Summary
Current in vitro islet differentiation protocols suffer from heterogeneity and low efficiency. Induced pluripotent stem cells (iPSCs) derived from pancreatic beta cells (BiPSCs) preferentially differentiate toward endocrine pancreas-like cells versus those from fibroblasts (FiPSCs). We interrogated genome-wide open chromatin in BiPSCs and FiPSCs via ATAC-seq and identified ∼8.3k significant, differential open chromatin sites (DOCS) between the two iPSC subtypes (false discovery rate [FDR] < 0.05). DOCS where chromatin was more accessible in BiPSCs (Bi-DOCS) were significantly enriched for known regulators of endodermal development, including bivalent and weak enhancers, and FOXA2 binding sites (FDR < 0.05). Bi-DOCS were associated with genes related to pancreas development and beta-cell function, including transcription factors mutated in monogenic diabetes (PDX1, NKX2-2, HNF1A; FDR < 0.05). Moreover, Bi-DOCS correlated with enhanced gene expression in BiPSC-derived definitive endoderm and pancreatic progenitor cells. Bi-DOCS therefore highlight genes and pathways governing islet-lineage commitment, which can be exploited for differentiation protocol optimization, diabetes disease modeling, and therapeutic purposes.
Keywords: human iPS cell, endoderm differentiation, diabetes, epigenetics, genomics, pancreas, beta-cell
Highlights
-
•
Beta cells and fibroblasts were reprogrammed to pluripotent cells (BiPSCs/FiPSCs)
-
•
ATAC-seq identified differential open chromatin sites in Bi-DOCS and Fi-DOCS
-
•
Bi-DOCS were enriched for genes expressed in BiPSC-derived cells
-
•
Bi-DOCS may explain the preferential differentiation of BiPSCs into islet cells
Beer and colleagues showed that iPSCs from beta cells (BiPSCs) preferentially differentiate into islet-lineage cells versus fibroblast iPSCs (FiPSCs). Differential open chromatin sites (DOCS) specific to BiPSCs (Bi-DOCS) were identified, enriched for pancreas development genes, and correlated with gene expression changes during directed differentiation. By governing developmental gene expression during differentiation, DOCS may explain the endodermal preference of BiPSCs and highlight novel beta-cell biology.
Introduction
Human pancreatic islets have been placed center stage in type 2 diabetes pathogenesis (Dimas et al., 2014). Current disease-modeling efforts are often frustrated by the limited availability of human physiologically authentic islet-like cells. Derivation of endocrine pancreas from iPSCs represents one solution for generating sufficient numbers of physiologically and disease-relevant human islet-like cells (Nostro et al., 2015, Pagliuca et al., 2014, Rezania et al., 2014). While directed in vitro differentiation of iPSCs routinely yields cells positive for islet hormones, such as insulin and glucagon, these cell populations are heterogeneous, contain poly-hormonal cells, and are functionally immature versus primary human islets (Rezania et al., 2014, van de Bunt et al., 2016). Differentiation efficiency also varies across iPSC lines (Bar-Nur et al., 2011, Burrows et al., 2016, Kim et al., 2010, Kyttala et al., 2016, Polo et al., 2010, Rouhani et al., 2014).
Possible causes for inconsistencies in differentiation capacity include technical factors such as reprogramming strategy (Balboa and Otonkoski, 2015), but also line-specific characteristics such as donor genotype (Burrows et al., 2016, Kyttala et al., 2016, Rouhani et al., 2014). There is also evidence to support an epigenetic “memory” in iPSCs (Bar-Nur et al., 2011, Kim et al., 2010, Polo et al., 2010), this comprising epigenomic and transcriptomic signatures of the original reprogrammed cell type, which may erode over prolonged periods of passaging in culture (Bar-Nur et al., 2011, Kim et al., 2010, Polo et al., 2010).
The epigenome plays an important role in establishing developmental competence in stem cells (Wang et al., 2015, Xie et al., 2013). Epigenetic memory could thus account for the enhanced propensity of beta-cell-derived iPSCs (BiPSCs) to differentiate down the endocrine pancreas lineage versus those derived from skin fibroblasts (FiPSCs; Bar-Nur et al., 2011). Here, we aimed to capitalize upon proposed BiPSC epigenetic memory to identify genes and pathways governing islet development and identity. By utilizing an assay for transposase accessible chromatin with high-throughput sequencing (ATAC-seq), we first aimed to define the open chromatin landscape in BiPSCs. Secondly, by comparison with global open chromatin in FiPSCs, we aimed to identify BiPSC-specific differential open chromatin sites (Bi-DOCS). We then integrated Bi-DOCS with publicly available genomic annotations to highlight regulatory elements, genes, and pathways that may explain preferential differentiation of BiPSCs toward endocrine pancreas-like cells.
Finally, to confirm that differences in open chromatin lead to changes in gene expression, we compared the transcriptome of cells derived from directed differentiation of both BiPSCs and FiPSCs toward two key stages of islet development (definitive endoderm and pancreatic progenitors). Our study improves understanding of human islet development, which may provide clues for improving in vitro differentiation protocols, facilitate more advanced disease modeling, and eventually contribute to therapeutic applications (Rezania et al., 2014, ViaCyte, 2014).
