Abstract
Intrahepatic cholangiocarcinoma (iCCA) is a deadly liver primary cancer associated with poor prognosis and limited therapeutic opportunities. Active transforming growth factor beta (TGFβ) signaling is a hallmark of the iCCA microenvironment. However, the impact of TGFβ on the transcriptome of iCCA tumor cells has been poorly investigated. Here, we have identified a specific TGFβ signature of genes commonly deregulated in iCCA cell lines, namely HuCCT1 and Huh28. Novel coding and noncoding TGFβ targets were identified, including a TGFβ‐induced long noncoding RNA (TLINC), formerly known as cancer susceptibility candidate 15 (CASC15). TLINC is a general target induced by TGFβ in hepatic and nonhepatic cell types. In iCCA cell lines, the expression of a long and short TLINC isoform was associated with an epithelial or mesenchymal phenotype, respectively. Both isoforms were detected in the nucleus and cytoplasm. The long isoform of TLINC was associated with a migratory phenotype in iCCA cell lines and with the induction of proinflammatory cytokines, including interleukin 8, both in vitro and in resected human iCCA. TLINC was also identified as a tumor marker expressed in both epithelial and stroma cells. In nontumor livers, TLINC was only expressed in specific portal areas with signs of ductular reaction and inflammation. Finally, we provide experimental evidence of circular isoforms of TLINC, both in iCCA cells treated with TGFβ and in resected human iCCA. Conclusion: We identify a novel TGFβ‐induced long noncoding RNA up‐regulated in human iCCA and associated with an inflammatory microenvironment. (Hepatology Communications 2018;2:254‐269)
Abbreviations
- CASC15
cancer susceptibility candidate 15
- CCA
cholangiocarcinoma
- circRNA
circular RNA
- EMT
epithelial to mesenchymal transition
- GSEA
gene set enrichment analysis
- HCC
hepatocellular carcinoma
- iCCA
intrahepatic cholangiocarcinoma
- IL8
interleukin 8
- ISH
in situ hybridization
- LCM
laser capture microdissection
- lncRNA
long non coding RNA
- miRNA, miR
micro RNA
- Q‐RT‐PCR
quantitative reverse‐transcription polymerase chain reaction
- RNase
ribonuclease
- SMAD
SMAD family member
- SNAI1
snail family transcriptional repressor 1
- TGFβ
transforming growth factor beta
- TLINC
transforming growth factor beta‐induced long noncoding RNA
- TLINC‐L
long TLINC isoform
- TLINC‐S
short TLINC isoform
Introduction
Cholangiocarcinomas (CCAs) comprise heterogeneous hepatobiliary tumors with cholangiocyte differentiation features. CCAs are classified as intrahepatic (iCCA), perihilar, and distal, based on their anatomic location.1, 2 In the liver, iCCA accounts for 5%‐10% of all malignant primary cancers and ranks second after hepatocellular carcinoma (HCC). Importantly, both incidence and mortality rates of iCCA have increased significantly worldwide during the past decades.2 To date, surgery is the best potentially curative treatment for iCCA but is feasible in less than 40% of cases. For these resectable early stage iCCAs, 5‐year survival is about 40%. However, for unresectable advanced iCCA, the median survival is less than 15 months.2 Although significant progress in understanding the molecular basis of iCCA pathogenesis has been achieved by high throughput strategies, including the identification of genetic, epigenetic, and genomic alterations (e.g., KRAS proto‐oncogene guanosine triphosphatase [KRAS], isocitrate dehydrogenase 1/2 [IDH1/2] mutations, fibroblast growth factor receptor 2 [FGFR2] fusions), there is no approved molecular targeted therapy that significantly improves patient survival in iCCA.1, 3 Thus, iCCA remains characterized by limited therapeutic options and very poor prognosis.1, 3 One histologic feature of iCCA is the presence of a prominent desmoplastic stroma that is known to contribute to tumor onset and progression.4, 5 While previous studies largely focused on identifying molecular alterations in tumor cells, we recently characterized genomic alterations that occur in the tumor microenvironment of iCCA.6 Thus, by combining laser microdissection and gene expression profiling, we demonstrated that the expression of transforming growth factor beta (TGFβ) in the stroma of iCCA predicts a poor prognosis, suggesting that TGFβ may represent a potential therapeutic target in iCCA.6
TGFβ is a pleiotropic cytokine that controls fundamental cellular processes associated with carcinogenesis (e.g., proliferation, apoptosis, invasion, angiogenesis, immunity).7 However, the actions of TGFβ in cancer are complex because TGFβ exhibits either tumor‐suppressive or tumor‐promoting properties, depending on the tumor stage. At an early stage, TGFβ exerts potent antiproliferative properties on precancerous epithelial cells, notably by inducing genes with cytostatic actions (e.g., cyclin‐dependent kinase inhibitor 1A [CDKN1A]). At an advanced stage, cells become resistant to the cytostatic effects of TGFβ, which then promotes tumor growth and metastatic progression, particularly as a potent inducer of epithelial–mesenchymal transition (EMT).7, 8 To date, the molecular mechanisms switching the TGFβ actions from tumor suppression toward tumor promotion are not fully understood and may depend on the cellular context.9, 10 Gene mutations in the canonical TGFβ signaling (e.g., SMAD family member 4 [SMAD4]) and crosstalk with proliferative pathways have been proposed to contribute to the loss of the tumor suppressor arm of TGFβ in several cancers.9 In addition, recent evidence demonstrated that long noncoding RNA (lncRNA) could mediate the prometastatic properties of TGFβ.11, 12 LncRNAs form a large and diverse class of long RNA molecules (>200 nucleotides) that do not encode proteins. LncRNAs emerged as key regulators involved in human carcinogenesis, including liver cancers.13, 14 Here, we characterized the transcriptional response of iCCA cell lines to TGFβ and identified novel TGFβ‐regulated genes, including a lncRNA that we named TGFβ‐induced lncRNA (TLINC, formerly known as LINC00340 and cancer susceptibility candidate 15 [CASC15]). TLINC is induced by TGFβ in several settings, and the expression of specific isoforms correlates with an EMT phenotype. TLINC is strongly up‐regulated in human iCCA and is associated with an enhanced expression of proinflammatory cytokines, including interleukin 8 (IL8). Finally, circular isoforms of TLINC are highlighted as promising biomarkers in patients with iCCA.
