Abstract
Background
Diagnosis of TB in children is challenging, and is largely based on positive history of contact with a TB case, clinical and radiological findings, often without microbiological confirmation. Diagnostic efforts are also undermined by challenges in specimen collection and the limited availability of high sensitivity, rapid diagnostic tests that can be applied with a quick turnaround time. The current project was undertaken in four major cities of India to address TB diagnostic challenges in pediatric population, by offering free of cost Xpert testing to pediatric presumptive TB cases, thereby paving the way for better TB care.
Methods
A high throughput lab was established in each of the four project cities, and linked to various health care providers across the city through rapid specimen transportation and electronic reporting linkages. Free Xpert testing was offered to all pediatric (0–14 years) presumptive TB cases (both pulmonary and extra-pulmonary) seeking care at public and private health facilities.
Results
The current project enrolled 42,238 pediatric presumptive TB cases from April, 2014 to June, 2016. A total of 3,340 (7.91%, CI 7.65–8.17) bacteriologically confirmed TB cases were detected, of which 295 (8.83%, CI 7.9–9.86) were rifampicin-resistant. The level of rifampicin resistance in the project cohort was high. Overall Xpert yielded a high proportion of valid results and TB detection rates were more than three-fold higher than smear microscopy. The project provided same-day testing and early availability of results led to rapid treatment initiation and success rates and very low rates of treatment failure and loss to follow-up.
Conclusion
The current project demonstrated the feasibility of rolling out rapid and upfront Xpert testing for pediatric presumptive TB cases through a single Xpert lab per city in an efficient manner. Rapid turnaround testing time facilitated prompt and appropriate treatment initiation. These results suggest that the upfront Xpert assay is a promising solution to address TB diagnosis in children. The high levels of rifampicin resistance detected in presumptive pediatric TB patients tested under the project are a major cause of concern from a public health perspective which underscores the need to further prioritize upfront Xpert access to this vulnerable population.
Background
Tuberculosis is an important cause of childhood morbidity and mortality. It is estimated that around 10% of the 10.4 million global incident TB cases and 250,000 of the 1.7 million TB deaths in 2016 were amongst children (<15 years). Globally, children accounted for only 6.9% of the new cases that were notified in 2016 [1]. Under and delayed diagnoses of TB in children remains an obstacle to effective management of childhood TB due to which cases often remain underreported [2–3]. In high TB burden settings, it is estimated that childhood TB contributes to 15–20% of all TB cases and is one of the leading causes of childhood mortality [4]. India, which is among the highest TB and DR-TB burden countries globally, notified 76,745 childhood TB cases in 2016, accounting for only 5% of the total notified TB cases in the country [5].
Diagnosis of TB in children is challenging, is largely based on triad of, a) positive history of contact with a TB case, b) clinical and radiological findings and c) tuberculin skin test [5–9]. This is often without microbiological confirmation due to practical concerns in obtaining a sputum specimen, necessitating collection of alternate types of specimen in children [10–11]. Additionally, clinical diagnosis of TB is challenging in children, as signs and symptoms of TB in children can be very non-specific and similar to other common childhood chest infections [2, 10]. Limited availability of high sensitivity rapid diagnostic tests that can be applied with a quick turnaround time often leads to microbiological confirmation not being attempted [12, 13]. These diagnostic challenges and over reliance on clinical diagnosis limit the possibility of diagnosis of rifampicin-resistant TB (RR-TB) [14,15].
The WHO has recommended upfront Xpert MTB/RIF (Xpert) testing for the diagnosis of TB in pediatric presumptive pulmonary and extra-pulmonary (EPTB) cases [16–17]. Upfront rapid testing with Xpert assay, offers a promising solution to achieve the global objective of early and accurate detection of TB and rifampicin-resistant TB which is crucial for the timely initiation of accurate treatment in this vulnerable population [12,18]. Against this backdrop, the current project was undertaken in four major cities of India, namely Delhi, Chennai, Kolkata and Hyderabad, to address TB diagnostic challenges in the pediatric population, by offering free of cost upfront Xpert testing to pediatric presumptive TB cases. This project was initially undertaken in a pilot mode, from April- November, 2014, provided promising results [19]. However, this initial pilot did not address several aspects which were crucial to scalability and replicability of the intervention, including sustainability of the rapid turnaround time demonstrated in the initial phase over an extended period of time, impact of early diagnosis on TB care and mortality, etc. Further, some of the key findings observed in the initial pilot needed to be further validated on a larger cohort. We report here the larger project data from April, 2014 to June, 2016 and our experiences in rolling out the WHO recommendations in programmatic settings for pediatric presumptive TB cases in four cities of India and building the capacity of the health system to routinely offer upfront Xpert testing for all types of pediatric specimens including non-sputum specimens.
Material and methods
The project was implemented in 4 major cities of India, namely Chennai, Delhi, Hyderabad and Kolkata, covering a population over 30 million, with the objective of providing all pediatric presumptive TB cases in these cities with upfront access to free of cost Xpert testing. High throughput Xpert laboratories were established under the project and were dedicated to the pediatric population. Using a hub and spoke model, one laboratory was established in each city which linked to various public and private sector health care providers in the city. While the exact size of pediatric population, including the burden of TB in children, in these large cities is unknown, geographic coverage of the project was ensured by means of rapid specimen transport linkages between the Xpert lab and linked public and private institutions/providers in these cities. All the presumptive pediatric TB and DR-TB cases referred from the collaborating clinics and hospitals (both public/private) were offered free of cost Xpert testing. Xpert test was performed on various types of specimens such as gastric aspirate/lavage (GA/GL), bronco-alveolar lavage (BAL), cerebrospinal fluid (CSF), sputum, lymph node aspirates, etc.
Children (age 0–14 yrs) presenting with signs and symptoms suggestive of TB to any of the public or private health facilities in the project areas between April 2014 to June 2016 were prospectively enrolled in the project. The different providers (both public and private) in the project cities were given an option of prescribing free of cost Xpert testing to the pediatric presumptive TB case identified by them in their health facilities. The health care providers were requested to refer the different types of patient specimens (sputum and non-sputum specimen) by leveraging rapid specimen transportation linkages for Xpert testing established as part of the project. Specimens were collected at the respective health facilities and transported to a centralized lab in each city for microscopy and Xpert testing. A number of sensitization workshops were organized with various mapped healthcare providers in the project cities to increase the project uptake. The specimen transportation linkages were planned across all four cities, taking into account feasible local transportation mechanisms acceptable to respective providers. The mechanisms deployed for rapid specimen transportation included use of commercial courier services and local volunteers whose incidental costs were reimbursed at a standard rate. The average cost of transportation covered under the project was one USD per specimen shipment received at the lab. The referring providers shared their contact details in the test request form. Specimens were subjected to smear microscopy using Ziehl-Neelsen (ZN) staining for comparison, with the first available specimen being tested on Xpert. A rapid reporting mechanism was established to ensure that all test results were promptly communicated back to providers utilizing e-mail and short messaging service (SMS).
Presumptive pediatric TB cases were defined as per the India’s Revised National TB Control Programme (RNTCP) guidelines [20,21]. This includes children presenting with fever and/or cough for ≥2 weeks, with or without weight loss or no weight gain, or showing symptoms suggestive of pulmonary and/or extra-pulmonary TB [18].
Bacteriologically confirmed TB (TB cases) were defined as having a pulmonary and/or extra pulmonary specimen positive for TB by smear microscopy, culture and/or Xpert MTB/RIF, or other WHO-approved rapid diagnostic test [18].