Results
Mapping the Open Chromatin Landscape in BiPSCs
We generated five BiPSC lines from three independent non-diabetic donors and five FiPSC lines from two independent non-diabetic donors using reprogramming strategies described previously (Bar-Nur et al., 2011, van de Bunt et al., 2016) (Table S1). Of note, because of differences in source, the BiPSCs were on average ten passages lower than the FiPSC lines. All iPSC lines passed quality control assessing pluripotency and differentiation capacity (Figures S1A–S1D). All but two lines (BiPSC-D1 and D2, Table S1) were karyotypically normal; however, as shown in the section on “In Silico and Cellular Validation of DOCS”, removing these two lines did not substantially alter the results reported in this study. As shown previously (Bar-Nur et al., 2011), BiPSCs showed enhanced spontaneous in vitro differentiation capacity into islet-lineage cells expressing FOXA2, PDX1, and INS, compared with FiPSCs (Figure S1E).
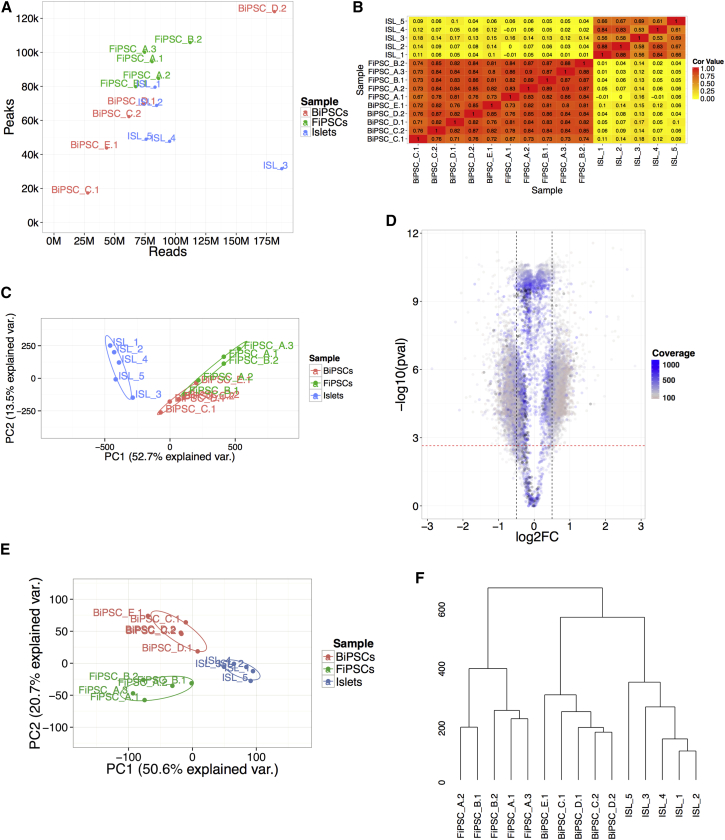
We performed ATAC-seq in all ten lines, as well as in five adult human islet samples. We generated between 28 and 186 million mapped and filtered reads per sample (Figure 1A), and identified between 17.3k and 123.9k open chromatin peaks (false discovery rate [FDR] < 0.01). Consistent with the pluripotent nature of both iPSC subtypes, we found the open chromatin pattern to be highly similar between BiPSCs and FiPSCs (median rho = 0.84, Figure 1B), and clearly distinct from primary human islets. Principal component analysis (PCA) across open chromatin peaks of all samples also confirmed that the two iPSC types were highly similar, and that iPSCs did not cluster by donor genotype (Figure 1C).
Figure 1.
The Open Chromatin Landscape of BiPSCs and FiPSCs
(A) Sample read (x axis) and peak number (y axis).
(B) Heatmap with mean sample correlation (rho) across peaks.
(C) Sample PCA plot based on peak read depth.
(D) DOCS Volcano plot with log2FC (x axis) and −log10 p value (y axis). DOCS, dashed black (log2FC ± 0.5) and red (FDR < 0.05) lines.
(E) Sample PCA plot based on DOCS read depth.
(F) Hierarchical clustering of samples based on DOCS read depth.
Color of cell type in (A, C, and E), BiPSCs (red), FiPSCs (green), human islets (blue).
Identifying BiPSC-Specific Open Chromatin
Despite the similarities in open chromatin between the two iPSC subtypes, we were able to identify differential open chromatin sites (DOCS) between BiPSCs and FiPSCs (Supplemental Experimental Procedures). We found 8.3k significant DOCS (FDR < 0.05) with a minimum absolute log2 fold change (log2FC) of 0.5 (Figure 1D). About 4.8k (58%) of DOCS were characterized by an increase in open chromatin in BiPSCs (Bi-DOCS), while the remaining 3.5k sites were more open in FiPSCs (Fi-DOCS). PCA and hierarchical clustering of normalized ATAC-seq read depth across DOCS showed that BiPSCs were more similar to human islets than FiPSCs (Figures 1E and 1F). Again, we did not observe sample clustering by donor. This suggests that genetic variation is not the predominant driver of differences in open chromatin in our study.