Materials and Methods
CELL CULTURE AND REAGENTS
HuCCT1 (RCB‐1960) and Huh28 (RCB‐1943) CCA cell lines were purchased at the RIKEN BioResource Center (Tsukuba‐shi, Japan). Cells were grown in Roswell Park Memorial Institute 1640 medium (HuCCT1) or minimal essential medium (Huh28) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum. HCC cell lines were purchased from ATCC and cultured as described.15 LX2 cells were grown as described.16 Freshly isolated human hepatocytes were purchased from Biopredic International (St. Grégoire, France). Cells were cultured at 37 °C in a 5% CO2 atmosphere, and overnight serum starvation was performed before each treatment. Unless otherwise specified, cells were treated with 1 ng/mL recombinant human TGFβ (R&D Systems, Minneapolis, MN) and 10 µM TGFβ inhibitors (Sigma‐Aldrich, St. Louis, MO).
TGFβ SENSOR CELL LINES AND LUCIFERASE ACTIVITY
Stable TGFβ sensor cell lines were generated by using a pCignal Lenti‐SMAD Reporter assay (Qiagen, Courtabeuf, France) according to the manufacturer's instructions. Briefly, cells were transduced with lentiviral particles (multiplicity of infection, 10; 8 µg/mL SureENTRY transduction reagent) and selected with 1 µg/mL puromycin for 5 days. Luciferase activity was determined with the Firefly Luciferase Assay System (Promega, Madison, WI) and normalized by the number of viable cells evaluated by a PrestoBlue cell viability reagent (Invitrogen, Carlsbad, CA).
DNA TRANSFECTION
DNA constructs were kindly provided by Dr. Kristina A. Cole (Children's Hospital of Philadelphia, Philadelphia, PA) and Dr. Sumio Sugano (University of Tokyo, Tokyo, Japan). All constructs were amplified by transformation of One Shot TOP10 Escherichia coli (Invitrogen) and validated by DNA sequencing (Supporting Table S1) after DNA purification (Nucleobond Xtra plasmid maxiprep kit; Macherey Nagel, Hoerdt, France). DNA transfection was performed by using Lipofectamine 2000 (Invitrogen).
PROTEIN ANALYSIS
A Quantikine enzyme‐linked immunosorbent assay human TGFβ1 immunoassay (R&D Systems) was used to evaluate the expression of TGFβ in the supernatant of cell cultures collected 24 hours after serum starvation. Protein analysis by immunoblotting was performed as described.17 Human anti‐E‐cadherin (CDH1) (AF648; R&D Systems) and anti‐vimentin (VIM) (B01P; Abnova) antibodies were used.
GENE EXPRESSION PROFILING
Total RNA, including lncRNA and micro RNA (miRNA, miR), was purified with an miRNAeasy kit (Qiagen). Genome‐wide expression profiling was performed using the low‐input QuickAmp labeling kit and human SurePrint G3 8x60K probes pangenomic microarrays (Agilent Technologies, Santa Clara, CA) as described.15 These microarrays include 26,083 Entrez genes and 30,606 lncRNAs. Gene expression data were processed using Feature Extraction and GeneSpring software (Agilent Technologies). Microarray data sets have been deposited into the public Gene Expression Omnibus database under accession numbers GSE102109 and GSE102110. Gene set enrichment analysis (GSEA) was performed using the Java tool developed at the Broad Institute (Cambridge, MA). The Enrichr algorithm (http://amp.pharm.mssm.edu/Enrichr) was also used to identify key genes associated with relevant signaling pathways.
QUANTITATIVE REVERSE‐TRANSCRIPTION POLYMERASE CHAIN REACTION
Quantitative reverse‐transcription polymerase chain reaction (Q‐RT‐PCR) was performed as described16 by using a SYBR Green master mix (Applied Biosystems, Carlsbad, CA). A mixture of deoxythymidine oligomer (250 ng) and random hexamers (100 ng) was used to prime the RT reaction of 1 µg total RNA (Superscript III RT; Invitrogen). A list of DNA primers used in this study is provided in Supporting Table S2.
