Summary
Tissue engineering offers an exciting possibility for cardiac repair post myocardial infarction. We assessed the effects of combined polyethylene glycol hydrogel (PEG), human induced pluripotent stem cell-derived cardiomyocyte (iPSC-CM), and erythropoietin (EPO) therapy in a rat model of myocardial infarction. PEG with/out iPSC-CMs and EPO; iPSC-CMs in saline; or saline alone was injected into infarcted hearts shortly after infarction. Injection of almost any combination of the therapeutics limited acute elevations in chamber volumes. After 10 weeks, attenuation of ventricular remodeling was identified in all groups that received PEG injections, while ejection fractions were significantly increased in the gel-EPO, cell, and gel-cell-EPO groups. In all treatment groups, infarct thickness was increased and regions of muscle were identified within the scar. However, no grafted cells were detected. Hence, iPSC-CM-encapsulating bioactive hydrogel therapy can improve cardiac function post myocardial infarction and increase infarct thickness and muscle content despite a lack of sustained donor-cell engraftment.
Keywords: myocardial infarction, rat, myocardial tissue engineering, hydrogel, induced pluripotent stem cell-derived cardiomyocytes, iPSC-CMs, MRI, erythropoietin
Highlights
-
•
Hydrogels tailored to myocardial properties do not impair normal heart function
-
•
Stem cell-encapsulating bioactive hydrogels can be delivered to infarcted hearts
-
•
Bioactive hydrogels increased cardiac function, infarct thickness, and muscle content
-
•
Cell-related benefits were observed even though donor cells did not stably engraft
Human iPSC-derived cardiomyocyte-encapsulating bioactive hydrogel therapy can improve cardiac function post myocardial infarction and increase infarct thickness and muscle content despite a lack of sustained donor-cell engraftment. This novel combination of injectable hydrogels, iPSCs, and the cardioprotective molecule EPO provides a highly translational strategy with excellent potential for prevention of cardiac failure.
Introduction
Pharmacological and surgical treatments have improved prognosis post myocardial infarction (MI), but there is still no effective method to replace the lost tissue, meaning that heart failure remains a leading cause of mortality and morbidity worldwide (Roger et al., 2012).
Regenerative medicine has the potential to repair damaged myocardium (Segers and Lee, 2008). Experimental studies show that grafting stem cells into the heart can directly replace the tissue damaged during MI (Orlic et al., 2001), while stimulation of innate repair mechanisms can activate resident progenitor cells and initiate regeneration (Ellison et al., 2013). Many cells with regenerative potential have been proposed, including those derived from blood, bone marrow, adipose, and cardiac tissue (Segers and Lee, 2008). Although each of these cell types has the ability to differentiate into new contractile cardiomyocytes, the efficiency of differentiation is low (Murry et al., 2006). Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have true cardiomyogenic potential (Zhang et al., 2009, Chong et al., 2014) and may offer a more effective cell type for cardiac regeneration. iPSCs can be expanded as highly purified and specific cell populations and avoid many of the immunological and ethical problems associated with ESCs (Yamanaka, 2009), making them suitable for clinical use (Chong et al., 2014).
A major current limitation of regenerative medicine is poor donor-cell retention. The most commonly used approach of directly injecting cells suspended in liquid solution into the infarcted heart results in the loss of 95%–99% of grafted cells within the first 24 hr (Sheikh et al., 2007). Tissue engineering can enhance cell retention and survival by delivering cells on or within biomaterials (Leor et al., 2005, Christman and Lee, 2006, Coulombe et al., 2014), including injectable hydrogels, tissue patches, cell sheets, or recellularized scaffolds (Jawad et al., 2008). These approaches can reduce immediate mechanical cell loss, provide a protective environment for cell survival, promote integration into the host tissue, and reduce myocardial wall stress by providing mechanical support for the damaged tissue (Place et al., 2009).
An advantage of hydrogels is the capacity for precise tailoring of mechanical properties and gelation times that allow them to be matched to the stiffness of the myocardium and delivery using current clinical catheter-based approaches, as opposed to the invasive procedures required for many other approaches (Hoffman, 2002, Frey et al., 2014). Synthetic hydrogels, including those based upon polyethylene glycol (PEG), have been used as a scaffold for drug and cell delivery (Lin and Anseth, 2009). They have excellent biocompatibility and safety records and are now well established for use in the medical field (Van Tomme et al., 2008). In addition, bioactive agents, such as growth factors, hormones, and small molecules, can easily be incorporated within the matrix. These can enhance donor-cell survival, integration, proliferation, and differentiation and modulate the host's immune and innate regenerative response (Laflamme et al., 2007). One such agent, erythropoietin (EPO), has already been successful in the clinic for reduction of cell death and remodeling post MI (Brines and Cerami, 2008) and has shown benefits in experimental infarction when delivered using a gelatin cardiac patch (Kobayashi et al., 2008).