Rifampicin-resistant TB cases were defined as bacteriologically confirmed TB cases with indication of rifampicin resistance on one or more of the following assays: Xpert MTB/RIF, line probe assay (LPA) or phenotypic drug susceptibility testing (DST) [18].
For the purpose of analysis, presumptive TB cases with only pulmonary specimens positive for TB were classified as pulmonary TB cases and cases with only extra-pulmonary specimens positive for TB were classified as extra-pulmonary TB cases. Presumptive TB cases with both pulmonary and extra-pulmonary specimens positive for TB were classified as mixed TB cases.
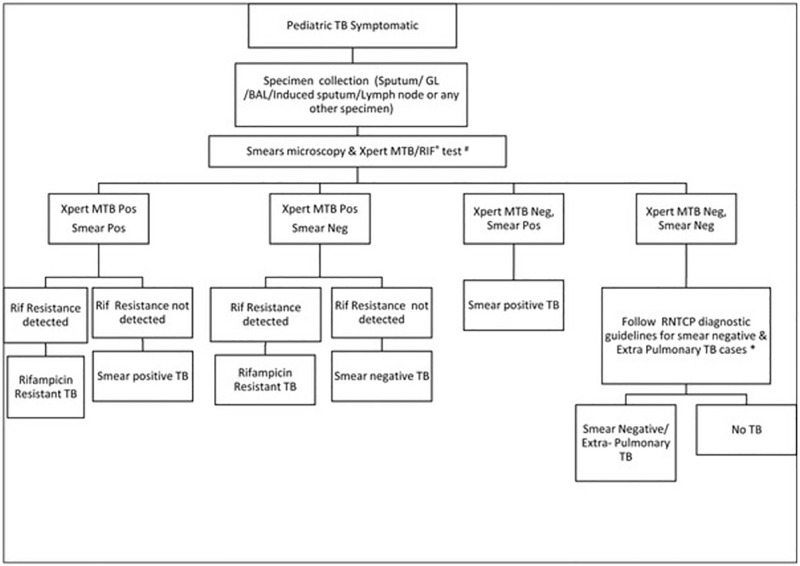
Specimens were collected at the referral facilities which were linked with the project Xpert lab in the city. Sputum smear microscopy was conducted using RNTCP smear microscopy guidelines with all functional components of quality assurance (QA) in place [22]. Xpert testing was performed as per the project diagnostic algorithm (Fig 1). In cases where specimens were less than 1ml in volume, preference was given to Xpert testing ahead of smear microscopy in line with WHO recommendations [15–16]. For a given patient, whenever multiple types of specimens were available, all types of available specimen were tested. In case of ‘error’ and ‘no result’ test result on Xpert, a repeat test was performed on the remaining sample–buffer mix. In case of ‘invalid’ and ‘rifampicin resistance indeterminate’ test result, repeat testing was performed on a second specimen as per the WHO recommendation [16]. Sputum specimens were tested by adding buffer in 1:2 proportions as recommended by the manufacturer (Cepheid manufacturer instructions). For non-sputum specimens, standard operating procedures (SOPs) developed by RNTCP and WHO were adopted [23]. Confirmatory DST for diagnosed rifampicin resistant cases was performed on LPA and/or Xpert and/or culture DST. Confirmatory DST was performed either on the remnant specimen, or additional specimen, if available.
Fig 1. Project diagnostic algorithm.
Treatment of all the diagnosed TB and rifampicin-resistant TB cases was initiated based on Xpert results in line with the project diagnostic algorithm as approved by RNTCP [24]. Any presumptive TB case receiving a negative result on Xpert and a positive result on smear microscopy were managed based on results of smear microscopy. Treatment of rifampicin resistant TB cases was based on initial Xpert rifampicin resistance results. Any case with rifampicin susceptible results on confirmatory DST was subsequently switched to appropriate regimen based on the decision of treating physicians.
Feasibility of Xpert implementation was assessed in terms of the ability of the assay to produce a valid result. The absence of a valid test result for any given assay performed was defined as a ‘test failure’ regardless of the underlying reason. The operational feasibility of offering Xpert testing to pediatric presumptive TB cases through a single lab in each of the 4 cities was assessed by analyzing the turnaround time (TAT) for specimen transportation, diagnosis and reporting of results to the providers.
Data management
Data for all presumptive TB and DR-TB cases were collected from the RNTCP lab request form (Annexure I). The project was carried out under uncontrolled programmatic field conditions covering health facilities in the selected geographic areas. The data were fully anonymized and could not be traced back to specific individuals, or limited group of patients. Microsoft Excel 2013 was used for cleaning the data and data were analyzed using “R” software. All confidence intervals were calculated based on the binomial distribution with 95% probability interval (S1 Data). For analytical purposes, pediatric patients were categorized into 3 groups: 0–4 years, 5–9 years, and 10–14 years of age. Odds ratios were calculated to determine the statistical significance and relation between two variables.
Ethical issues
Xpert testing for pediatric presumptive TB cases is an approved intervention under RNTCP. The current project was undertaken by FIND, after approval from and in collaboration with RNTCP. As such, the results presented here are our experience-sharing of implementing approved interventions in a programmatic setting within the existing accredited RNTCP TB diagnostic lab network. Since the observations described here are a part of implementation of approved interventions under RNTCP and a part of Standard of TB care in India, separate ethical clearance was not required.
Results
Overall 42,238 pediatric presumptive TB cases and DR-TB cases were provided access to project interventions, across the four cities from April 2014 to June 2016. Of the 42,238 presumptive TB cases tested through the project, significantly higher proportion of cases came from public sector facilities (38047, 90.1%; CI 89.8–90.4) than private sector (4191, 9.9%; CI 9.6–10.2). Amongst them, the proportion of males (23,018, 54.5%) was higher than females (19,218, 45.5%). Similar proportions of presumptive TB cases from the three pediatric sub-age-groups were enrolled (Table 1). Overall, the median age of enrolled children was 7 years (IQR 3–11 years). Males were marginally, but not significantly younger (median 7, IQR 3–10) than females (median 8, IQR 4–11).
Table 1. Presumptive pediatric TB cases enrolled under the project and TB cases diagnosed on Xpert, stratified by age, gender, referring sector and prior history of TB treatment.