FUNCTIONAL TESTS
Cell viability was evaluated using a PrestoBlue reagent (Invitrogen). Data were expressed as relative cell viability compared to untreated cells transfected with the control plasmid set as 1. Cell migration and invasion were evaluated using BD BioCoat Matrigel Invasion Chambers (BD Biosciences) as described.16 Briefly, 4 × 104 cells were plated in serum‐free medium either on an 8‐µm pore‐size positron emission tomography membrane insert coated with a layer of matrigel basement membrane matrix (invasion test) or on an 8‐µm pore‐size positron emission tomography control membrane insert (migration test). The lower compartment contained medium with 10% fetal bovine serum as a chemoattractant. After incubation for 48 hours, we removed the cells remaining on the upper surface of the membrane with a cotton swab, fixed cells in 4% paraformaldehyde that had passed through to the lower surface of the membrane, and stained the fixed cells with 1% crystal violet. We scored invasive and migrating cells per membrane under a light microscope at a 5 × magnification (n = 3 independent experiments). The number of migrating and invasive cells after transfection of TLINC was normalized relative to the number of migrating and invasive cells after transfection of the control plasmid, which was set to 1.
HUMAN iCCA SAMPLES AND TISSUE MICROARRAY
Freshly frozen and formalin‐fixed paraffin‐embedded human iCCA samples were obtained through the national liver biobanks network. Written informed consent was obtained from all patients. The study protocol fulfilled national laws and regulations and was approved by the local ethics committee and the institutional review board of the Institut National de la Santé et de la Recherche Médicale (IRB00003888). Tissue microarrays were performed using a Minicore 3 Tissue Arrayer (Excilone, Vicq, France) as described.6
LASER CAPTURE MICRODISSECTION
Laser capture microdissection (LCM) was performed using an Arcturus Veritas Microdissection system (Applied Biosystems) as described.6 Briefly, serial sections of 10‐µm frozen tissues were mounted onto PEN membrane glass slides prior to LCM of biliary epithelial cells and fibrous tissue from tumor and nontumor parts. Total RNA was purified using an Arcturus Picopure RNA isolation kit (Applied Biosystems).
IN SITU HYBRIDIZATION
Specific in situ hybridization (ISH) riboprobes were designed to evaluate the expression of TLINC‐S and TLINC‐T transcripts (Supporting Table S1). Sense and antisense riboprobes were generated by in vitro transcription from PCR products incorporating the promoter of T7 RNA polymerase. In vitro transcription was performed with 40 units T7 RNA Polymerase (Agilent) in the presence of 0.35 mM digoxygenin‐11‐UTP (Roche Diagnostics, Meylan, France). ISH was performed on the Discovery Automated IHC Stainer using the Ventana Detection Kit (Ventana Medical Systems, Tucson, AZ). Staining was performed using an anti‐digoxygenin‐horseradish peroxidase antibody (Roche Diagnostics), and signal detection was performed using a nitro‐blue tetrazolium/5‐bromo‐4‐chloro‐3′‐indolyphosphate or a Discovery purple kit (Ventana Medical Systems).
CIRCULAR RNA ANALYSIS
RT‐PCR using convergent and divergent primers combined with ribonuclease (RNase) R (Epicentre, Middleton, WI) treatment (1 µ/µg, 37 °C, 1 hour) was used to detect linear and circular transcripts of TLINC and actin beta (ACTB). The sequence of DNA primers is provided in Supporting Table S2.
Results
ENDOGENOUS TGFβ ACTIVATION IN Huh28 CELLS
HuCCT1 and Huh28 cells were used to study the impact of TGFβ on iCCA cells. The two cell lines have different phenotypic features that reflect tumor heterogeneity. HuCCT1 cells exhibit an epithelioid‐like phenotype associated with a cobblestone‐like morphology and numerous cell‐to‐cell junctions compared to Huh28 cells, which exhibit a mesenchymal‐like phenotype associated with a spindle and polygonal morphology together with prominent cytoskeleton fibers (Fig. 1A). Epithelioid and mesenchymal‐like phenotypes were further characterized by the expression of CDH1 and VIM markers in HuCCT1 cells and Huh28 cells, respectively, both at the RNA and protein level (Fig. 1A). Marked differences in pangenomic expression profiles were also highlighted between HuCCT1 and Huh28 cells and validated by Q‐RT‐PCR (Fig. 1B; Supporting Fig. S1A). Interestingly, GSEA, using the Kyoto Encyclopedia of Genes and Genomes signaling pathways, demonstrated that a TGFβ signature was significantly enriched (P < 0.001) at the basal level in mesenchymal Huh28 cells (Fig. 1B). Endogenous activation of the TGFβ pathway in Huh28 cells was further validated by a significantly enhanced secretion of TGFβ (Fig. 1C) that correlated with enhanced luciferase activity as detected in engineered SMAD sensor cell lines (Fig. 1D). A higher expression of TGFβ target genes (e.g., snail family transcriptional repressor 1 [SNAI1], collagen type IV alpha 1 chain [COL4A1], SMAD7) in Huh28 cells was also validated by Q‐RT‐PCR (Fig. 1E; Supporting Fig. S1A) as well as a reduced expression of SMAD7, a well‐known TGFβ transcriptional target, when cells were cultured in the presence of TGFβ inhibitors (Fig. 1F). Altogether, these data demonstrate that Huh28 cells exhibit a mesenchymal phenotype associated with basal and endogenous activation of the TGFβ pathway.
Figure 1.