The aim of the current study was to determine whether combining mechanically tailored injectable hydrogels, iPSC therapy, and the cardioprotective molecule EPO could provide a novel strategy to prevent cardiac failure in a rat model of MI.
Results
Hydrogels Can Be Tailored to the Properties Desired for Cardiac Repair
Hydrogels were formed by copolymerization of 4-arm PEG acrylate with PEG dithiol in stoichiometrically balanced ratios. Rheometry analysis of the evolution of storage modulus G′ and loss modulus G″ was used to calculate the gelation times for 5%, 10%, 20%, and 30% w/v hydrogels (n = 4) as 352 ± 123, 195 ± 42, 140 ± 14, and 138 ± 29 s, respectively. Shear modulus (maximum G′) for 5%, 10%, 20%, and 30% hydrogels was 0.8 ± 0.3, 6.9 ± 1.8, 17.2 ± 1.2, and 34.9 ± 1.2 kPa, respectively. Wet weight measurements indicated that the gel degradation rate decreased with increased hydrogel percentage, with a time to total degradation of 8, 16, 21, and 22 days for 5%, 10%, 20%, and 30% hydrogels (n = 4), respectively (Figure S2). The optimal hydrogel would have a gelation time of around 180 s to permit catheter-based delivery with rapid crosslinking once in situ; a calculated shear modulus between normal (6 kPa) and infarcted (18 kPa) myocardium (Berry et al., 2006); and a total degradation time greater than 10 days to allow donor-cell integration. The 10% PEG hydrogel most closely matched these parameters and was used in all later experiments.
EPO Protects Against Oxidative Stress and Cell Death In Vitro
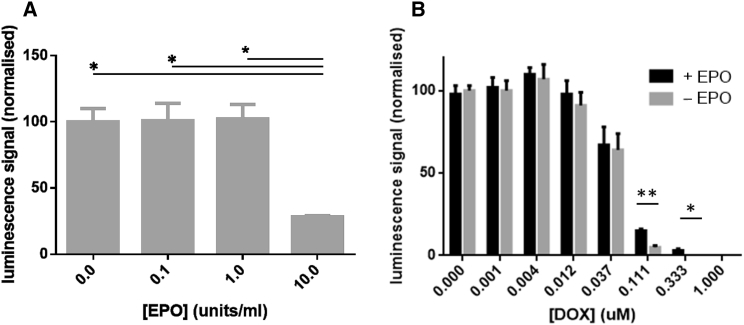
The iPSC-cardiomyocytes (iPSC-CMs) used in these studies were purchased from Cellular Dynamics Inc. and offer a highly purified cardiomyocyte cell line in which 98.2 ± 1.1% of cells expressed the cardiac-specific marker α-major histocompatibility complex (MHC). To assess the effects of EPO, iPSC-CMs were exposed to 0.1 to 10 units/mL EPO. Cell viability was unaltered up to 1 unit/mL EPO but was significantly reduced by 10 units/mL EPO (Figure 1A). To assess whether EPO was cardioprotective under cell-stress conditions, iPSC-CMs were exposed to doxorubicin. Cell viability was reduced in the presence of doxorubicin. However, addition of 1 unit/mL EPO significantly increased cell viability (Figure 1B). This suggests that EPO has therapeutic potential for use in MI treatment and in tandem with cell therapy.
Figure 1.
Effect of EPO on iPSC-CM Viability
(A) EPO dose response for iPSC-CMs in vitro. Increasing concentrations of EPO were added to iPSC-CM cultures for 6 hr (n = 4 individual replicates for each group). Cell viability was unaffected at 0.1 and 1 units/mL but compromised at 10 units/mL.
(B) Effect of 0.1 units/mL EPO on doxorubicin (DOX)-induced injury of iPSC-CMs. iPSC-CM cultures were exposed to increasing concentrations of doxorubicin for 6 hr with or without the presence of 1 unit/mL EPO. Cell viability was greater in the presence of EPO.
Data are presented as mean ± SD. ∗∗p < 0.01, ∗p < 0.05.
Hydrogel Injection Does Not Inhibit Normal Heart Function
To test potentially deleterious effects of biomaterial injection, PEG hydrogels were injected into Langendorff perfused hearts ex vivo (n = 5) and naive rat hearts in vivo (n = 5). No arrhythmia or significant change in heart rate or left ventricular (LV) pressure was observed in the Langendorff preparation, with heart rate 102% and mean LV pressure 93% of pre-injection values. No alteration to in vivo cardiac function was identified using cardiac magnetic resonance imaging (MRI), with end diastolic volume (EDV) of 412 ± 24 μL before and 400 ± 54 μL after and ejection fraction (EF) of 73% ± 5% before and 78% ± 11% after.