| Variables | Number of patients tested | Number of diagnosed TB cases | Positivity rate, % (95% CI) | Proportion of total cases, % (95% CI) | Number of DR cases | Positivity of all TB cases, % (95% CI) | Proportion of total cases, % (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Total | 42238 | 3340 | 7.9 (7.7–8.2) | 295 | 8.8 (7.9–9.9) | |||
| Sub-Age group | 0–4 | 14082 | 681 | 4.8 (4.5–5.2) | 20.4 (19.0–21.8) | 42 | 6.2 (4.5–8.3) | 14.2 (10.6–18.9) |
| 5–9 | 14045 | 751 | 5.4 (5.0–5.7) | 22.5 (21.1–24.0) | 66 | 8.8 (6.9–11.1) | 22.4 (17.8–27.6) | |
| 10–14 | 14111 | 1908 | 13.5 (13.0–14.1) | 57.1 (55.4–58.8) | 187 | 9.8 (8.5–11.2) | 63.4 (57.6–68.8) | |
| Gender | Male | 23018 | 1293 | 5.6 (5.3–5.9) | 38.7 (37.1–40.4) | 119 | 9.2 (7.7–10.9) | 40.3 (34.7–46.2) |
| Female | 19218 | 2047 | 10.7 (10.2–11.1) | 61.3 (59.6–62.9) | 176 | 8.6 (7.4–9.9) | 59.7 (53.8–65.3) | |
| Transgender | 2 | 0 | 0 | 0 | ||||
| Sector | Public | 38047 | 3034 | 8.0 (7.7–8.3) | 90.8 (89.8–91.8) | 270 | 8.9 (7.9–10.0) | 91.5 (87.6–94.3) |
| Private | 4191 | 306 | 7.3 (6.5–8.1) | 9.2 (8.2–10.2) | 25 | 8.2 (5.5–12.0) | 8.5 (5.7–12.4) | |
| Smear Status | Positive | 964 | 964 | 1 (0.1–1) | 28.9 (27.3–30.4) | 120 | 12.4 (10.5–14.7) | 40.7 (35.1–46.5) |
| Negative | 38999 | 2219 | 5.7 (5.5–5.9) | 66.4 (64.8–68.0) | 160 | 7.2 (6.2–8.4) | 54.2 (48.4–60) | |
| NA | 2275 | 157 | 6.9 (5.9–8.0) | 4.7(4.0–5.5) | 15 | 9.6 (5.6–15.5) | 5.1 (3.0–8.4) | |
| Prior H/O TB Rx | Yes | 1295 | 530 | 40.9 (38.2–43.7) | 15.9 (14.7–17.2) | 95 | 17.9 (14.8–21.5) | 32.2 (27.0–37.9) |
| No | 40838 | 2808 | 6.9 (6.6–7.1) | 84.1 (82.8–85.3) | 200 | 7.1 (6.2–8.2) | 67.8 (62.1–73.0) | |
| NA | 105 | 2 | 1.9 (0.3–7.4) | 0.1 (0.0–0.02) | ||||
NA- not available
Of the 42,238 presumptive TB cases enrolled, 3,340 (7.9%, CI 7.7–8.2) pediatric TB cases were diagnosed. Among 3,340 pediatric TB cases, TB positivity in females (10.7%; CI 10.2–11.1) was observed to be almost two-fold higher than males (5.6%; CI 5.3–5.9); TB detection rates were similar in patients catered to by public and private sectors. Significantly higher TB positivity was observed in children in the 10–14 years age group (13.5%; CI 13.0–14.1) as compared to children in the 5–9 years age group (5.4%; CI 5.0–5.7) and 0–4 years age group (4.8%; CI 4.5–5.2). (Table 1)
Amongst the 3,340 pediatric TB cases diagnosed under the project, 295 (8.8%; CI 7.9–9.9) were found to be DR-TB cases. Higher levels of rifampicin resistance were observed in children in the 10–14 years age group (9.8%; CI 8.5–11.2) and 5–9 years age group (8.8%; CI 6.9–11.1) as compared to children in the 0–4 years age group (6.2%; CI 4.5–8.3). Similar levels of rifampicin resistance were observed in males (9.2%; CI 7.7–10.9) and females (8.6%; CI 7.4–9.9) and in patients from both public and private sectors facilities. (Table 1). Of the 295 cases, 95 (32.2%) had past history of TB treatment.
Of the 3340 TB cases detected under the project, 2534 (75.9% CI: 74.4–77.3) were pulmonary TB cases of which 210 (8.3% CI 7.3–9.4) were rifampicin resistant; 734 (22.0% CI 20.6–23.4) TB cases were extra-pulmonary TB cases of which 71 (9.7% CI 7.7–12.0) were rifampicin resistant. A total of 72 (2.2% CI: 1.7–2.7) TB cases had both pulmonary and extra pulmonary specimens positive for TB, of which 14 (19.4% CI: 12.0–30.0) were rifampicin-resistant. Of the 3340 TB cases, 185 (5.5%) were cases of TB meningitis of which 14 (7.6%) were found to be rifampicin-resistant. Confirmatory DST was performed on specimens showing rifampicin resistance. Overall findings indicate that majority of the valid TB-positive results on LPA were found to have concordant rifampicin results with Xpert. The detailed sub-analysis is on-going.
Project turnaround time
Same-day turnaround for Xpert testing including specimen collection, transportation, testing and reporting was the norm (0 days (IQR 0–0 days)). Overall, the median days between reporting of results and treatment initiation was 3 days (IQR 1–6 days) and 8 days (IQR 4–16 days) for TB cases and DR-TB cases, respectively (Table 2).
Table 2. Project turnaround time: Median time between events in diagnostic cascade, median (IQR).
| Variables | Days between collection and receipt | Days between receipt and testing | Days between testing and reporting | Days between reporting and treatment initiation (for those who initiated treatment after reporting) | Days between reporting and treatment initiation for DR TB (for those who initiated treatment after reporting) |
|---|---|---|---|---|---|
| Total | 0 (0,0) | 0 (0,0) | 0 (0,0) | 3 (1,6) | 8 (4,16) |
| Age | |||||
| 0–4 | 0 (0,0) | 0 (0,0) | 0 (0,0) | 2 (1,4) | 8 (2,115) |
| 5–9 | 0 (0,0) | 0 (0,0) | 0 (0,0) | 3 (1,6) | 9 (4.5,16) |
| 10–14 | 0 (0,0) | 0 (0,0) | 0 (0,0) | 3 (1,6) | 8 (4.3,15) |
| Gender | |||||
| Male | 0 (0,0) | 0 (0,0) | 0 (0,0) | 3 (1,6) | 7 (4,12) |
| Female | 0 (0,0) | 0 (0,0) | 0 (0,0) | 3 (1,6) | 9 (4,16) |
| Transgender | N/A | N/A | N/A | N/A | N/A |
| Sector | |||||
| Public | 0 (0,0) | 0 (0,0) | 0 (0,0) | 3 (1,6) | 8 (4,15.5) |
| Private | 0 (0,0) | 0 (0,0) | 0 (0,0) | 1 (1,3) | 9 (4.5,15.5) |
N/A- not applicable
Specimen wise analysis
For the 42,238 pediatric presumptive TB cases tested under the project, a total of 46,879 specimens were tested on Xpert and 41,918 specimens were tested using smear microscopy. The overall specimen-wise TB positivity rate on Xpert was 3,653/46,879 (7.8%; CI 7.6–8.0) and TB positivity rate on smear microscopy was 1,062/41,918 (2.5%; CI 2.4–2.7) (Table 3). TB case detection was more than threefold higher on Xpert as compared to smear microscopy independent of specimen types.
Table 3. Comparison of Xpert and smear positivity in different types of specimen.