Bi-DOCS Are Enriched in Chromatin States Involved in Developmental Competence
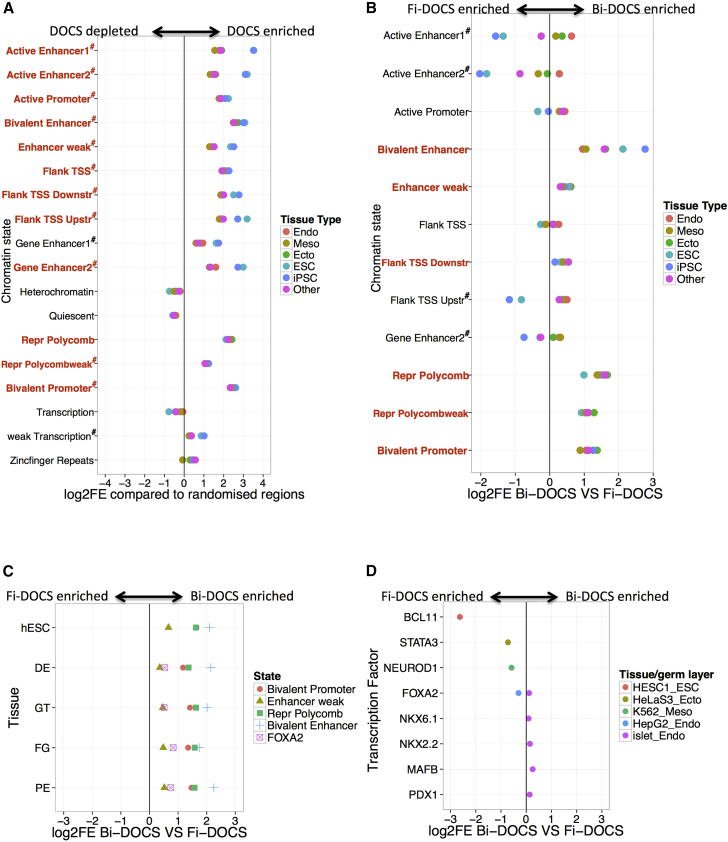
To uncover the potential regulatory landscape of DOCS, we obtained data on 18 predefined chromatin states from 98 Epigenome Roadmap cell types, which include human islets (Kundaje et al., 2015). Samples were grouped according to pluripotency (iPSCs and embryonic stem cells [ESCs], cell types per group = 4–5), germ layer (mature cells and tissues, cell types per group = 17–42), or “other” (germ layer status unclear, cell type number = 10). We found through permutation that DOCS were significantly enriched in enhancer, promoter, flanking transcription start site (TSS) and polycomb-repressed chromatin states, compared with random regions (mean FDR adjusted p value across all cell types of a given chromatin and germ layer/stem cell type < 0.05 and log2 fold enrichment (log2FE) > 0; Figures 2A and S2A).
Figure 2.
Enrichment of Bi-DOCS and Fi-DOCS in Chromatin States
(A) Enrichment (x axis) of DOCS in Epigenome Roadmap chromatin states (y axis) grouped according to pluripotent cell/germ layer type. Bold red states were enriched across all germ layer and pluripotent stem cell states while # indicates that ESC/iPSC states had highest enrichment.
(B) Log2FE ratio (x axis) of Bi-DOCS versus Fi-DOCS across chromatin states (y axis) enriched in (A). Bold red states are enriched for Bi-DOCS while # shows states most enriched for Fi-DOCS in ESC/iPSC lines.
(C) Log2FE ratio (x axis) of Bi-DOCS versus Fi-DOCS across chromatin states and FOXA2 TFBS (shape/color) identified during different stages (y axis) of endodermal development. human stem cells (hESC, FOXA2 not available), definitive endoderm (DE), gut tube (GT), fore gut (FG), and pancreatic endoderm (PE).
(D) Log2FE ratio (x axis) of Bi-DOCS versus Fi-DOCS across HESC-H1 (stem cell, red), HeLaS3 (ectoderm, dark yellow), K562 (mesoderm, green), HepG2 (endoderm, blue), and islet (endoderm, purple) TFBS (y axis).
We observed that across groups Bi-DOCS were enriched for bivalent enhancers, bivalent promoters, weak enhancers, flanking TSS downstream elements, and polycomb-repressed regions versus Fi-DOCS (mean log2FE ratio of Bi-DOCS versus Fi-DOCS (log2FE ratio) > 0, Figure 2B). In contrast, Fi-DOCS showed significant enrichment in ESC and iPSC active enhancer chromatin states compared with Bi-DOCS (log2FE ratio < 0, Figure 2B). These results show that Bi-DOCS overlap important regulatory elements known to be involved in developmental competence, including endoderm developmental competence (weak enhancer and bivalent states; Wang et al., 2015, Xie et al., 2013).
Bi-DOCS Are Enriched for Regulators of Early Endodermal Lineage Commitment
Endoderm developmental competence is regulated by epigenetic factors, including weak enhancers (marked by H3K4me1), bivalent regions marked by H3K27me3 (bivalent enhancers/promoters), and changes in gene expression governed by lineage-specific transcription factors such as FOXA2 (Wang et al., 2015, Xie et al., 2013). To confirm whether Bi-DOCS were enriched for such early endodermal regulatory states, we identified FOXA2 transcription factor binding sites (TFBS) and chromatin regulatory states across discrete stages of a previously published model of pancreatic endoderm development (Figure S2B; Supplemental Experimental Procedures) (Wang et al., 2015, Xie et al., 2013). We found that Bi-DOCS (versus Fi-DOCS) showed significant enrichment in weak enhancers, bivalent enhancers, bivalent promoters, polycomb-repressed regions, and FOXA2 TFBS as identified across all stages of pancreatic endoderm development (FDR < 0.05, log2FE ratio > 0, Figures 2C and S2C). Enrichment for these regulatory regions in Bi-DOCS may explain the preferential endodermal lineage commitment of BiPSCs.
Bi-DOCS Enrichment Is Specific to Pancreatic Endoderm Lineage Commitment
To show that Bi-DOCS are specifically enriched for pancreatic endoderm regulatory annotations and not regulatory annotations in other cell types or mature islets, we obtained publicly available TFBS information for four Encode cell lines (representative of pluripotency or germ layer commitment) (Encode Project Consortium, 2012) and TFBS active in human islets (Pasquali et al., 2014; Figures 2D and S2D).