Bioactive Hydrogel Injections Improve Heart Function Post MI
Forty-three male nude rats survived MI or sham surgery and underwent cardiac MRI within 48 hr. As expected, EF was lower and infarct size higher in all infarcted groups. Injection of almost any combination of gel, cells, or EPO slightly reduced the expected increase in EDV and ESV at this early time point (Table 1 and Figure 2).
Table 1.
In Vivo and Histological Assessment of Cardiac Structure, Function, and Viability
| Sham n = 4/4 | MI n = 8/7 | Gel n = 8/6 | Gel-EPO n = 6/5 | Cell n = 9/7 | Gel-Cell-EPO n = 8/5 | ANOVA | ||
| End diastolic volume (μL) | acute | 290 ± 45 | 407 ± 51a | 348 ± 66 | 324 ± 49 | 328 ± 72 | 327 ± 39 | 0.018 |
| chronic | 422 ± 36 | 632 ± 65a | 598 ± 89 | 565 ± 146 | 649 ± 155a | 592 ± 60 | 0.038 | |
| End systolic volume (μL) | acute | 88 ± 15 | 222 ± 44a | 194 ± 65a | 151 ± 62 | 165 ± 55 | 181 ± 34 | 0.003 |
| chronic | 101 ± 10 | 334 ± 54a | 283 ± 85a | 220 ± 135 | 298 ± 103a | 272 ± 77 | 0.005 | |
| Late gadolinium enhancement (%) | acute | 3.2 ± 1.7 | 36.2 ± 7.3a | 38.3 ± 8.0a | 29.1 ± 9.3a | 38.4 ± 12.5a | 37.0 ± 8.1a | <0.001 |
| chronic | 1.4 ± 1.1 | 27.1 ± 4.4a | 28.3 ± 4.4a | 16.7 ± 7.2a,b | 21.1 ± 5.1a | 21.6 ± 2.6a | <0.001 | |
| Ejection fraction (%) | acute | 69.8 ± 1.1 | 45.4 ± 7.8a | 45.2 ± 10.2a | 54.6 ± 12.6 | 50.6 ± 8.9a | 44.8 ± 8.4a | <0.001 |
| chronic | 76.2 ± 0.9 | 47.3 ± 6.1a | 53.1 ± 10.5a | 63.5 ± 11.6b,d | 55.3 ± 8.0a,d | 54.7 ± 8.1a,d | <0.001 | |
| Infarct thickness (mm) | chronic | – | 1.34 ± 0.27 | 1.99 ± 0.34b | 1.66 ± 0.37b | 1.92 ± 0.35b | 1.98 ± 0.41b | <0.001 |
| Infarct muscle content (%) | chronic | – | 9.2 ± 3.3 | 22.7 ± 10.1b | 18.1 ± 10.5 | 24.2 ± 3.4b | 36.4 ± 6.3b,c | <0.001 |
A one-way between-subjects ANOVA test to compare the values between all groups. Post hoc analysis (Tukey's test), statistically significant (p < 0.05) difference between group versus sham (a); group versus MI (b); group versus all others (c). Student's t test analysis, statistically significant (p < 0.05) difference between acute (<48 hr) and chronic (10 weeks) time points (d).
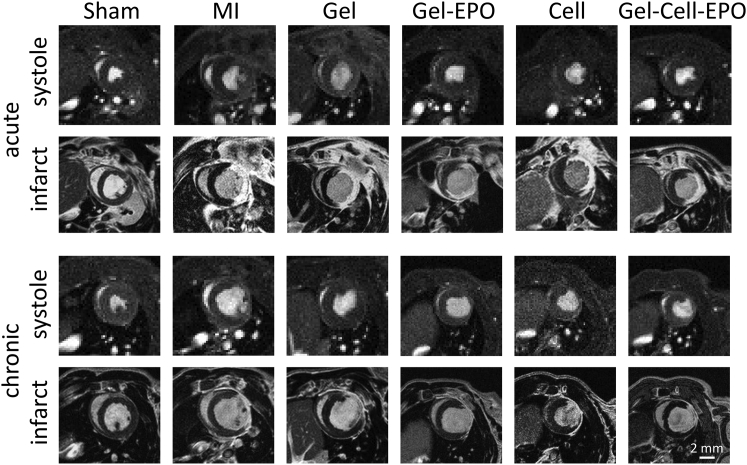
Figure 2.
MRI Characterization of Cardiac Structure, Function, and Viability Acutely and Chronically after MI
The systolic image shows the end systolic frame of a mid-ventricular cine-MR image. The infarct image shows a late gadolinium-enhanced MRI of the hyper-enhanced infarct region at the same position as the cine-MR image.