| Type of specimen | Total Specimens on Xpert | Total Positive | % (95% CI) | Rif cases | % (95% CI) | Total Tests on Smear | Smear Pos | % (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Total | 46879 | 3653 | 7.8% (7.6–8) | 431 | 11.8% (10.8–12.9) | 41918 | 1062 | 2.5% (2.4–2.7) |
| Gastric Aspirate/ Lavage | 19703 | 1131 | 5.7% (5.4–6.1) | 99 | 8.8% (7.2–10.6) | 17462 | 243 | 1.4% (1.2–1.6) |
| Induced Sputum/Sputum | 19178 | 1499 | 7.8% (7.5–8.2) | 206 | 13.7% (12.1–15.6) | 18658 | 685 | 3.7% (3.4–4) |
| CSF | 3171 | 221 | 7.0% (6.1–7.9) | 22 | 10.0% (6.5–14.9) | 2282 | 0 | |
| Pus/FNAC/Lymph Node/Cervical Asp | 1332 | 519 | 39.0% (36.3–41.6) | 72 | 13.9% (11.1–17.2) | 998 | 87 | 8.7% (7.1–10.7) |
| Pericardial Fluid | 74 | 8 | 10.8% (5.1–20.7) | 0 | 52 | 1 | 1.9% (0–11.6) | |
| BAL | 1193 | 158 | 13.2% (11.4–15.3) | 10 | 6.3% (3.3–11.7) | 869 | 23 | 2.6% (1.7–4.0) |
| Pleural Fluid | 1184 | 56 | 4.7% (3.6–6.1) | 10 | 17.9% (9.3–30.9) | 1109 | 8 | 0.7% (0.3–1.5) |
| Ascitic Fluid | 297 | 9 | 3.0% (1.5–5.9) | 1 | 11.1% (0.6–49.3) | 214 | 1 | 0.5% (0–3) |
| Endo-tracheal Secretion/ Tracheal Aspirate | 141 | 11 | 7.8% (4.2–13.9) | 4 | 36.4% (12.4–68.4) | 80 | 4 | 5.0% (1.6–13) |
| Others* | 606 | 41 | 6.8% (5–9.2) | 7 | 17.1% (7.7–32.6) | 194 | 10 | 5.2% (2.6–9.5) |
*Abscess, bone, Bone Marrow, Chyle Fluid, Cystic Fluid, Knee Aspirate, Liver Biopsy, Peritoneal Fluid, Pleural Biopsy, Right colonic ulceration, Serum, Skin Biopsy, Synovial Fluid, Thoracic swab, Tissue
First and second specimens were positive for 259 presumptive TB patients and 3 tests were positive for 28 children
A total of 46,879 different specimens were Xpert tested, of which 19,178 (40.9%) were sputum and 27,701 (59.1%) were various kinds of non-sputum specimens. Among the sputum specimens tested with Xpert, 1,499 (7.8%; CI 7.5–8.2) were found to be positive for TB and of the non-sputum specimens tested under the project the overall TB positivity on Xpert was 2,154 (7.8%; CI 7.5–8.1). Smear microscopy yielded fewer positive diagnoses compared to Xpert testing. Of the 41,918 different specimens subjected to smear microscopy, 18,658 (44.5%) were sputum specimens and 23,260 (55.5%) were non-sputum specimens. Among 18,658 sputum specimens, 685 (3.7%; CI 3.4–4.0) were smear positive, while among 23,260 non-sputum specimens tested on smear microscopy, 377 (1.6%; CI 1.5–1.8) were TB positive. (Table 3)
Higher TB positivity on Xpert were observed on Pus/FNAC/lymph nodes (39.0%; CI 36.3–41.6), followed by BAL (13.2%; CI 11.4–15.3) specimens. Further, as compared to smear microscopy, significantly higher TB positivity on Xpert testing was observed on pericardial fluid, tissue, tracheal aspirate and CSF. However, low TB positivity was observed on pleural and ascitic fluid specimens. (Table 3)
Specimen-wise analysis showed that of 3,653 specimens found positive for TB on Xpert, 431 (11.8%; CI 10.8%-12.9%) specimens were rifampicin-resistant. Of these, 225 (52.2% CI 47.4–57.0) rifampicin-resistant specimens were non-sputum specimens (Table 3). Of a total of 46,879 different specimens tested on Xpert, 40,215 (85.8%; CI 85.5–86.1) were pulmonary (sputum, induced sputum, BAL, tracheal aspirate & gastric aspirate) and 6,664 (14.2%; CI 13.9–14.5) were non-pulmonary specimens. Higher Xpert TB positivity was observed amongst non-pulmonary specimens as compared to pulmonary specimens (854, 12.8%; CI 12.0–13.7 vs. 2,799, 7.0%; CI 6.7–7.2).
Yield of valid results on Xpert
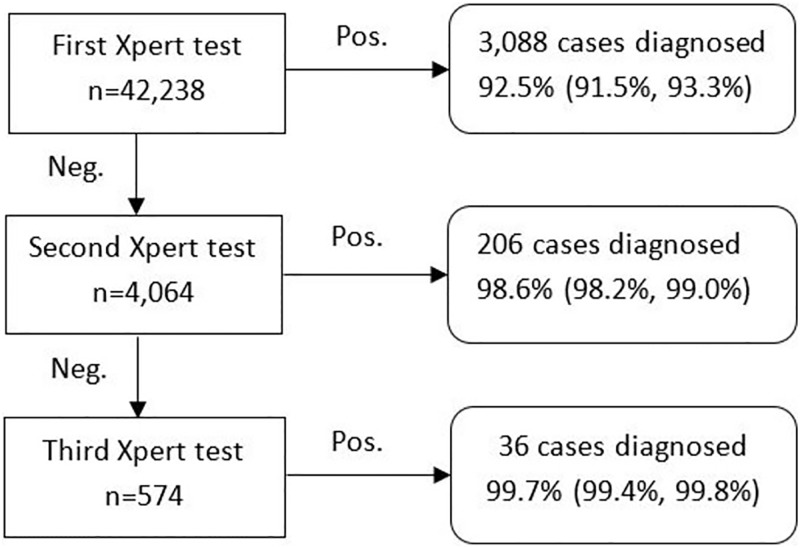
Overall out of the 42,238 pediatric presumptive TB cases tested in the project, valid test results were provided to 99.7% of the cases by ensuring retesting of initial test failures. Of these, a single specimen was tested for 38,173 patients, 3489 patients had two specimens tested and 576 patients had three specimens tested. Overall, of the 3340 paediatric TB cases detected under the project 92.5%; (3,088, CI 91.5%-93.3%) were detected on the first Xpert test, and an additional 206 cases after the second Xpert test, with a cumulative positivity rate 98.6% (CI 98.2%-99.0%) and an incremental yield of 6.1% on a second Xpert test. Additional 36 cases were diagnosed after the third test, i.e. incremental yield of 1.1% (Fig 2).
Fig 2. Incremental yields among first, second & third round Xpert testing.
Treatment details on pediatric TB cases
Among 3,045 rifampicin-sensitive TB cases, 2,738 (89.9%; CI 88.8–90.9) patients were initiated on treatment, 60 (2.0%, CI 1.5–2.5) died, 118 (3.9%; CI 3.2–4.6) were lost to follow-up prior to treatment initiation and 6 (0.2%, CI 0.1–0.5) were ‘transferred out’ for treatment initiation to their respective TB project management units under RNTCP. Treatment initiation for a total of 123 pediatric TB cases (4.0%) could not be confirmed as these cases could not be traced. The treatment initiation rates were similar for male and females; however, significantly lower treatment initiation rates were observed in the 0–4 years age group as compared to the other pediatric sub-age-groups. Lower treatment initiation rates in this age-group in the project cohort was likely due to higher pre-treatment mortality and higher initial loss to follow up rate (Table 4). Similarly, among 295 rifampicin-resistant TB cases, 257 (87.1%; CI 82.6–90.6) cases were confirmed to be initiated on treatment. Of the remaining cases, 18 (6.1%; CI 3.8–9.6) died before treatment initiation and 14 (4.8%; CI 2.7–8.0) were lost to follow up prior to treatment initiation. The remaining 6 (2.0%; CI 0.8–4.6) could not be traced for treatment initiation verification and follow-up. The proportion of DR-TB cases initiated on treatment was significantly lower in 0–4 years age group as compared to other pediatric sub-age groups again due to higher rates of pre-treatment mortality and initial loss to follow-up. (Table 5)
Table 4. Treatment initiation status of bacteriologically confirmed rifampicin sensitive TB cases.