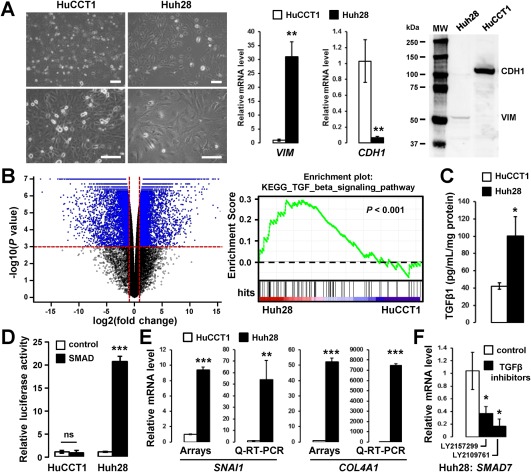
HuCCT1 and Huh28 cell lines exhibit an epithelioid and a mesenchymal‐like phenotype respectively, the later correlating with endogenous activation of the TGFβ pathway. (A) Phase contrast micrographs of HuCCT1 and Huh28 cells (left panel; scale bar, 100 µm) and expression of vimentin and E‐cadherin at the RNA (middle panel) and protein level (right panel). (B) Volcano plot of genes differentially expressed between HuCCT1 and Huh28 (left panel) and GSEA highlighting a TGFβ signature enriched in the gene expression profile of Huh28. (C) Enzyme‐linked immunosorbent assay demonstrating increased secretion of TGFβ1 in the supernatant of Huh28 cells 24 hours after overnight serum starvation. (D) Relative luciferase activity of HuCCT1 and Huh28 engineered cell lines in which a luciferase gene is under the transcriptional control of a TATA box alone (white bars) or with SMAD responsive elements (black bars). Luciferase activity was measured at the basal level without exogenous addition of TGFβ after overnight serum starvation. (E) Gene expression analysis of TGFβ target genes in HuCCT1 and Huh28 cells at the basal level by microarray and Q‐RT‐PCR. (F) Q‐RT‐PCR analysis of TGFβ target gene SMAD7 in Huh28 cells treated with 10 µM TGFβ inhibitors (black bars). Statistical analysis was performed by t test (*P < 0.05; **P < 0.01; ***P < 0.001); n ≥ 3 replicates. Data represent mean ± SD. Abbreviations: mRNA, messenger RNA; MW, molecular weight.
NEW TGFβ TARGET GENES IN iCCA CELLS
HuCCT1 and Huh28 cells were treated with 1 ng/mL TGFβ for 16 hours. Gene expression profiling using pangenomic microarrays identified 859 probes in HuCCT1 cells (Fig. 2A) and 491 probes in Huh28 cells (Fig. 2B) that were significantly deregulated by TGFβ (P < 0.001; fold change >2). A Venn diagram analysis, including genes differentially expressed between HuCCT1 and Huh28 cells at a basal level (Fig. 1B), identified a set of 48 probes (42 nonredundant annotated genes) that were recurrently deregulated by TGFβ in all three experimental settings (Fig. 2C; Table 1). The deregulation of well‐known positive (e.g., serpin family E member 1 [SERPINE1], connective tissue growth factor [CTGF]) and negative (e.g., baculoviral inhibitor of apoptosis protein repeat containing 3 [BIRC3]) TGFβ target genes was confirmed by Q‐RT‐PCR on independent RNA samples (Fig. 2D; Supporting Fig. S1B). GSEA was also performed in all specific gene subsets and demonstrated that the 42 common genes were significantly associated with TGFβ and SMAD2/3 and related to EMT (Supporting Fig. S2). Importantly, this common signature comprised genes not known to be regulated by TGFβ, including several lncRNAs (e.g., LINC00312, LINC00340). Notably, two microarray probes for LINC00340 were induced by TGFβ (Table 1).
Figure 2.
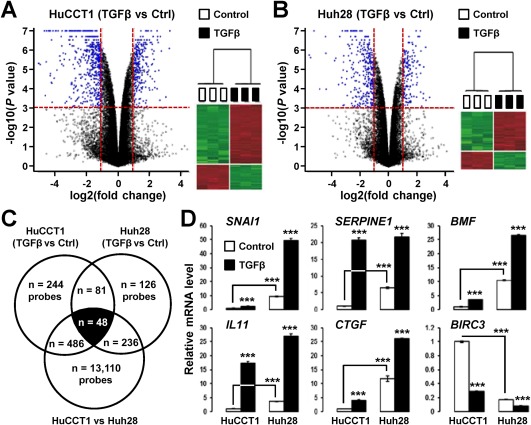
A TGFβ‐specific gene expression signature in cholangiocarcinoma cell lines. (A,B) Volcano plot and hierarchical clustering of genes differentially expressed by TGFβ (1 ng/mL, 16 hours) in (A) HuCCT1 and (B) Huh28 cells. Horizontal dashed lines on volcano plots, P < 0.001; vertical dashed lines, fold change >2. (C) Venn diagram analysis of genes differentially expressed by TGFβ in HuCCT1 and Huh28 cells. A common TGFβ signature made of 48 probes (42 nonredundant well‐annotated genes) was identified. (D) Expression of six well‐known TGFβ target genes in HuCCT1 and Huh28 cells after 16 hours of culture in absence (white bars) or presence (black bars) of 1 ng/mL TGFβ. Statistical analysis was performed by t test (*P < 0.05; **P < 0.01; ***P < 0.001); n ≥ 3 replicates. Data represent mean ± SD. Abbreviation: Ctrl, control.
Table 1.
