patel T.P., Tanik S.A., Riddle D.M., Stieber A., Meaney D.F., Trojanowski J.Q., Lee V.M. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning G.K., Stefanova N., Jellinger K.A., Poewe W., Schlossmacher M.G. Multiple system atrophy: a primary oligodendrogliopathy. Ann. Neurol. 2008;64:239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- Wilkins A., Majed H., Layfield R., Compston A., Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J. Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary OPC culture was incubated for 62 hr with proliferation medium containing PDGF-AA and FGF-2. Images were acquired at defined positions every 10 min.
Primary OPC culture was incubated for 62 hr with differentiation medium containing CNTF and T3. Images were acquired at defined positions every 10 min.