| Variables | Total | Initiated treatment | % (95% CI) | Died | % (95% CI) | Lost to follow-up | % (95% CI) | Transferred Out | % (95% CI) | NA | % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 3045 | 2738 | 89.9 (88.8–90.9) | 60 | 2.0 (1.5–2.5) | 118 | 3.9 (3.2–4.6) | 6 | 0.2 (0.1–0.5) | 123 | 4.0 (3.4–4.8) |
| Age | |||||||||||
| 0–4 | 639 | 523 | 81.9 (78.6–84.7) | 32 | 5.0 (3.6–7.0) | 39 | 6.1 (4.4–8.3) | 2 | 0.3 (0.1–1.3) | 43 | 6.7 (5.0–9.0) |
| 5–9 | 685 | 620 | 90.5 (88.0–92.6) | 13 | 2.0 (1.1–3.3) | 22 | 3.2 (2.1–4.9) | 0 | 30 | 4.4 (3.0–6.3) | |
| 10–14 | 1721 | 1595 | 92.7 (91.3–93.8) | 15 | 0.9 (0.5–1.5) | 57 | 3.3 (2.5–4.3) | 4 | 0.2 (0.1–0.6) | 50 | 2.9 (2.2–3.9) |
| Gender | |||||||||||
| Female | 1871 | 1689 | 90.3 (88.8–91.6) | 38 | 2.0 (1.5–2.8) | 69 | 3.7 (2.9–4.7) | 1 | 0.1 (0–0.03) | 74 | 4.0 (3.1–5.0) |
| Male | 1174 | 1049 | 89.4(87.4–91.0) | 22 | 1.9 (1.2–2.9) | 49 | 4.2 (3.1–5.5) | 5 | 0.4 (0.2–1.1) | 49 | 4.2 (3.1–5.5) |
| Sector | |||||||||||
| Private | 281 | 260 | 92.5 (88.6–95.2) | 11 | 3.9 (2.2–7.1) | 1 | 0.4 (0.1–2.3) | 9 | 3.2 (1.6–6.2) | ||
| Public | 2764 | 2478 | 89.7 (88.4–90.8) | 60 | 2.2 (1.7–2.8) | 107 | 3.9 (3.2–4.7) | 5 | 0.2 (0.1–0.5) | 114 | 4.1 (3.4–5.0) |
NA- not available
Table 5. Treatment initiation status of bacteriologically confirmed rifampicin resistance TB cases.
| Variables | Total | Initiated treatment | % (95% CI) | Died | % (95% CI) | Lost to follow-up | % (95% CI) | NA | % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Overall | 295 | 257 | 87.1 (82.6–90.6) | 18 | 6.1(3.8–9.6) | 14 | 4.8 (2.7–8.0) | 6 | 2.0 (0.8–4.6) |
| Age | |||||||||
| 0–4 | 42 | 31 | 73.8 (57.7–85.6) | 6 | 14.3 (6.0–29.2) | 5 | 11.9 (4.5–26.4) | 0 | 0 |
| 5–9 | 66 | 57 | 86.4 (75.2–93.2) | 4 | 6.1 (2.0–15.6) | 4 | 6.1 (2.0–15.6) | 1 | 1.5 (0.1–9.3) |
| 10–14 | 187 | 169 | 90.4(85.0–94.0) | 8 | 4.3 (2–8.6) | 5 | 2.7 (1.0–6.5) | 5 | 2.7 (1.0–6.5) |
| Sex | |||||||||
| Female | 176 | 157 | 89.2 (83.4–93.2) | 11 | 6.3 (3.3–11.2) | 3 | 1.7 (0.4–5.3) | 5 | 2.8 (1.1–6.9) |
| Male | 119 | 100 | 84.0 (75.9–89.9) | 7 | 5.9 (2.6–12.2) | 11 | 9.2 (4.9–16.3) | 1 | 0.8 (0.04–5.3) |
| Sector | |||||||||
| Private | 25 | 19 | 76.0 (54.5–89.8) | 3 | 12 (3.6–32.3) | 3 | 12 (3.2–32.3) | 0 | 0 |
| Public | 270 | 238 | 88.2 (83.5–91.6) | 15 | 5.6 (3.3–9.2) | 11 | 4.1 (2.2–7.4) | 6 | 2.2 (0.9–5.0) |
NA- not available
Treatment outcome
Treatment status for 1,164 pediatric TB cases initiated on first-line anti-TB treatment during April 2014 to 15 December 2015 was available at the time of data analysis. Of these, 1,006 (86.4%; CI 84.3–88.3) cases had successfully completed the treatment, 30 (2.6%; CI 1.8–3.7) were still on treatment and 74 (6.4%; CI 5.1–8.0) had died during treatment. A total of 45 (3.9%; CI 2.9–5.2) cases were transferred out to during treatment to their respective RNTCP program management unit, as they were not residents of the same cities. Overall treatment loss to follow-up and failure rates were low: 3 (0.3%; CI 0.1–0.8) and 6 (0.5%; CI 0.2–1.2), patients respectively (Table 6).
Table 6. Treatment outcome in rifampicin sensitive cases.
| Variables | Grand Total | Completed | % (95% CI) | On Treatment | % (95% CI) | Lost to follow-up | % (95% CI) | Failure | % (95% CI) | Died | % (95% CI) | Transferred out | % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grand Total | 1164 | 1006 | 86.4 (84.3–88.3) | 30 | 2.6 (1.8–3.7) | 3 | 0.3(0.1–0.8) | 6 | 0.5 (0.2–1.2) | 74 | 6.4 (5.1–8.0) | 45 | 3.9 (2.9–5.2) |
| Age | |||||||||||||
| 0–4 | 214 | 168 | 78.5 (72.3–83.7) | 9 | 4.2 (2.1–8.1) | 0 | 0 | 27 | 12.6 (8.6–18) | 10 | 4.7 (2.4–8.7) | ||
| 5–9 | 259 | 220 | 84.9 (79.9–89.0) | 8 | 3.1 (1.4–6.2) | 0 | 3 | 1.2 (0.3–3.6) | 17 | 6.6 (4.0–10.5) | 11 | 4.3 (2.3–7.7) | |
| 10–14 | 691 | 618 | 89.4 (85–91.6) | 13 | 1.9 (1.1–3.3) | 3 | 0.4 (0.1–1.4) | 3 | 0.4 (0.1–1.4) | 30 | 4.3 (3.1–6.2) | 24 | 3.5 (2.3–5.2) |
| Sex | |||||||||||||
| F | 736 | 644 | 87.5 (84.8–89.9) | 12 | 1.6 (0.9–3.1) | 2 | 0.3 (0.1–1.1) | 6 | 0.8 (0.3–2) | 42 | 5.7(4.2–7.7) | 30 | 4.1 (2.8–5.8) |
| M | 428 | 362 | 84.6 (80.1–87.8) | 18 | 4.2 (2.6–6.7) | 1 | 0.2 (0.0–1.5) | 0 | 32 | 7.5 (5.3–10.6) | 15 | 3.5 (2.0–5.9) |
Similarly, 21 TB cases started on second line TB treatment were included for treatment outcomes analysis (treatment initiation during April 2014 to 30th June 2014). Of these 10 (47.6%; CI 26.4–69.7) cases had completed the treatment, 7 (33.3%; CI 15.5–56.9) had died, 2 (9.5%; CI 1.7–31.8) cases transferred out, 1 (4.8%; CI 0.8–22.7) failed treatment and 1 (4.8%; CI 0.8–22.7) was still on treatment. Since the majority of cases were not eligible for treatment outcome, this aspect was excluded from the scope of current manuscript.