TGFβ SIGNATURE IN CHOLANGIOCARCINOMA CELL LINES
| (A) Genes induced by TGFβ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Agilent Probe | Gene Name | Gene Symbol | Accession No. | HuCCT1 (TGFβ/Ctrl) | Huh28 (TGFβ/Ctrl) | Huh28/HuCCT1 | Known TGFβ Target | |||
| P Value | FC | P Value | FC | P Value | FC | |||||
| A_24_P411561 | Hepatitis A virus cellular receptor 2 | HAVCR2 | NM_032782 | 1.00E‐07 | 38.46 | 5.00E‐07 | 7.14 | 1.40E‐06 | 11.25 | yes |
| A_24_P158089 | Serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1 | SERPINE1 | NM_000602 | < 1e‐07 | 20.83 | 7.00E‐07 | 3.33 | 1.00E‐07 | 6.63 | yes |
| A_21_P0011334 | na | XLOC_l2_004757 | BC043257 | 4.50E‐05 | 18.52 | < 1e‐07 | 5.88 | 8.21E‐04 | 5.20 | no |
| A_33_P3243887 | Interleukin 11 | IL11 | NM_000641 | < 1e‐07 | 17.24 | < 1e‐07 | 7.14 | 1.40E‐06 | 3.54 | yes |
| A_21_P0001676 | na | XLOC_000166 | na | < 1e‐07 | 15.15 | 5.10E‐06 | 2.08 | < 1e‐07 | 300.67 | no |
| A_23_P153571 | IGF‐like family member 2 | IGFL2 | NM_001002915 | 3.40E‐06 | 12.82 | 5.35E‐05 | 2.27 | 4.69E‐05 | 5.42 | no |
| A_33_P3243454 | IGF‐like family member 3 | IGFL3 | NM_207393 | 5.30E‐06 | 11.76 | 1.00E‐07 | 3.70 | 4.00E‐07 | 57.37 | no |
| A_33_P3255384 | BPI Fold Containing Family C | BPIFC | NM_174932 | 1.00E‐07 | 10.20 | 7.56E‐05 | 2.78 | 2.00E‐07 | 12.20 | no |
| A_32_P185637 | Collagen, type XX, alpha 1 | COL20A1 | NM_020882 | 1.50E‐06 | 10.10 | < 1e‐07 | 6.25 | 3.10E‐06 | 8.09 | no |
| A_23_P110777 | Leukocyte cell‐derived chemotaxin 2 | LECT2 | NM_002302 | 2.57E‐04 | 8.33 | 1.23E‐05 | 7.69 | 2.30E‐06 | 7.21 | no |
| A_33_P3345534 | Keratin 14 | KRT14 | NM_000526 | 1.00E‐07 | 7.69 | 9.30E‐06 | 2.70 | 1.00E‐07 | 10.82 | no |
| A_23_P96158 | Keratin 17 | KRT17 | NM_000422 | < 1e‐07 | 7.69 | 8.70E‐06 | 2.63 | < 1e‐07 | 9.50 | no |
| A_24_P887857 | na | na | na | < 1e‐07 | 7.69 | 1.65E‐05 | 2.70 | 1.00E‐07 | 9.67 | no |
| A_23_P205074 | Solute carrier family 46, member 3 | SLC46A3 | NM_181785 | 3.15E‐04 | 7.69 | 1.00E‐06 | 2.33 | 3.55E‐04 | 7.54 | no |
| A_24_P882732 | na | na | DR007925 | < 1e‐07 | 7.14 | 2.28E‐05 | 2.70 | 1.00E‐07 | 9.50 | no |
| A_21_P0011578 | na | XLOC_l2_006021 | DB117598 | 3.00E‐07 | 7.14 | 3.00E‐05 | 2.70 | 4.00E‐07 | 8.77 | no |
| A_33_P3857239 | Keratin 42 pseudogene | KRT42P | NR_033415 | < 1e‐07 | 6.67 | 9.70E‐06 | 2.86 | 1.00E‐07 | 8.69 | no |
| A_33_P3235905 | na | na | na | 6.20E‐05 | 6.67 | 6.82E‐05 | 2.44 | 3.22E‐04 | 3.90 | no |
| A_21_P0008663 | na | XLOC_011331 | na | 2.90E‐06 | 6.67 | < 1e‐07 | 5.56 | 3.00E‐07 | 19.21 | no |
| A_32_P62963 | Keratin 16 pseudogene 2 | KRT16P2 | NR_029392 | 1.00E‐07 | 5.88 | 2.19E‐05 | 2.70 | 4.00E‐07 | 5.88 | no |
| A_23_P381489 | Long intergenic nonprotein coding RNA 313 | LINC00313 | NR_026863 | 4.10E‐06 | 5.88 | 8.91E‐05 | 2.08 | 2.19E‐04 | 2.29 | no |
| A_23_P166779 | Long intergenic nonprotein coding RNA 312 | LINC00312 | NR_024065 | 3.06E‐05 | 4.17 | 1.00E‐07 | 5.00 | 1.36E‐04 | 2.86 | no |
| A_23_P19663 | Connective tissue growth factor | CTGF | NM_001901 | 4.00E‐07 | 4.00 | 1.91E‐05 | 2.22 | 1.00E‐07 | 11.67 | yes |
| A_19_P00321671 | na | na | na | 1.56E‐04 | 4.00 | 3.60E‐06 | 3.03 | 5.06E‐05 | 5.57 | no |
| A_21_P0012601 | na | XLOC_l2_010751 | na | 1.20E‐06 | 4.00 | 6.60E‐06 | 3.03 | 5.00E‐07 | 5.23 | no |
| A_32_P76627 | na | na | CR597597 | 1.00E‐07 | 3.70 | 2.60E‐06 | 2.78 | 3.00E‐07 | 4.00 | no |