While we found strong enrichment of all DOCS in all TFBS (Figure S2D), Bi-DOCS (compared with Fi-DOCS) were not enriched for TFBS from any of the four tested cell lines (Figure 2D), and showed only weak global enrichment in TFBS from adult primary human islets (max log2FE ratio = 0.3; Figures 2D and S2D).
These data confirm that Bi-DOCS are highly and specifically enriched for TFBS and chromatin states active in early pancreatic endoderm development (see previous section). However, despite this global pattern, we also found a number of Bi-DOCS associated with mature beta-cell genes including INS and PDX1 as described below.
Bi-DOCS Are Enriched for Genes and Pathways Regulating Pancreatic Islet Development and Function
To understand which genes and pathways are regulated by regions mapping to Bi-DOCS, we conducted a pathway enrichment test (Supplemental Experimental Procedures). We found that the 4.8k Bi-DOCS were significantly enriched in 27 Gene Ontology (GO) Biological Process and 2 MSigDB Pathway terms (binomial and hypergeometric FDR < 0.05 and binomial region fold enrichment and minimum gene enrichment > 2; Tables 1 and S2). Stratification of Bi-DOCS into different regulatory annotations highlighted additional terms in which Bi-DOCS were significantly enriched (hypergeometric FDR < 0.05 and enrichment > 2, compared with background; Tables S3 and S4, Supplemental Experimental Procedures). These include diabetes-relevant terms such as maturity-onset diabetes of the young (MODY), a form of monogenic diabetes caused mainly by mutations in islet transcription factors (Murphy et al., 2008). In addition, terms associated with glucose sensing and insulin metabolism and secretion were identified, as well as those relating to endocrine pancreas fate decisions and beta-cell development. For instance, FOXA2 (Lee et al., 2002), NKX2-2 (Sussel et al., 1998), and PDX1 (Stoffers et al., 1997) were highlighted, each of these genes serving important roles in islet development. WNT and NOTCH signaling terms were also enriched; these pathways are important in the induction of posterior endoderm and pancreatic progenitors, as well as pancreatic endocrine versus exocrine fate choices (Cras-Meneur et al., 2009, Nostro et al., 2011). Multiple terms relating to neuronal development were also enriched; this is consistent with gene expression profiles observed between developing pancreatic islets and neurons (van Arensbergen et al., 2010).
Table 1.
Bi-DOCS-Associated GO Biological Processes and MSigDB Pathways Terms
| Term | Pathway Description | DOCS Trend | Gene Number | Region Enrichment | Gene Enrichment | Regional FDR | Gene FDR | Associated Function |
|---|---|---|---|---|---|---|---|---|
| GO | neural tube patterning | Bi-DOCS | 80 | 3.27 | 2.69 | 8.7 × 10−17 | 2.4 × 10−5 | neuro-developmental |
| GO | positive regulation of embryonic development | Bi-DOCS | 51 | 4.75 | 3.23 | 1.0 × 10−16 | 9.3 × 10−3 | developmental |
| GO | non-canonical Wnt receptor signaling pathway | Bi-DOCS | 65 | 3.55 | 2.10 | 2.4 × 10−15 | 3.9 × 10−2 | WNT/NOTCH signaling |
| GO | hindbrain morphogenesis | Bi-DOCS | 82 | 2.84 | 2.32 | 4.1 × 10−14 | 4.1 × 10−4 | neuro-developmental |
| GO | branching involved in mammary gland duct morphogenesis | Bi-DOCS | 68 | 3.20 | 2.53 | 5.2 × 10−14 | 3.0 × 10−3 | developmental |
| GO | cerebellum morphogenesis | Bi-DOCS | 75 | 2.86 | 2.36 | 4.9 × 10−13 | 6.8 × 10−4 | neuro-developmental |
| GO | mammary gland duct morphogenesis | Bi-DOCS | 94 | 2.39 | 2.47 | 6.2 × 10−12 | 1.8 × 10−4 | developmental |
| GO | mammary gland epithelium development | Bi-DOCS | 123 | 2.10 | 2.12 | 9.1 × 10−12 | 1.4 × 10−4 | developmental |
| GO | dorsal spinal cord development | Bi-DOCS | 51 | 3.42 | 2.57 | 1.4 × 10−11 | 3.8 × 10−3 | neuro-developmental |
| GO | cerebellum development | Bi-DOCS | 110 | 2.10 | 2.02 | 1.4E-10 | 1.1 × 10−4 | neuro-developmental |
| GO | cerebellar cortex morphogenesis | Bi-DOCS | 58 | 2.91 | 2.31 | 1.9E-10 | 6.6 × 10−3 | neuro-developmental |