Analysis of case mortality
Of the total 3,340 pediatric TB cases diagnosed under the project, a total of 190 (5.7% CI 4.9–6.5) deaths were observed (148 in rifampicin-sensitive TB cases and 42 rifampicin-resistant TB cases). Of these, 78 (41.1%, CI 34.1–48.4) deaths occurred before treatment initiation and 112 (59.0%, CI 51.6–66.0) deaths were after treatment initiation. Of 112 children who died after treatment initiation, exact date of death could be ascertained for 80 children (Table 7). For these 80 children, 20% (16/80; CI: 12.2%-30.7%) had died within a week of treatment initiation, cumulatively 41.3% (33/80; CI: 30.5–52.8) had died within 15 days of treatment initiation. Overall, 111 of 190 deaths (58.4% CI: 51.1–65.4) had occurred between specimen collection and 15 days of treatment.
Table 7. Mortality analysis stratified by treatment initiation of positive cases, age group, sex and past history of treatment.
| Variables | Pretreatment mortality, n (%) | On-treatment mortality, n (%) | ||||
|---|---|---|---|---|---|---|
| Rif Sen | Rif Res | Total | Rif Sen | Rif Res | Total | |
| Total | 60 (2.0%) | 18 (6.1%) | 78 (2.3%) | 88 (3.2%) | 24 (9.3%) | 112 (3.7%) |
| Age Group | ||||||
| 0–4 | 32 (5.0%) | 6 (14.3%) | 38 (5.6%) | 33 (6.3%) | 8 (25.8%) | 41 (7.4%) |
| 5–9 | 13 (1.9%) | 4 (2.1%) | 17 (1.9%) | 23 (3.7%) | 5 (3.0%) | 28 (3.5%) |
| 10–14 | 15 (0.9%) | 8 (12.1%) | 23 (1.3%) | 32 (2.0%) | 11 (19.3%) | 43 (2.6%) |
| Sex | ||||||
| F | 38 (2.0%) | 11 (6.3%) | 49 (2.4%) | 51 (3.0%) | 13 (8.3%) | 64 (3.5%) |
| M | 22 (1.9%) | 7 (5.9%) | 29 (2.2%) | 37 (3.5%) | 11 (11.0%) | 48 (4.2%) |
| Past history | ||||||
| No | 58 (2.2%) | 18 (9.0%) | 76 (2.7%) | 71 (3.1%) | 18 (11.1%) | 89 (3.6%) |
| Yes | 2 (0.5%) | 0 | 2 (0.4%) | 17 (4.0%) | 6 (6.3%) | 23 (4.4%) |
Discussion
In spite of recent progress in the development of TB diagnostic tools and available evidence and guidance on their application specifically in the context of pediatric TB and EP-TB, under and delayed diagnosis of childhood tuberculosis remains a major road-block in its effective management [2, 5]. Globally, there remains a major gap between the estimated burden and notification of paediatric TB cases [10, 25]. The current project was undertaken by FIND in coordination with the RNTCP to address this important implementation gap and focused on four major cities of India. This project, dedicated to the pediatric population, offered free of cost, upfront Xpert-based TB diagnosis to all symptomatic presumptive pediatric TB cases from a large number of linked facilities across these cities through a hub-and-spoke model. The current project represents one of largest global efforts exclusively dedicated to the implementation of WHO guidance on the use of upfront Xpert testing for pediatric population and the large project cohort provides useful insights into the potential impact of its effective implementation. The project design ensured rapid specimen transportation, testing, reporting and linkages to treatment and successfully extended routine Xpert testing to large numbers of pediatric specimens, especially non-sputum specimens.
Under the project, application of upfront Xpert testing led to three-fold higher TB detection as compared to smear microscopy along with detection of significant number of pediatric drug-resistant TB cases with a rapid turnaround time. Diagnostic challenges in the management of pediatric TB are well documented [26–29]. Clinical diagnosis in the absence of microbiological confirmation has been relied on extensively over the years for the diagnosis of TB in children, however clinical symptoms in children are often non-specific due to which TB diagnosis is complicated and/or delayed [2, 10–11]. Different clinical definitions used for diagnosis of TB in children have shown inconsistent and variable performance in different settings [13]. These factors often led to significant TB diagnostic delay in children, contributing to TB related morbidity and mortality [5,26]. Further diagnosis and management of rifampicin-resistant TB in the absence of laboratory confirmation poses a major challenge in this highly vulnerable age-group [5]. The findings from the current project demonstrate the ability of a rapid diagnostic assay in effectively addressing these diagnostic challenges in presumptive pediatric TB cases.
TB detection rates in female pediatric presumptive TB cases was two-fold higher as compared to males. Similar findings have earlier been earlier from India and Afghanistan [30,31]. This finding could not be attributed to variations in age, prior history of TB treatment or sectoral variations. While no clear explanation of this finding could be projected from the project data, it seems probable that a combination of different gender-related factors like access to health care may be part of the explanation. Thus it is not known whether this is a genuine difference in incidence or the result of gender variation in patient pathways or a combination of both.
More than half of the samples tested under the project were non-sputum specimens. Xpert performance on both sputum and non-sputum specimens provided a high proportion of valid results, similar to earlier reports [32–35]. The project implemented over two years in uncontrolled settings, demonstrates the feasibility of routinely applying Xpert testing to both sputum and non-sputum specimen. TB and DR-TB detection rates were similar in both sputum and non-sputum specimens. High TB positivity was observed in most specimens especially gastric aspirates/lavage, BAL, pus, lymph node, and pericardial fluid. Further, the project data clearly demonstrates limited utility of subjecting non-sputum specimen to smear microscopy. Xpert positivity rates on pleural fluid and ascitic fluid in the current study were very low, with limited additional gain as compared to smear microcopy, in line with WHO guidance and similar other studies [16,36]. A large number of rifampicin-resistant TB cases would have been missed in the absence of Xpert testing of non-sputum specimens, especially cases of rifampicin-resistant TB meningitis. These findings also have relevance in the context of adult extra-pulmonary TB (EP-TB) cases where higher levels of drug resistance are likely to be observed as compared to a pediatric population.
Under the project we observed alarmingly high levels of rifampicin-resistance in the diagnosed TB cases in the enrolled cohort of presumptive pediatric TB cases. The levels were high across the four cities, and didn’t show any clustering around particular facilities or time period or sector (public or private). Levels of rifampicin resistance were high in all three pediatric sub-age-groups. The majority of the DR-TB cases were smear negative and had no past history of previous TB treatment. Resistance levels were higher in cases with history of past TB treatment; however, the majority of the rifampicin-resistant cases were treatment naïve. While the project cohort wasn’t drawn purposively to capture a representative sample/estimate of children in India or these cities, levels of rifampicin-resistant TB observed in this large cohort is of great concern and suggests high levels of ongoing transmission. Children are not a priority target group for DR-TB control interventions, with the majority of pediatric TB cases being clinically diagnosed in the absence of microbiological confirmation. There is no systematic routine monitoring and reporting of rifampicin resistance in the pediatric population. The levels of rifampicin resistance observed in this project suggest a need for further research in this area and routine monitoring of DR-TB in pediatric population, both from programmatic management and epidemiological perspective [24,37]. Further, specimen-wise analysis showed that more than half of the rifampicin-resistant results were observed in non-sputum specimens. Amongst the sputum specimens, more than half of the rifampicin-resistant results were observed amongst specimen which were negative on smear microscopy. Such specimens are generally not subjected to upfront drug susceptibility testing in routine practice and such cases are either managed clinically or diagnosis is based on histopathological examination, which potentially can lead to missed diagnosis of drug-resistant TB, leading to sub-optimal TB treatment.