Nine rats did not survive until the 10 week MRI scan. This level of death is expected in this model and was not specific to any treatment group. At 10 weeks, EDV in saline and cell-alone treated hearts was increased compared with shams. However, EDVs were not significantly higher than shams in animals that received any form of gel injection, suggesting reduction of LV remodeling in groups that received hydrogel. EF significantly increased from the acute to the chronic MRI time points in the gel-EPO, cell, and gel-cell-EPO groups, although not in the control MI and gel groups. Percentage infarct sizes reduced in all groups owing to thinning of the infarcted tissue and hypertrophy of viable myocardium (Table 1 and Figure 2). Regional analysis of contrast-enhanced MRI demonstrated increased muscle mass within the infarcted apical region of the left ventricle, suggesting that functional improvements may have been due to greater viable muscle within the infarcted regions (Figure S3).
Therapies Increase Infarct Thickness and Muscle Content but Not Through Donor-Cell Engraftment
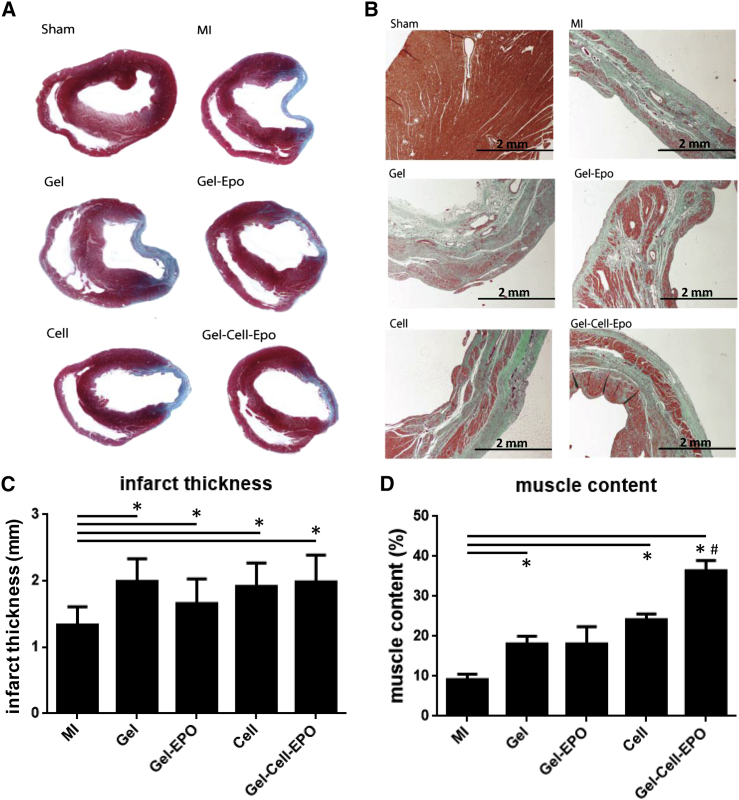
At 10 weeks, Masson's trichrome staining demonstrated that the thickness of the infarcted region was greater in all treated groups compared with control MI hearts (Figure 3C). Further, the percentage of muscle content within the infarcted region was also greater in every group except gel-EPO, suggesting that some form of tissue salvage or regeneration had occurred (Figure 3D). Muscle content within the infarcted region was greatest in the gel-cell-EPO group and significantly enhanced in comparison with any other treatment group (p < 0.005).
Figure 3.
Masson's Trichrome Staining of Infarct Size
(A) Masson's trichrome staining of mid-ventricular cardiac sections 10 weeks after infarction. Collagen fibers stain light blue and muscle fibers stain red.
(B) Magnified views (×4) show muscle fibers (red) within the collagen-rich scar (blue).
(C) Infarct thickness of hearts stained with Masson's trichrome stain after the chronic imaging time point. Each therapy increased the thickness of the infarcted tissue.
(D) The proportion of the infarct scar containing muscle fibers in the Masson's stained heart sections shows that all therapies, except gel-EPO, increased the viable tissue content within the infarct.
Data are presented as mean ± SD. Statistically significant difference between group versus MI(∗p < 0.05); group versus all other groups (#p < 0.005).
However, immunohistochemical staining for human cardiac troponin T within iPSC-CM grafted rat tissue was unable to identify any engrafted human cells at 10 weeks after injection. In addition, qPCR for expression of known early and late cardiac markers, including atrial natriuretic factor (NPPA), myosin-6 (MYH6), myosin-7 (MYH7), and alpha-1A and 1B adrenergic receptors (ADRA1a and ADRA1b) was unable to identify any human gene transcripts within the rat tissue. Staining and qPCR for human cells performed on a subset of rat hearts taken shortly after iPSC-CM injection was able to detect grafted human cells (Figure S4).