| A_23_P379649 | Bcl2 modifying factor | BMF | NM_001003940 | 1.65E‐05 | 3.57 | 3.00E‐07 | 2.56 | 8.00E‐07 | 10.75 | yes |
| A_21_P0009087 | na | XLOC_012049 | na | 1.72E‐04 | 3.45 | 1.06E‐05 | 3.03 | 2.81E‐05 | 5.86 | no |
| A_19_P00318323 | Long intergenic nonprotein coding RNA 340 | LINC00340 | NR_015410 | 2.06E‐05 | 3.33 | 8.60E‐06 | 2.27 | 1.00E‐07 | 19.17 | no |
| A_23_P107744 | Sphingosine‐1‐phosphate receptor 5 | S1PR5 | NM_030760 | 6.04E‐04 | 3.03 | 1.50E‐06 | 3.57 | 1.35E‐05 | 12.24 | no |
| A_32_P309404 | Solute carrier family 22 (extraneuronal monoamine transporter), member 3 | SLC22A3 | NM_021977 | 5.00E‐07 | 3.03 | 1.78E‐05 | 2.04 | 1.60E‐06 | 3.48 | no |
| A_24_P838448 | Long intergenic nonprotein coding RNA 340 | LINC00340 | NR_015410 | 1.04E‐04 | 2.86 | 1.30E‐06 | 2.44 | 2.00E‐07 | 12.50 | no |
| A_23_P124642 | RAS guanyl releasing protein 1 (calcium and DAG‐regulated) | RASGRP1 | NM_005739 | 1.40E‐05 | 2.70 | 1.32E‐05 | 2.08 | < 1e‐07 | 27.00 | no |
| A_23_P344531 | Synaptopodin | SYNPO | NM_007286 | 7.10E‐06 | 2.50 | 1.60E‐06 | 2.56 | < 1e‐07 | 24.38 | no |
| A_23_P303548 | Nucleolar protein 4‐like | NOL4L | XM_003403733 | 2.34E‐04 | 2.44 | 5.14E‐05 | 2.08 | 4.81E‐04 | 2.31 | no |
| A_24_P239606 | Growth arrest and DNA‐damage‐inducible, beta | GADD45B | NM_015675 | 3.00E‐06 | 2.33 | 6.10E‐05 | 2.44 | 3.69E‐05 | 2.93 | yes |
| A_23_P131846 | Snail homolog 1 (Drosophila) | SNAI1 | NM_005985 | 4.20E‐06 | 2.33 | 1.00E‐07 | 5.26 | < 1e‐07 | 9.48 | yes |
| A_21_P0009196 | na | XLOC_012515 | na | 6.91E‐04 | 2.27 | 2.06E‐05 | 2.08 | 8.00E‐06 | 7.67 | no |
| (B) Genes repressed by TGFβ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Agilent Probe | Gene Name | Gene Symbol | Accession No. | HuCCT1 (TGFβ/Ctrl) | Huh28 (TGFβ/Ctrl) | Huh28 / HuCCT1 | Known TGFβ Target | |||
| P Value | FC | P Value | FC | P Value | FC | |||||
| A_33_P3663974 | Thyroid hormone receptor beta | THRB | NM_001128177 | 8.10E‐06 | 0.49 | 1.75E‐04 | 0.47 | 1.70E‐06 | 0.42 | no |
| A_21_P0010449 | na | XLOC_014399 | XR_109732 | 2.70E‐06 | 0.47 | 1.05E‐04 | 0.38 | 1.00E‐07 | 0.08 | no |
| A_21_P0000841 | Hypothetical LOC100505483 | LOC100505483 | NR_038926 | 7.60E‐06 | 0.45 | 3.13E‐05 | 0.40 | 2.25E‐05 | 0.43 | no |
| A_23_P121064 | Pentraxin 3, long | PTX3 | NM_002852 | 1.50E‐06 | 0.45 | 2.00E‐06 | 0.32 | 4.90E‐06 | 0.42 | yes |
| A_23_P121657 | Heparan sulfate (glucosamine) 3‐O‐sulfotransferase 1 | HS3ST1 | NM_005114 | 3.00E‐07 | 0.42 | 3.07E‐04 | 0.45 | < 1e‐07 | 0.00 | no |
| A_23_P52266 | Interferon‐induced protein with tetratricopeptide repeats 1 | IFIT1 | NM_001548 | 1.19E‐04 | 0.40 | 1.66E‐05 | 0.36 | 1.72E‐05 | 0.27 | no |
| A_23_P345460 | Pleckstrin homology domain‐containing, family G (with RhoGef domain) member 4 | PLEKHG4 | NM_015432 | 6.00E‐07 | 0.36 | 2.90E‐04 | 0.50 | 1.00E‐07 | 0.21 | no |
| A_23_P98350 | Baculoviral inhibitor of apoptosis protein repeat‐containing 3 | BIRC3 | NM_001165 | 1.00E‐07 | 0.29 | 2.70E‐06 | 0.45 | < 1e‐07 | 0.17 | yes |
| A_33_P3398181 | Hypothetical LOC100127909 | LOC100127909 | XR_111781 | 8.81E‐05 | 0.26 | 7.13E‐05 | 0.35 | 1.89E‐05 | 0.27 | no |
| A_23_P415021 | Methyltransferase‐like 7A | METTL7A | NM_014033 | 2.00E‐07 | 0.22 | 4.00E‐06 | 0.32 | 1.00E‐07 | 0.19 | no |
Abbreviations: Ctrl, control; FC, fold change; na, not applicable.