Under the project, in cases of high clinical suspicion and negative test result on Xpert, treating providers were encouraged to have additional patient specimens tested. Significant gains in the diagnostic yield on testing a second patient specimen were observed; however, on testing the third specimen the gain in diagnostic yield was only marginal. Similar other studies have also reported gains in sensitivity on testing an additional patient specimen [34, 38].
Since the outset of the project, same day diagnosis was one of the key project components. This was pertinent as the project was being implemented in the largest cities of the country, requiring turnaround time to be regularly monitored in the patients’ interest. Rapid turnaround time to test result was achieved by synergy of interventions such as local consultative approach in developing same day transportation linkages between the city labs and linked facilities utilizing services of volunteers, having high throughput labs to accommodate any intra-day workload surges, provision of having need based extended working hours, and 100% electronic reporting of results. This rapid diagnostic time in turnaround facilitated prompt treatment initiation both for rifampicin-sensitive and resistant TB cases. Availability of lab results with upfront information on rifampicin susceptibility enabled prompt initiation of appropriate treatment regimens, effectively addressing the diagnostic challenges faced by treating providers, minimizing the need for subjectively interpreting clinical presentations of the disease, thereby simplifying the patient pathway bottlenecks and delays which have been flagged in various studies [39–42]. Infield implementation of the project design over two years in the largest cities of India demonstrates the feasibility of replicating the project design in other similar and even smaller settings.
Quick turnaround time to test results and upfront availability of information on rifampicin susceptibility, leading to appropriate and rapid treatment initiation contributed to high treatment success rates. Overall loss to follow-up and treatment failure rates were exceptionally low (less than 1%).
However, in spite of rapid diagnostic time and prompt treatment initiation, significant levels of mortality were also observed in the project cohort. Mortality rates were higher in the 0–4 years, followed by 5–9 years and 10–14 years age-group. Review of overall mortality in the project cohort of diagnosed cases revealed that more than 5% of the children diagnosed with TB died (before or during treatment). In spite of the very quick diagnostic turnaround time and prompt access to treatment, it was concerning to see majority (58%) of these deaths occurring within two weeks of diagnosis. High proportion of mortality occurring early in the course of patient care suggests either patient health seeking delays or provider associated delays in prescribing free of cost Xpert testing which needs to be better understood and addressed. Similar, high mortality rates were observed in previous studies and suggested the need to intensify efforts at improving notification and treatment outcomes in children [43,44]. Other studies have also suggested that major advances needs to be made in the understanding of the epidemiology, diagnosis and treatment of childhood TB [45]. In this regard, a separate study on the pediatric patient pathways was undertaken as part of this project which will likely provide more insight on this aspect.
Conclusion
The current project implemented over more than two years demonstrated the feasibility of rolling out rapid and upfront Xpert testing for pediatric presumptive TB cases. The project design of a single Xpert lab per city efficiently covered each of the four large cities of India. The hub and spoke model implemented as part of the project ensured a rapid turnaround testing time, which in turn facilitated prompt and appropriate treatment initiation. These results further suggest that the upfront Xpert assay is a promising solution to address TB diagnostic challenges in children, testing both sputum and non-sputum specimens. The high levels of rifampicin resistance detected in pediatric TB cases, while being a major cause of concern from epidemiological prospective, also underscores the need to further prioritize upfront Xpert access to this vulnerable population.
Supporting information
(XLSB)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The project was funded by United States Agency for International Development (USAID) under Challenge TB project. FIND was responsible for implementation, training, coordination, monitoring, data analysis and writing of the report in close coordination with Central TB Division, Ministry of Health and Family Welfare, Government of India.
References
- 1.Global tuberculosis report 2017. Geneva: World Health Organization; 2017. http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf?ua=1
- 2.Connell TG, Zar HJ, Nicol MP. Advances in the Diagnosis of Pulmonary Tuberculosis in HIV-Infected and HIV-Uninfected Children. J Infect Dis.2011; 204 (suppl_4): S1151–S1158. Available: https://doi.org/10.1093/infdis/jir413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicol MP, Workman L, Isaacs W, Munro J, Black F, Eley B, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet 2011; 11:819–24. Available: https://www.ncbi.nlm.nih.gov/pubmed/21764384 doi: 10.1016/S1473-3099(11)70167-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Tuberculosis report 2013, http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1, Accessed 25 August 2014.
- 5.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infect Dis. 2008;8:498e510 Available: https://www.ncbi.nlm.nih.gov/pubmed/18652996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004;8:636e47 Available: https://www.ncbi.nlm.nih.gov/pubmed/15137548 [PubMed] [Google Scholar]
- 7.Starke JR. Diagnosis of tuberculosis in children. Pediatr Infect Dis J. 2000;19:1095e6 Available: https://www.ncbi.nlm.nih.gov/pubmed/11099094 [DOI] [PubMed] [Google Scholar]
- 8.Chow F, Espiritu N, Gilman RH, Gutierrez R, Lopez S, Escombe AR, et al. La cuerda dulceea tolerability and acceptability study of a novel approach to specimen collection for diagnosis of paediatric pulmonary tuberculosis. BMC Infect Dis. 2006;6:67 Available: https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-6-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int J Tuberc Lung Dis. 2002;6(12):1038–1045. Available: https://www.ncbi.nlm.nih.gov/pubmed/12546110 [PubMed] [Google Scholar]
- 10.Brent AJ. Childhood TB surveillance: bridging the knowledge gap to inform policy. J Trop Med. 2012; 2012: 865436 Available: https://www.hindawi.com/journals/jtm/2012/865436/ doi: 10.1155/2012/865436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med 2012; 367: 348–61. Available: http://www.nejm.org/doi/full/10.1056/NEJMra1008049 [DOI] [PubMed] [Google Scholar]
- 12.Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future microbiology. 2011;6(9):1067–1082. Available: https://www.ncbi.nlm.nih.gov/pubmed/21958145 doi: 10.2217/fmb.11.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar MK, Kumar P, Singh A. Recent advances in the diagnosis and treatment of childhood tuberculosis. J Nat SciBiol Med. 2015. Jul-Dec; 6(2): 314–320. Available: https://www.ncbi.nlm.nih.gov/pubmed/26283820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoang TTT, Nguyen NV, Dinh SN, Nguyen HB, Cobelens F, Thwaites G, Nguyen HT, Nguyen AT, Wright P, Wertheim HFL. Challenges in detection and treatment of multidrug resistant tuberculosis patients in Vietnam. BMC Public Health.2015; 15:980 Available: https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-015-2338-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pai M. Extrapulmonary Tuberculosis: New Diagnostics and New Policies, 2014; Indian J Chest Dis Allied Sci. 2014; 56:71–73. Available: https://www.ncbi.nlm.nih.gov/pubmed/25230546 [PubMed] [Google Scholar]
- 16.World Health Organization. Policy update: automated real time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/ RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children 2013. http://apps.who.int/iris/bitstream/10665/112472/1/9789241506335_eng.pdf [PubMed]
- 17.World Health Organization. Xpert MTB/RIF implementation manual, Technical and operational ‘how to’ practical consideration, 2014. http://apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf [PubMed]
- 18.Central TB Division Ministry of Health and Family Welfare, Managing the RNTCP in your area—A training course (Module 1–4). https://tbcindia.gov.in/showfile.php?lid=2907
- 19.Raizada N, Sachdeva KS, Swaminathan S, Kulsange S, Khaparde SD, Nair SA, et al. Piloting Upfront Xpert MTB/RIF Testing on Various Specimens under Programmatic Conditions for Diagnosis of TB & DR-TB in Paediatric Population. PLoS ONE 2015; 10(10): e0140375 doi: 10.1371/journal.pone.0140375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amdekar Y. Consensus statement on childhood tuberculosis. Indian Pediatrics.2010; vol. 47, no. 1, pp. 41–55. Available: http://www.ncbi.nlm.nih.gov/pubmed/20139477 [DOI] [PubMed] [Google Scholar]
- 21.Uria G A, Azcona J M, Midde M, Naik P, Reddy S, Reddy R. Rapid Diagnosis of Pulmonary and Extrapulmonary Tuberculosis in HIV-Infected Patients. Comparison of LED Fluorescent Microscopy and the GeneXpert MTB/RIF Assay in a District Hospital in India, 2012; Hindawi Publishing Corporation Tuberculosis Research and Treatment Volume 2012, Article ID 932862, 4 pages, Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3433122/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Geneva Definitions and reporting framework for tuberculosis -2013 revision. 213; S.n.p.3. http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf.