| GO | spinal cord association neuron differentiation | Bi-DOCS | 38 | 3.83 | 2.69 | 5.6E-10 | 1.6 × 10−2 | neuro-developmental |
| GO | dorsal/ventral neural tube patterning | Bi-DOCS | 50 | 2.99 | 2.83 | 2.0 × 10−9 | 9.5 × 10−4 | neuro-developmental |
| GO | negative regulation of Notch signaling pathway | Bi-DOCS | 34 | 3.73 | 2.55 | 1.1 × 10−8 | 1.0 × 10−2 | WNT/NOTCH signaling |
| GO | cell differentiation in spinal cord | Bi-DOCS | 93 | 2.07 | 2.83 | 1.1 × 10−8 | 2.6 × 10−9 | neuro-developmental |
| GO | cell differentiation in hindbrain | Bi-DOCS | 55 | 2.61 | 2.64 | 2.4 × 10−8 | 1.6 × 10−3 | neuro-developmental |
| GO | cerebellar cortex development | Bi-DOCS | 65 | 2.38 | 2.02 | 2.8 × 10−8 | 8.8 × 10−3 | neuro-developmental |
| GO | somitogenesis | Bi-DOCS | 93 | 2.00 | 2.05 | 4.6 × 10−8 | 4.1 × 10−4 | developmental |
| GO | white fat cell differentiation | Bi-DOCS | 29 | 3.37 | 2.69 | 1.3 × 10−6 | 4.5 × 10−2 | developmental |
| GO | embryonic axis specification | Bi-DOCS | 65 | 2.10 | 2.02 | 2.0 × 10−6 | 1.9 × 10−2 | developmental |
| GO | ventral spinal cord interneuron fate commitment | Bi-DOCS | 34 | 2.91 | 3.67 | 2.8 × 10−6 | 3.1 × 10−4 | neuro-developmental |
| GO | regulation of insulin receptor signaling pathway | Bi-DOCS | 44 | 2.44 | 2.02 | 5.0 × 10−6 | 3.3 × 10−2 | diabetes/insulin |
| GO | positive regulation of focal adhesion assembly | Bi-DOCS | 25 | 3.46 | 2.80 | 5.7 × 10−6 | 1.9 × 10−2 | other |
| GO | cerebellar cortex formation | Bi-DOCS | 40 | 2.54 | 2.43 | 6.4 × 10−6 | 1.8 × 10−2 | neuro-developmental |
| GO | regulation of Notch signaling pathway | Bi-DOCS | 58 | 2.06 | 2.21 | 1.4 × 10−5 | 1.1 × 10−3 | WNT/NOTCH signaling |
| GO | spinal cord dorsal/ventral patterning | Bi-DOCS | 39 | 2.34 | 2.55 | 4.3 × 10−5 | 1.0 × 10−2 | neuro-developmental |
| GO | ventral spinal cord interneuron differentiation | Bi-DOCS | 35 | 2.46 | 3.42 | 5.0 × 10−5 | 4.1 × 10−4 | neuro-developmental |
| MSigDB pathway | Wnt signaling network | Bi-DOCS | 53 | 2.68 | 2.31 | 9.4 × 10−8 | 2.8 × 10−2 | WNT/NOTCH signaling |
| MSigDB pathway | maturity-onset diabetes of the young | Bi-DOCS | 40 | 2.15 | 2.43 | 3.0 × 10−4 | 2.5 × 10−2 | diabetes/insulin |
To evaluate signatures of epigenetic memory, which could explain the enhanced endodermal differentiation capacity of BiPSCs, we tested whether Bi-DOCS-associated genes relevant for endodermal development were also open in human islets. We found that 99% of Bi-DOCS-associated genes, which are specifically expressed during endocrine pancreas development (van de Bunt et al., 2016), were also associated with open chromatin in human islets. In addition, when comparing the total number of Bi-DOCS and adult islet open chromatin-associated genes, we found that Bi-DOCS-associated genes were significantly enriched in stage-specific endocrine pancreas developmental genes versus those associated with adult islet open chromatin (Fisher test odds ratio [OR] = 1.3, p = 1.9 × 10−13).
The overlap of Bi-DOCS-associated genes with those open in human islets suggests BiPSCs could retain an epigenetic signature of their cell type of origin. In addition, the enrichment of Bi-DOCS for endocrine pancreas developmental stage-specific genes could explain the enhanced propensity of BiPSCs to differentiate down this same developmental lineage.
In Silico and Cellular Validation of DOCS
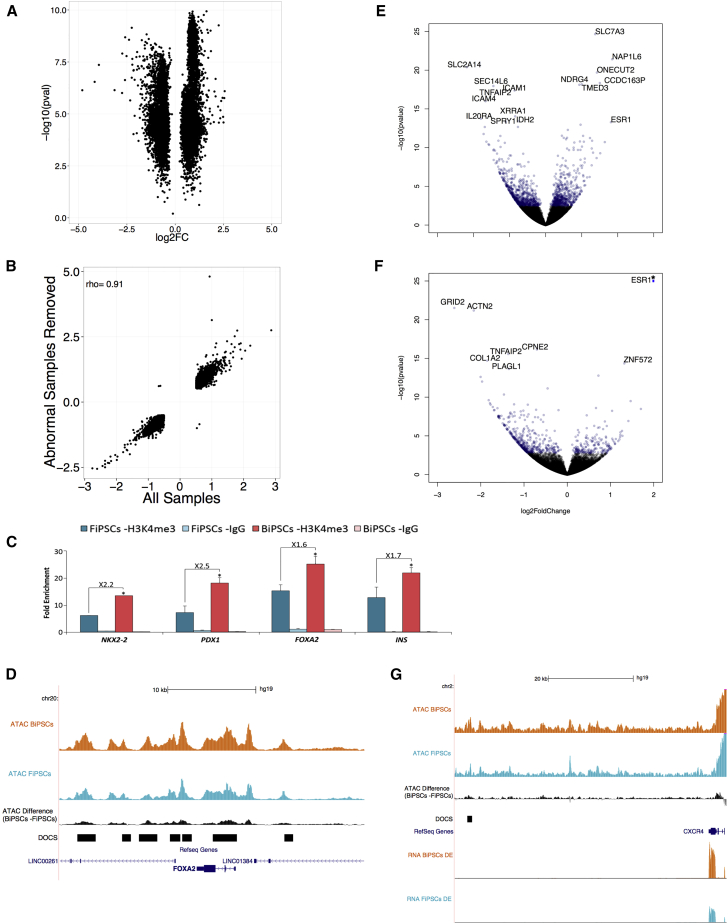
Using random permutations of sample labels to account for biases caused by donor-dependent factors, we found that the number of DOCS identified in permuted samples was lower than that observed in the original analysis (mean random sites = 2.0k versus 8.3k in the original analysis). This confirms that donor-specific factors are not the major driver in the differential open chromatin analysis in our study. Furthermore, we found that removing samples with an abnormal karyotype (lines BiPSC-D 1–2) yielded results similar to the original analysis (at 4.8k/8.3k overlapping DOCS) including all samples (Figures 3A and 3B, Supplemental Experimental Procedures).