Discussion
Determining the optimal and most clinically relevant methods for enhancing the potential of stem cell-mediated cardiac repair is essential for the future of regenerative medicine. We compared the efficacy of clinically applicable biomaterials, biologics, and human iPSC-CMs in a rat model of MI using state-of-the-art pre-clinical cardiac MRI.
Although many cell types with potential to repair the damaged heart have been identified, the challenge of efficiently delivering, protecting, and nurturing them within the hostile post-MI environment has not been met. Tissue engineering is being intensely investigated as a way to address this problem, but such approaches must use clinically acceptable materials, delivery approaches, biologics, and cell types. Here, we engineered a clinically approved PEG hydrogel to have gelation time comparable with catheter-based delivery, shear modulus similar to the host myocardium, and a degradation profile that would support donor-cell integration; all properties desirable for cardiac repair.
Hydrogel injections did not disrupt normal heart function, with no observed arrhythmia or alterations to heart rate, mean LV pressure, or cardiac function. Injection of the hydrogel into the myocardium acutely post MI tempered the expected early increase in EDV and ESV seen in infarcted hearts. This suggests that the compliance of the biomaterial is suited to reducing wall stress acutely post MI and can limit initial chamber dilation. At 10 weeks, EDV was not significantly greater than sham-operated animals in any of the groups that received a gel injection, while control MI and cell-injected MI hearts had increased EDV. This provides further evidence that a 10% PEG hydrogel can limit LV remodeling post MI. These results support a previous in silico study predicting that the injection of non-contractile biomaterials into a finite element model simulation of the infarcted heart would reduce EDV (Wall et al., 2006). In addition, acellular alginate-based hydrogels have been tested clinically in MI patients and show preservation of LV indices (Frey et al., 2014). Taken together with the data presented here, these results support the use of injectable acellular biomaterials to reduce detrimental remodeling post MI.
Delivery of any therapy did not significantly alter the acute late gadolinium-enhanced (LGE) MRI infarct size compared with control MIs, suggesting therapeutic benefits were not directly related to early myocardial salvage. Between 48 hr and 10 weeks, EF significantly increased in the gel-EPO, cell, and gel-cell-EPO groups but not in the control MI or gel groups. This suggests that incorporation of a biological component within the gel is essential for an active increase in cardiac function, as opposed to the passive restriction of remodeling that occurred in the gel-alone group where EDV was reduced but EF was not enhanced. The degree of EF enhancement at the time points studied was similar across the gel-EPO, cell, gel-cell-EPO groups, suggesting that the mechanism of improved contraction was not cumulative or directly linked to a single component of the regenerative platform.
There have been previous reports of a tissue engineering approach combining biomaterials, soluble factors, and cell therapy for cardiac failure. In a rat model of MI, Davis et al. (2006) demonstrated a significant improvement in function in a combined nanofiber, insulin-like growth factor 1, and neonatal cardiomyocyte therapy group after 3 weeks, implying that the combination of all three therapies was required for significant benefit. Takehara et al. (2008) used a porcine model of MI to investigate the combination of a gelatin hydrogel, basic fibroblast growth factor (bFGF), and human cardiosphere-derived cells (hCDCs). After 4 weeks, they demonstrated a synergistic effect of hCDCs and the bFGF-loaded hydrogel upon EF and infarct volume. More recently, Kraehenbuehl et al. (2010) utilized a combination of a matrix-metalloproteinase-sensitive PEG hydrogel, thymosin β4, and human ESC-derived vascular cells in a rat model of MI. Using cardiac MRI, they demonstrated significant effects after 6 weeks upon infarct size, EDV, and EF, which was most pronounced in the combined hydrogel-thymosin β4-cell therapy group. The current study is the first to combine PEG hydrogels with iPSC-CMs and EPO. Rapid translation of this combination therapy to human trials is feasible as PEG-based compounds are already in extensive use in human medicine (Lin and Anseth, 2009), and EPO has already demonstrated safety and efficacy in the treatment of other human diseases (Coleman and Brines, 2004).
At 10 weeks, the thickness of the infarct in histological sections was increased in all treated groups. Further analysis of the extent of muscle fibers within the collagenous infarct zone revealed muscle salvage or regeneration was greater in the gel, cell, and gel-cell-EPO groups than control MI, and that gel-cell-EPO injection resulted in significantly more infarct muscle content than any other treatment. This suggests that the combination gel-cell-EPO therapy is optimal for salvaging or regenerating the infarct. Although gel-cell-EPO therapy did not lead to greater functional improvement in vivo after 10 weeks than that seen with other groups, it might be anticipated that benefits in function and survival would become apparent in more chronic post-MI remodeling. The possibility that the observed benefits resulted directly from PEG-mediated enhancement of cell retention cannot be answered by this study as a gel-cell group was not included. Likewise, the omission of a cell-EPO group precludes further discussion of the benefits of EPO on cell retention and resultant myocardial viability.