- 23.Revised National Tuberculosis Control Program. Standard Operating Procedure. 2014. http://www.tbcindia.nic.in/showfile.php?lid=3255. Accessed 15 January 2017.
- 24.Central TB Division Ministry of Health and Family Welfare, New Delhi, India, Revised National TB Control Programme, Guidelines on Programmatic Management of Drug Resistant TB (PMDT) in India, 2012; http://tbcindia.nic.in/pdfs/Guidelines%20for%20PMDT%20in%20India%20-%20May%202012.pdf
- 25.Jenkins HE. Global burden of childhood tuberculosis. Pneumonia.2016;8:24 doi: 10.1186/s41479-016-0018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swaminathan S, Rekha B. Pediatric tuberculosis: Global overview and challenges. Clin Infect Dis. 2010;50(Suppl 3):S184–94. Available: https://www.ncbi.nlm.nih.gov/pubmed/20397947 [DOI] [PubMed] [Google Scholar]
- 27.Marais BJ, Pai M. Recent advances in the diagnosis of childhood tuberculosis. Arch Dis Child. 2007;92:446–52. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2083717/ doi: 10.1136/adc.2006.104976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman N, Pedersen KK, Rosenfeldt V, Johansen IS. Challenges in diagnosing tuberculosis in children. Dan Med J. 2012. July;59(7):A4463 Available: https://www.ncbi.nlm.nih.gov/pubmed/22759843 [PubMed] [Google Scholar]
- 29.Poerksen G, Kazembe P, Graham S. Challenges of Childhood TB/HIV Management in Malawi. Malawi Medical Journal : The Journal of Medical Association of Malawi. 2007; 19(4):142–148. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3345928/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah I, Chilkar S. Clinical profile of drug resistant tuberculosis in children. Indian Pediatric. 2012. September; 49(9): 741–4. Epub 2012 Jun 10, Available: http://www.ncbi.nlm.nih.gov/pubmed/22728624. [DOI] [PubMed] [Google Scholar]
- 31.Sabawoon W, Sato H. Sex Difference in Tuberculosis in Afghanistan: A National Cohort Study. Mycobac Dis. 2012; 2:3 Available: http://dx.doi.org/10.4172/2161-1068.1000115 [Google Scholar]
- 32.Scott LE, Beylis N, Nicol M, Nkuna G, Molapo S, Berrie L, et al. The diagnostic accuracy of Xpert MTB/ RIF on extra pulmonary tuberculosis specimens: Establishing a laboratory testing algorithm for South Africa. Journal of Clinical Microbiology. 2014; Available: https://www.ncbi.nlm.nih.gov/pubmed/24622091 doi: 10.1128/JCM.03553-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid Molecular Detection of Tuberculosis and Rifampin Resistance, 2010; New England Journal of Medicine, 363: 1005–1015 Available: http://www.nejm.org/doi/full/10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahrili R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study, 2011; Lancet 377: 1495–1505 Available: http://www.ncbi.nlm.nih.gov/pubmed/21507477 doi: 10.1016/S0140-6736(11)60438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M DN. Xpert ® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults (Review). Chochrane Database Syst Rev, 2013; Available: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD009593.pub2/pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J ClinMicrobiol. 2011;49:4138–41. Available: http://jcm.asm.org/content/49/12/4138.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cobelens FGJ, Heldal E, Kimerling ME, Mitnick CD, Podewils LJ, Ramachandran R, et al. Scaling Up Programmatic Management of Drug-Resistant Tuberculosis: A Prioritized Research Agenda. PLoS Med. 2008; 5(7): e150 Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2443187/ doi: 10.1371/journal.pmed.0050150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez-Pastrana D, Torronteras R, Caro P, Luisa Anguita ML, Maria Lopz-Barrio AM, Andres A, et al. Comparison of amplicor, in-house polymerase chain reaction, and conventional culture for the diagnosis of tuberculosis in children. Clin Infect Dis. 2001; 32:17–22. Available: cid.oxfordjournals.org/content/32/1/17.full.pdf doi: 10.1086/317526 [DOI] [PubMed] [Google Scholar]
- 39.Arora V, Agarwal S. Chapter 12-Paediatric Tuberculosis: An Experience from LRS Institute of Tuberculosis and Respiratory Diseases. Tuberculosis Control In India. 2005; Vol. 330 pp. 115–118. [Google Scholar]
- 40.Nelliyanil M, Sharada MP, Joseph N, Basagoudar SS, Jayaram S, Patil DC A study of the socio-demographic profile and treatment outcome of paediatric tuberculosis patients in Bangalore Mahanagar Palike area. Indian J Tuberc. 2012; October; 59(4):207–13. Available: http://www.ncbi.nlm.nih.gov/pubmed/23342540 [PubMed] [Google Scholar]
- 41.Dangisso MH, Datiko DG, Lindtjorn B. Low case notification rates of childhood tuberculosis in southern Ethiopia. BMC Pediatrics December. 2015;15:142 Available: https://bmcpediatr.biomedcentral.com/articles/10.1186/s12887-015-0461-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalra A. Care seeking and treatment related delay among childhood tuberculosis patients in Delhi, India. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2017;21: 645–650. Available: https://www.ncbi.nlm.nih.gov/pubmed/28482958 doi: 10.5588/ijtld.16.0563 [DOI] [PubMed] [Google Scholar]
- 43.Hailu D, Abegaz WE, Belay M. Childhood tuberculosis and its treatment outcomes in Addis Ababa: a 5 years retrospective study. BMC Pediatr. 2014; 14: 61 Available: https://bmcpediatr.biomedcentral.com/articles/10.1186/1471-2431-14-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adejumo OA, Daniel OJ, Adebayo BI, Adejumo EN, Jaiyesimi EO, Akang G et al. Treatment Outcomes of Childhood TB in Lagos, Nigeria. J Trop Pediatr. 2016; 62 (2): 131 Available: https://www.ncbi.nlm.nih.gov/pubmed/26705331 doi: 10.1093/tropej/fmv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritz N, Curtis N. Novel concepts in the epidemiology, diagnosis and prevention of childhood tuberculosis. Swiss Med Wkly. 2014; 144, w14000 Available: https://www.ncbi.nlm.nih.gov/pubmed/25207918 doi: 10.4414/smw.2014.14000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSB)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.