Figure 3.
Validation of DOCS
(A) DOCS Volcano plot with log2FC (x axis) and −log10 p value (y axis) of karyotypically normal samples only.
(B) Log2FC correlation of overlapping DOCS identified from all samples (x axis) or only karyotypically normal samples (y axis).
(C) ChIP-qPCR analysis of H3K4me3 in BiPSCs (n = 5) and FiPSCs (n = 4). Values are means ± SEM and normalized to CRYAA and TEX15. ∗p < 0.05.
(D) FOXA2 region with normalized read depth of BiPSCs (orange) and FiPSCs (blue) samples. In black, ATAC read depth difference between iPSC types. Bottom tracks: DOCS (black bar) and genes (blue).
(E and F) Volcano plot with log2FC (x axis) and −log10 p value (y axis) of differentially expressed genes between FiPSC- and BiPSC-derived DE (E) and PP (F) cells. ∗ESR1 at PP stage out-of-scale in (F) (true log2FC = 5.74, −log10 p value = 100.9).
(G) CXCR4 region comparing normalized ATAC-seq read depth of BiPSCs (orange) and FiPSCs (blue, top). In black (middle), read depth difference between BiPSCs and FiPSCs. DOCS (black bar) are shown in the middle. Bottom tracks: CXCR4 RNA-seq data of BiPSC- (orange) and FiPSC (blue)-derived DE cells.
ATAC-seq data and prediction of Bi-DOCS were validated in vitro using H3K4me3 chromatin immunoprecipitation (ChIP)-qPCR and primers flanking a subset of DOCS near the promoters of PDX1, NKX2-2, FOXA2, and INS; these genes highlighted by the gene enrichment analysis. All five BiPSC lines showed higher chromatin enrichment in the promoter regions of PDX1, NKX2-2, FOXA2, and INS versus FiPSCs (Figure 3C). Figure 3D visualizes seven DOCS between FiPSCs and BiPSCs around the FOXA2 promoter.
Bi-DOCS Affect Gene Expression at Key Stages of Islet Development
To confirm that Bi-DOCS contain regulatory annotations that have an impact on gene expression during development, we performed directed differentiation of a subset of FiPSCs and BiPSCs cell lines toward islet-like cells and collected RNA at two key developmental stages: definitive endoderm (DE) and pancreatic progenitors (PP) (Table S1). RNA-seq and differential expression analysis identified 1,247 protein-coding genes differentially expressed (FDR < 0.05) between BiPSCs- and FiPSCs-derived cells at the DE stage (567 genes upregulated in BiPSC-derived and 680 in FiPSC-derived cells, Figure 3E), and 607 genes at the PP stage (181 genes upregulated in BiPSC-derived cells, 426 in FiPSC-derived cells, Figure 3F). Genes upregulated in BiPSCs-derived cells at both stages were significantly enriched in those mapping to Bi-DOCS (hypergeometric FDRDE = 3.4 × 10−7, FDRpp = 9.1 × 10−4), while genes upregulated in FiPSC-derived cells were significantly enriched in Fi-DOCS genes (hypergeometric FDRDE = 2.5 × 10−14, FDRpp = 5.0 × 10−8, Table S6). We detected a proximal Bi-DOCS site for 126 of the 567 genes upregulated at BiPSC-derived DE, and a Fi-DOCS site for 145 of the 680 genes upregulated at FiPSC-derived DE. Bi-DOCS-associated genes include those of known endoderm developmental biology, such as CXCR4 (Katsumoto and Kume, 2011). CXCR4 was significantly upregulated in BiPSCs at the DE stage (log2FC = 1.01, padj = 7.4 × 10−6, Figure 3G) and has a Bi-DOC site that overlaps a DE weak enhancer; a type of chromatin state involved in endodermal development (Wang et al., 2015).
Finally, genes upregulated in BiPSCs at the DE stage were significantly enriched for FOXA2 target genes expressed at this stage (hypergeometric FDR = 1.4 × 10−53, Table S6) which is in line with the FOXA2 TFBS enrichment in Bi-DOCS.
Discussion
Here, we systematically cataloged sites of open chromatin across the genome of BiPSCs to explain the preferential endodermal lineage commitment of BiPSCs versus FiPSCs. We were able to identify Bi-DOCS and showed that these Bi-DOCS were enriched for genomic regulatory annotations such as weak enhancers, bivalent enhancers, bivalent promoters, and FOXA2 binding sites shown to be active during endoderm lineage commitment (Wang et al., 2015, Xie et al., 2013).