LINC00340/CASC15/TLINC IS A NOVEL TGFβ‐INDUCED lncRNA
LINC00340 is known as cancer susceptibility candidate 15 (CASC15) located on chromosome 6p22. CASC15 was reported to act as a tumor suppressor in neuroblastoma18 and to promote tumor progression and phenotype switching in melanoma.19 To further characterize LINC00340 biological activities in iCCA, we first validated the microarray expression data by Q‐RT‐PCR. LINC00340 was induced by TGFβ in both HuCCT1 and Huh28 cells, and a significant higher expression in Huh28 cells at the basal level was confirmed (Fig. 3A). Repression of LINC00340 and SMAD7 was observed in Huh28 cells treated with inhibitors of the TGFβ pathway (Fig. 3B). Time‐course experiments also demonstrated increased expression of LINC00340 by TGFβ, both in HuCCT1 and Huh28 cells (Fig. 3C). Interestingly, LINC00340 was induced by TGFβ in nontransformed human primary hepatocytes, and the induction was abolished in the presence of a TGFβ inhibitor (Fig. 3D). In addition, LINC00340 was induced by TGFβ in several human HCC cell lines as well as in LX2‐activated hepatic stellate cells (Fig. 3E). Finally, induction by TGFβ occurred also in nonhepatic cell lines, such as pancreatic Panc‐1 (Fig. 3E). Altogether, these data demonstrate that LINC00340 is a novel and general transcriptional target of TGFβ. We therefore named it TGFβ‐induced lncRNA or TLINC.
Figure 3.
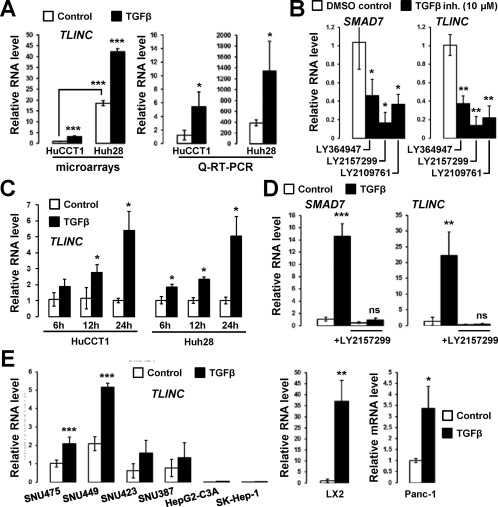
LINC00340/CASC15/TLINC is a novel TGFβ‐induced lncRNA. (A) Analysis of LINC00340 expression in HuCCT1 and Huh28 cells after 16 hours of culture in absence (white bars) or presence (black bars) of 1 ng/mL TGFβ, as evaluated by microarray and Q‐RT‐PCR. (B) Analysis of SMAD7 and LINC00340 expression in Huh28 cells after 72 hours treatment with 10 µM TGFβ inhibitors. (C) Time‐course analysis of LINC00340 expression in HuCCT1 and Huh28 cells (1 ng/mL TGFβ). (D) Analysis of SMAD7 and LINC00340 expression in primary human hepatocytes (n = 3 independent donors) in the presence of 1 ng/mL TGFβ and 10 µM TGFβ inhibitor LY2157299 for 16 hours. (E) Analysis of LINC00340 expression in HCC cell lines (left panel) and LX2 and Panc‐1 (pancreatic cancer) cells (right two panels) (1 ng/mL TGFβ for 16 hours). Statistical analysis was performed by t test (*P < 0.05; **P < 0.01; ***P < 0.001); n ≥ 3 replicates. Data represent mean ± SD. Abbreviations: DMSO, dimethyl sulfoxide; inh., inhibitor; mRNA, messenger RNA; ns, not significant.
INDUCTION OF TWO TLINC ISOFORMS LINKED TO AN EMT PHENOTYPE
Previous studies demonstrated that TLINC is located on a complex locus that produces multiple transcripts by alternative splicing, including a short (TLINC‐S) and a long (TLINC‐L) isoform, as shown in Fig. 4A.18, 19 These two isoforms have been functionally associated with either tumor suppressive (TLINC‐S)18 or tumor promoting (TLINC‐L)19 functions in neuroblastoma and melanoma, respectively. Because our previous gene expression analysis by microarray (Fig. 2) and Q‐RT‐PCR (Fig. 3) could not distinguish the two isoforms (primer set L + S), we next evaluated TLINC‐S and TLINC‐L with specific sets of primers (Fig. 4A). TLINC‐L was induced by TGFβ in HuCCT1 cells and TLINC‐S in Huh28 cells, as demonstrated by Q‐RT‐PCR (Fig. 4B). ISH demonstrated that the two isoforms were expressed in both the cytoplasm and the nucleus (Fig. 4C).
Figure 4.
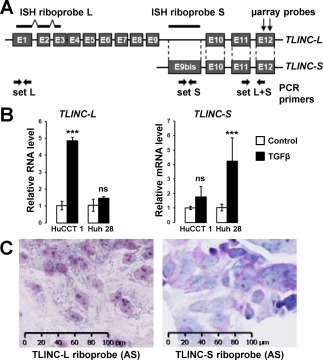
Expression and cellular localization of TLINC isoforms. (A) Simplified representation of TLINC locus highlighting two reported isoforms: TLINC‐L and TLINC‐S. ISH riboprobes, sets of PCR primers, and microarray probes are also represented. (B) Analysis of TLINC‐L and TLINC‐S expression in HuCCT1 and Huh28 cells after 16 hours of culture in the absence (white bars) or presence (black bars) of 1 ng/mL TGFβ, as evaluated by Q‐RT‐PCR using specific sets of primers. (C) In situ hybridization using TLINC‐L and TLINC‐S riboprobes highlights a nuclear and cytoplasmic location of both TLINC isoforms (HuCCT1 cells treated with 1 ng/mL TGFβ). Scale, 100 µm. Statistical analysis was performed by t test (*P < 0.05; **P < 0.01; ***P < 0.001); n ≥ 3 replicates. Data represent mean ± SD. Abbreviations: (AS), antisense; mRNA, messenger RNA; ns, not significant.