Importantly, immunohistochemistry and qPCR (methods that are able to detect cell grafts acutely after injection; Figure S4) did not detect any cells of human origin within the grafted hearts at 10 weeks. This indicates that the muscle fibers identified within the infarct region of treated hearts were not derived from integration of the grafted human iPSC-CMs but likely signifies either salvaged myocardium protected from cell death during acute MI, regenerated myocardium derived from the host endogenous stem cells (Ellison et al., 2013), or enhanced tissue vascularity, although unfortunately these processes were not measured in this study. It is disappointing that the advanced biomaterials approach employed here was unable to facilitate stable engraftment of iPSC-CMs within the host tissue, especially as we have previously shown enhanced cell retention within the myocardium using PEG hydrogels (Speidel et al., 2017). However, it is encouraging that the strategy did lead to increased cardiac function and infarct muscle content.
The use of cardiac MRI adds accuracy and clinical relevance to this study, as similar non-invasive assessments of function and tissue viability are employed in leading clinical trials of cell therapy for the heart (Bolli et al., 2011, Makkar et al., 2012). The ability to measure acute infarct size in vivo using LGE MRI can ensure that surgically induced infarct sizes are similar shortly after infarction, and that subsequent changes in function are due to therapy, rather than variation in initial infarct size. In this study, LGE infarct size was similar acutely after MI in the MI, gel, cell, gel-cell-EPO groups. However, the gel-EPO group had smaller LGE infarct size acutely after MI. This could be the result of variability in surgical procedure or might indicate that EPO can limit the degree of acute cell death during MI, as has previously been reported by others (Brines and Cerami, 2008, Robey et al., 2008).
A wide variety of cell types and biologics have been shown to initiate improvements in cardiac function post MI, both in the pre-clinical setting and in humans (Sanganalmath and Bolli, 2013). However, it is frequently reported that the number of grafted cells that remain within the heart is minimal at later time points (Gnecchi et al., 2005, Passier et al., 2008, Carr et al., 2011, Kawamura et al., 2012, Noseda et al., 2015). Taken in context, the data presented here indicate that the beneficial effects of grafting this bioactive hydrogel derive from a paracrine mechanism, with gel, cell, and EPO delivery initiating protective pathways that reduce cell death and stimulate endogenous repair, resulting in increased contractility and muscle content in the absence of sustained engraftment of donor cells.
Experimental Procedures
Expanded methods are available in the Supplemental Information.
Hydrogel Preparation
PEG hydrogels were formed using a Michael addition reaction by adding PEG dithiol (molecular weight = 1,019; Sigma-Aldrich, Dorset, UK) dissolved in 1% triethanolamine to 4-arm PEG acrylate (molecular weight = 23,300; Arab, AL, USA) dissolved in PBS. Viscoelastic properties were assessed using a rheometer (AR 2000ex; TA Instruments, Crawley, UK) at 37°C. Gel properties were examined by frequency sweep experiments at 1–10 Hz (ω = 6.3–62.83 rad/s) at a fixed strain amplitude of 0.001 (0.1%) in order to ensure uniform mechanical properties were maintained at frequencies present in human (1 Hz, 6.3 rad/s) and rat (8 Hz, 2.3 rad/s) heart.
Cell Culture
iPSC-CMs (Cellular Dynamics Inc, Madison, WI, USA) were thawed and plated onto 0.1% gelatin-coated surfaces at a density of ∼55,000 cells per cm2 for 1 week prior to injection. 98.2% ± 1.1% of iPSC-CMs expressed α-MHC, and viability was not significantly affected when cells were passed through the injection needle as determined using an automated cell counter (Luna-FL, Logos Biosystems) and acridine orange/propidium iodide staining.
Animal Model of Myocardial Infarction
All animal experiments were conducted under Home Office Project License 70/6568 and in accordance with local the Imperial College Ethics Committee and the ARRIVE Guidelines. The study design is illustrated in Figure S1. Eight-week old male nude rats (Charles River, Germany) underwent MI by occlusion of the left anterior descending artery as described (Carr et al., 2011). Sham surgery was performed by passing a suture and needle through the myocardium without ligation.