We also confirmed that Bi-DOCS could be linked to gene expression changes at two stages of in vitro differentiation into islet-like cells. We also observed a significant enrichment of the FOXA2 targets reported at the DE stage within the genes upregulated in BiPSCs at this stage. FOXA2 is one of the master regulators of endodermal development (Raum et al., 2006), with FOXA2 binding sites being enriched at endodermal poised weak enhancers, thereby priming cells to receive extracellular cues for differentiation into endodermal lineage organs (Wang et al., 2015). Bi-DOCS were also enriched for GO terms relating to early pancreatic endoderm signaling pathways (Nostro et al., 2015, Pagliuca et al., 2014, Rezania et al., 2014), as well as MODY genes. This suggests that Bi-DOCS represent signatures of epigenetic memory and drive the differences in gene expression, which may account for the enhanced propensity of BiPSCs to differentiate toward endodermal lineage. Bi-DOCS may thus provide clues to genes and pathways involved in islet-lineage development.
The extent of epigenetic “memory” in reprogrammed iPSCs remains a subject of debate. Alongside data suggesting donor genotype may drive differentiation potential (Burrows et al., 2016, Kyttala et al., 2016, Rouhani et al., 2014), technical artifacts such as culture conditions, cell-cycle phase, and reprogramming technology may also give rise to transcriptional and epigenetic differences between iPSC lines. Additional work will be required to determine whether the differences reported here are solely dependent on the donor cell type. Specifically, the two iPSC types in our study differ substantially in passage number, and prolonged passaging has been suggested to eradicate iPSC epigenetic memory (Polo et al., 2010).
Future work should focus on increasing sample number and integrating our data with additional genomic annotations to further elucidate the mechanisms driving developmental competence and differentiation capacity. In conclusion, our findings provide a valuable resource for improving endocrine pancreas lineage differentiation protocols and may lead to the development of enhanced islet cell models, and ultimately improved therapeutic approaches for diabetes.
Experimental Procedures
iPSC Generation and Human Islet Sample Collection
As described in Supplemental Experimental Procedures, relevant Ethics and Institutional Review Boards (IRBs) approved the use of human islets and human iPSCs in this study. BiPSC and FiPSC lines were generated by reprogramming beta cells and skin fibroblasts as described previously (Bar-Nur et al., 2011, van de Bunt et al., 2016). Five human islets were freshly isolated from cadaveric donors as previously described (van de Bunt et al., 2016). All samples were processed for ATAC-seq as described in the Supplemental Experimental Procedures.
Computational and Statistical Analysis
ATAC-seq and RNA-seq reads were processed and aligned to the genome (build hg19). We then predicted open chromatin regions, identified DOCS, and differentially expressed genes as described in the Supplemental Experimental Procedures. Statistical analysis was performed in R version 3.0.2 unless stated otherwise. Data have been deposited in public repositories (Supplemental Experimental Procedures).
Author Contributions
M.T., L.S., A.L.G., M.I.M., N.L.B., and S.E. designed the study. A.B. and A.J.B. obtained the human islet samples and performed quality control. L.S. and N.L.B. performed iPSC experiments. M.T. performed bioinformatics analysis of ATAC-seq data. A.W.-A. analyzed the RNA-seq data. M.T., L.S., and N.L.B. wrote the manuscript. A.W.A., M.I.M., A.L.G., and S.E. gave conceptual advice and edited the manuscript.
Acknowledgments
We thank the High-Throughput Genomics Group at the Wellcome Trust Centre for Human Genetics (funded by Wellcome Trust grant 090532) for the generation of the sequencing data. We thank Tamar Golan-Lev and Nissim Benvenisty for karyotyping the BiPSC lines. The research here received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115439, resources of which are composed of financial contribution from the European Union's Seventh Framework Program (FP7/2007–2013) and EFPIA companies in kind contribution. This publication reflects only the authors' views and neither the IMI JU nor EFPIA nor the European Commission is liable for any use that may be made of the information contained therein. This work was also supported by the Wellcome Trust (099673; 095101; 200837; 098381; 1060130; 099673/Z/12/Z), the Medical Research Council (MR/L020149/1), and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Program. A.L.G. is a Wellcome Trust Senior Fellow in Basic Biomedical Research; M.I.M. is a Wellcome Trust Senior Investigator. N.L.B. was a Naomi Berrie Fellow in Diabetes Research and is now an employee of Novo Nordisk (although all experimental work was carried out under employment at University of Oxford). M.T. was supported by a Wellcome Trust Doctoral Studentship. This study was performed as part of the requirements for the PhD thesis of L.S.
Published: October 26, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, two figures, and six tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2017.09.020.
Accession Numbers
Data have been deposited at the EBI hosted European Genome-phenome Archive (EGA, http://www.ebi.ac.uk/ega/) and European Nucleotide Archive (ENA, http://www.ebi.ac.uk/ena). EGA: EGAS00001002591 (islet ATAC-seq and iPSC RNA-seq). ENA: PRJEB21856 (iPSC ATAC-seq).
Supplemental Information
References
- Balboa D., Otonkoski T. Human pluripotent stem cell based islet models for diabetes research. Best Pract. Res. Clin. Endocrinol. Metab. 2015;29:899–909. doi: 10.1016/j.beem.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Bar-Nur O., Russ H.A., Efrat S., Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Burrows C.K., Banovich N.E., Pavlovic B.J., Patterson K., Gallego Romero I., Pritchard J.K., Gilad Y. Genetic variation, not cell type of origin, underlies the majority of identifiable regulatory differences in iPSCs. PLoS Genet. 2016;12:e1005793. doi: 10.1371/journal.pgen.1005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cras-Meneur C., Li L., Kopan R., Permutt M.A. Presenilins, Notch dose control the fate of pancreatic endocrine progenitors during a narrow developmental window. Genes Dev. 2009;23:2088–2101. doi: 10.1101/gad.1800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimas A.S., Lagou V., Barker A., Knowles J.W., Magi R., Hivert M.F., Benazzo A., Rybin D., Jackson A.U., Stringham H.M. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes. 2014;63:2158–2171. doi: 10.2337/db13-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encode Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumoto K., Kume S. Endoderm and mesoderm reciprocal signaling mediated by CXCL12 and CXCR4 regulates the migration of angioblasts and establishes the pancreatic fate. Development. 2011;138:1947–1955. doi: 10.1242/dev.058719. [DOI] [PubMed] [Google Scholar]
- Kim K., Doi A., Wen B., Ng K., Zhao R., Cahan P., Kim J., Aryee M.J., Ji H., Ehrlich L.I. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundaje A., Meuleman W., Ernst J., Bilenky M., Yen A., Heravi-Moussavi A., Kheradpour P., Zhang Z., Wang J., Ziller M.J. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttala A., Moraghebi R., Valensisi C., Kettunen J., Andrus C., Pasumarthy K.K., Nakanishi M., Nishimura K., Ohtaka M., Weltner J. Genetic variability overrides the impact of parental cell type and determines iPSC differentiation potential. Stem Cell Reports. 2016;6:200–212. doi: 10.1016/j.stemcr.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.S., Sund N.J., Vatamaniuk M.Z., Matschinsky F.M., Stoffers D.A., Kaestner K.H. Foxa2 controls Pdx1 gene expression in pancreatic beta-cells in vivo. Diabetes. 2002;51:2546–2551. doi: 10.2337/diabetes.51.8.2546. [DOI] [PubMed] [Google Scholar]
- Murphy R., Ellard S., Hattersley A.T. Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:200–213. doi: 10.1038/ncpendmet0778. [DOI] [PubMed] [Google Scholar]
- Nostro M.C., Sarangi F., Ogawa S., Holtzinger A., Corneo B., Li X., Micallef S.J., Park I.H., Basford C., Wheeler M.B. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nostro M.C., Sarangi F., Yang C., Holland A., Elefanty A.G., Stanley E.G., Greiner D.L., Keller G. Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Reports. 2015;4:591–604. doi: 10.1016/j.stemcr.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali L., Gaulton K.J., Rodriguez-Segui S.A., Mularoni L., Miguel-Escalada I., Akerman I., Tena J.J., Moran I., Gomez-Marin C., van de Bunt M. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat. Genet. 2014;46:136–143. doi: 10.1038/ng.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo J.M., Liu S., Figueroa M.E., Kulalert W., Eminli S., Tan K.Y., Apostolou E., Stadtfeld M., Li Y., Shioda T. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat. Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raum J.C., Gerrish K., Artner I., Henderson E., Guo M., Sussel L., Schisler J.C., Newgard C.B., Stein R. FoxA2, Nkx2.2, and PDX-1 regulate islet beta-cell-specific mafA expression through conserved sequences located between base pairs -8118 and -7750 upstream from the transcription start site. Mol. Cell. Biol. 2006;26:5735–5743. doi: 10.1128/MCB.00249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A., O'Dwyer S., Quiskamp N., Mojibian M., Albrecht T. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- Rouhani F., Kumasaka N., de Brito M.C., Bradley A., Vallier L., Gaffney D. Genetic background drives transcriptional variation in human induced pluripotent stem cells. PLoS Genet. 2014;10:e1004432. doi: 10.1371/journal.pgen.1004432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers D.A., Zinkin N.T., Stanojevic V., Clarke W.L., Habener J.F. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat. Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- Sussel L., Kalamaras J., Hartigan-O'Connor D.J., Meneses J.J., Pedersen R.A., Rubenstein J.L., German M.S. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- van Arensbergen J., Garcia-Hurtado J., Moran I., Maestro M.A., Xu X., Van de Casteele M., Skoudy A.L., Palassini M., Heimberg H., Ferrer J. Derepression of Polycomb targets during pancreatic organogenesis allows insulin-producing beta-cells to adopt a neural gene activity program. Genome Res. 2010;20:722–732. doi: 10.1101/gr.101709.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Bunt M., Lako M., Barrett A., Gloyn A.L., Hansson M., McCarthy M.I., Beer N.L., Honore C. Insights into islet development and biology through characterization of a human iPSC-derived endocrine pancreas model. Islets. 2016;8:83–95. doi: 10.1080/19382014.2016.1182276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ViaCyte. (2014). A Safety, Tolerability, and Efficacy Study of VC-01™ Combination Product in Subjects with Type I Diabetes Mellitus. https://clinicaltrials.gov/ct2/show/NCT02239354.
- Wang A., Yue F., Li Y., Xie R., Harper T., Patel N.A., Muth K., Palmer J., Qiu Y., Wang J. Epigenetic priming of enhancers predicts developmental competence of hESC-derived endodermal lineage intermediates. Cell Stem Cell. 2015;16:386–399. doi: 10.1016/j.stem.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R., Everett L.J., Lim H.W., Patel N.A., Schug J., Kroon E., Kelly O.G., Wang A., D'Amour K.A., Robins A.J. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12:224–237. doi: 10.1016/j.stem.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.