Injection of Therapeutics
A 10% 4-arm PEG acrylate solution was prepared by 1:1 mixing of 20% 4-arm PEG acrylate solution with cell suspension, and the appropriate volume of 10% PEG dithiol was added to this mixture just prior to injection to initiate the gelling process. Hydrogels (3 × 50 μL), cells (5 × 105), or a hydrogel and cell mixture was injected around the infarct border zone using a 27G needle. The experimental groups were as follows: sham, sham-operated control group that received PBS injection; MI, myocardial infarction group that received PBS injection; gel, MI group that received 10% PEG hydrogel injection; gel-EPO, MI group that received 10% PEG hydrogel containing 1 unit/mL EPO; cell, MI group that received 5 × 105 iPSC-CMs in PBS; gel-cell-EPO, MI group that received 10% PEG hydrogel containing 1 unit/mL EPO and 5 × 105 iPSC-CMs.
Cardiac MRI
Cardiac MRI was performed at 48 hr and 10 weeks as described (Stuckey et al., 2014) in anesthetized rats using a 4.7 T DirectDrive Varian MRI System (Palo Alto, CA, USA). Cardiac and respiratory-gated cine-MRI was used to measure cardiac structure and function. Infarct size was assessed by LGE MRI performed 25 min after intraperitoneal injection of 0.5 mg/kg Gd-DTPA-BMA (Omniscan) using a multi-slice inversion recovery sequence (Stuckey et al., 2014). Data were analyzed using ImageJ. MRI acquisition and analysis were performed by an investigator blinded to the experimental groups.
Histology and qPCR
Ten weeks after infarction, the animals were killed; hearts were fixed and cryosectioned for immunostaining or Masson's trichrome staining. RNA was extracted (RNeasy Mini Kit, QIAGEN) and qPCR performed in triplicate using pre-validated TaqMan assays (Life Technologies) for NPPA, MYH6, MYH7, and ADRA1a and b.
Statistical Analysis
Using GraphPad Prism, one-way between groups ANOVA(α = 0.05) was performed for group comparison. If the results of the ANOVA were found to be significant, post hoc analysis was performed using the Tukey multiple comparisons test to compare results among all groups.
Author Contributions
A.C., D.J.S., A.D., S.E.H., M.M.S., and T.A. planned and designed experiments. A.C., D.J.S., M.R., R.J.J., and C.A.M. performed and analyzed experiments. A.C., D.J.S., E.K., S.E.H., M.M.S., and T.A. wrote the manuscript.
Acknowledgments
The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London and grants from the BHF Regenerative Medicine Centre (RM/13/1/30157) and The Wellcome Trust Clinical PhD Fellowship scheme. D.J.S. is a BHF Intermediate Basic Science Research Fellow (FS/15/33/31608). M.M.S. is supported by a Wellcome Trust Senior Investigator Award (098411/Z/12/Z). Raw data is available upon request from rdm-enquiries@imperial.ac.uk.
Published: October 5, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at https://doi.org/10.1016/j.stemcr.2017.09.003.
Contributor Information
Sian E. Harding, Email: sian.harding@imperial.ac.uk.
Molly M. Stevens, Email: m.stevens@imperial.ac.uk.
Supplemental Information
References
- Berry M.F., Engler A.J., Woo Y.J., Pirolli T.J., Bish L.T., Jayasankar V., Morine K.J., Gardner T.J., Discher D.E., Sweeney H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- Bolli R., Chugh A.R., D'Amario D., Loughran J.H., Stoddard M.F., Ikram S., Beache G.M., Wagner S.G., Leri A., Hosoda T. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brines M., Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J. Intern. Med. 2008;264:405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- Carr C.A., Stuckey D.J., Tan J.J., Tan S.C., Gomes R.S., Camelliti P., Messina E., Giacomello A., Ellison G.M., Clarke K. Cardiosphere-derived cells improve function in the infarcted rat heart for at least 16 weeks–an MRI study. PLoS One. 2011;6:e25669. doi: 10.1371/journal.pone.0025669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J.J., Yang X., Don C.W., Minami E., Liu Y.W., Weyers J.J., Mahoney W.M., Van Biber B., Cook S.M., Palpant N.J. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman K.L., Lee R.J. Biomaterials for the treatment of myocardial infarction. J. Am. Coll. Cardiol. 2006;48:907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Coleman T., Brines M. Science review: recombinant human erythropoietin in critical illness: a role beyond anemia? Crit. Care. 2004;8:337–341. doi: 10.1186/cc2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe K.L., Bajpai V.K., Andreadis S.T., Murry C.E. Heart regeneration with engineered myocardial tissue. Annu. Rev. Biomed. Eng. 2014;16:1–28. doi: 10.1146/annurev-bioeng-071812-152344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.E., Hsieh P.C., Takahashi T., Song Q., Zhang S., Kamm R.D., Grodzinsky A.J., Anversa P., Lee R.T. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc. Natl. Acad. Sci. USA. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison G.M., Vicinanza C., Smith A.J., Aquila I., Leone A., Waring C.D., Henning B.J., Stirparo G.G., Papait R., Scarfo M. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Frey N., Linke A., Suselbeck T., Muller-Ehmsen J., Vermeersch P., Schoors D., Rosenberg M., Bea F., Tuvia S., Leor J. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circ. Cardiovasc. Interv. 2014;7:806–812. doi: 10.1161/CIRCINTERVENTIONS.114.001478. [DOI] [PubMed] [Google Scholar]
- Gnecchi M., He H., Liang O.D., Melo L.G., Morello F., Mu H., Noiseux N., Zhang L., Pratt R.E., Ingwall J.S., Dzau V.J. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Hoffman A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- Jawad H., Lyon A.R., Harding S.E., Ali N.N., Boccaccini A.R. Myocardial tissue engineering. Br. Med. Bull. 2008;87:31–47. doi: 10.1093/bmb/ldn026. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Miyagawa S., Miki K., Saito A., Fukushima S., Higuchi T., Kawamura T., Kuratani T., Daimon T., Shimizu T. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126(11 Suppl 1):S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Minatoguchi S., Yasuda S., Bao N., Kawamura I., Iwasa M., Yamaki T., Sumi S., Misao Y., Ushikoshi H. Post-infarct treatment with an erythropoietin-gelatin hydrogel drug delivery system for cardiac repair. Cardiovasc. Res. 2008;79:611–620. doi: 10.1093/cvr/cvn154. [DOI] [PubMed] [Google Scholar]
- Kraehenbuehl T.P., Ferreira L.S., Hayward A.M., Nahrendorf M., van der Vlies A.J., Vasile E., Weissleder R., Langer R., Hubbell J.A. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials. 2010;32:1102–1109. doi: 10.1016/j.biomaterials.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme M.A., Chen K.Y., Naumova A.V., Muskheli V., Fugate J.A., Dupras S.K., Reinecke H., Xu C., Hassanipour M., Police S. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Leor J., Amsalem Y., Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol. Ther. 2005;105:151–163. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Lin C.C., Anseth K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar R.R., Smith R.R., Cheng K., Malliaras K., Thomson L.E., Berman D., Czer L.S., Marban L., Mendizabal A., Johnston P.V. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry C.E., Reinecke H., Pabon L.M. Regeneration gaps: observations on stem cells and cardiac repair. J. Am. Coll. Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Noseda M., Harada M., McSweeney S., Leja T., Belian E., Stuckey D.J., Abreu Paiva M.S., Habib J., Macaulay I., de Smith A.J. PDGFRalpha demarcates the cardiogenic clonogenic Sca1+ stem/progenitor cell in adult murine myocardium. Nat. Commun. 2015;6:6930. doi: 10.1038/ncomms7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S.M., Li B., Pickel J., McKay R., Nadal-Ginard B., Bodine D.M. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Passier R., van Laake L.W., Mummery C.L. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- Place E.S., Evans N.D., Stevens M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- Robey T.E., Saiget M.K., Reinecke H., Murry C.E. Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell. Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanganalmath S.K., Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers V.F., Lee R.T. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- Sheikh A.Y., Lin S.A., Cao F., Cao Y., van der Bogt K.E., Chu P., Chang C.P., Contag C.H., Robbins R.C., Wu J.C. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–2684. doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speidel A.T., Stuckey D.J., Chow L.W., Jackson L.H., Noseda M., Abreu Paiva M., Schneider M.D., Stevens M.M. Multimodal hydrogel-based platform to deliver and monitor cardiac progenitor/stem cell engraftment. ACS Cent. Sci. 2017;3:338–348. doi: 10.1021/acscentsci.7b00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckey D.J., McSweeney S.J., Thin M.Z., Habib J., Price A.N., Fiedler L.R., Gsell W., Prasad S.K., Schneider M.D. T(1) mapping detects pharmacological retardation of diffuse cardiac fibrosis in mouse pressure-overload hypertrophy. Circ. Cardiovasc. Imaging. 2014;7:240–249. doi: 10.1161/CIRCIMAGING.113.000993. [DOI] [PubMed] [Google Scholar]
- Takehara N., Tsutsumi Y., Tateishi K., Ogata T., Tanaka H., Ueyama T., Takahashi T., Takamatsu T., Fukushima M., Komeda M. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J. Am. Coll. Cardiol. 2008;52:1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Van Tomme S.R., Storm G., Hennink W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008;355:1–18. doi: 10.1016/j.ijpharm.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Wall S.T., Walker J.C., Healy K.E., Ratcliffe M.B., Guccione J.M. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wilson G.F., Soerens A.G., Koonce C.H., Yu J., Palecek S.P., Thomson J.A., Kamp T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.