FUNCTIONAL CHARACTERIZATION OF TLINC ISOFORMS
Gain of function experiments were performed using DNA constructs designed to specifically overexpress TLINC‐L and TLINC‐S (Supporting Fig. S3A‐C). No significant effect was observed on cell viability (Supporting Fig. S3D) and cell invasion (Fig. 5A); however, overexpression of TLINC‐L increased cell migration (Fig. 5A). In order to get insights into the molecular mechanisms involved, gene expression profiling was performed on HuCCT1 cells after TLINC‐L overexpression. Numerous genes were repressed (Fig. 5B), whereas several inflammation‐associated genes, including cytokines and chemokines (e.g., IL6, IL8, C‐X‐C motif chemokine ligand 1 [CXCL1]) were induced together with LINC00340/TLINC (Fig. 5B). Accordingly, an immune response gene signature was significantly enriched in the gene expression profiles of HuCCT1 cells overexpressing TLINC‐L (Fig. 5C; Supporting Table S3). The enrichment of a described20 cell migration signature supported the phenotype observed after TLINC‐L overexpression (Fig. 5C). Although no obvious change was observed on cell morphology after TLINC‐L transfection, EMT‐associated SNAI2 was enriched in the gene expression profiles of TLINC‐L transfected cells (Supporting Table S4). A significant enrichment of TGFβ target genes was also observed, suggesting that TLINC‐L may act as a feedback regulator of the TGFβ pathway (Fig. 5C). However, experimental data using SMAD sensor cell lines refuted this hypothesis (Supporting Fig. S3E). In agreement with the identified immune signature, increased expression of IL8 was confirmed after TLINC overexpression (Fig. 5D). As a potent inducer of cell motility, IL8 may contribute to the observed phenotype by creating a gradient for the migration of cancer cells.
Figure 5.
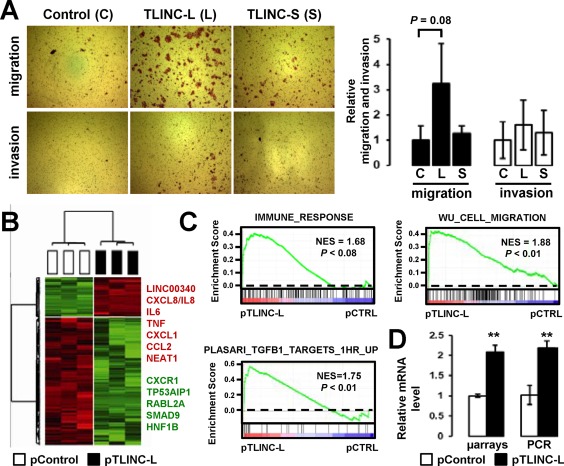
Functional analysis of TLINC. (A) Effects of TLINC‐L and TLINC‐S overexpression on migration and invasion of HuCCT1 cells 48 hours after transfection of the DNA constructs. (B) Gene expression analysis of HuCCT1 cells 24 hours after transfection of the TLINC‐L DNA construct. Hierarchical clustering was based on the expression of 431 genes (P < 0.01; fold change >1.5). (C) GSEA of specific signatures significantly enriched in the gene expression profile of HuCCT1 cells overexpressing TLINC‐L. (D) Analysis of IL8 expression in HuCCT1 cells after transfection of the TLINC‐L DNA construct, as evaluated by microarray and Q‐RT‐PCR. Statistical analysis was performed by t test (*P < 0.05; **P < 0.01; ***P < 0.001); n ≥ 3 replicates. Data represent mean ± SD. Abbreviations: C, control; CCL2, chemokine (C‐C motif) ligand 2; CTRL, control; CXCR1, chemokine (C‐X‐C motif) receptor 1; HNF, hepatocyte nuclear factor; L, long; mRNA, messenger RNA; NES, normalized enrichment score; p, plasmid [for pControl and pTLINC‐L]; RABL2A, RAB, member of RAS oncogene family like 2A; S, short; TP53AIP1, tumor protein p53 regulated apoptosis inducing protein 1; TNF, tumor necrosis factor.
INCREASED EXPRESSION OF TLINC IN HUMAN iCCA
We next evaluated the clinical relevance of TLINC expression in human resected iCCA. TLINC was shown to be induced both in tumor biliary epithelial cells and in the stroma compared to their nontumor counterparts (Fig. 6A). Overexpression of TLINC in iCCA tumors was validated by ISH on tissue microarrays (Fig. 6A). Interestingly, Q‐RT‐PCR on 22 resected human iCCA demonstrated that the expression of TLINC‐L and TLINC‐S was tightly correlated (Fig. 6B). A detailed analysis by ISH on human resected iCCA confirmed that both TLINC isoforms were expressed in tumor cholangiocytes and in cells from the tumor microenvironment. In the nontumor liver, TLINC was only expressed in remodeled portal areas with signs of ductular reaction and inflammation (Fig. 6C; Supporting Fig. S4).
Figure 6.
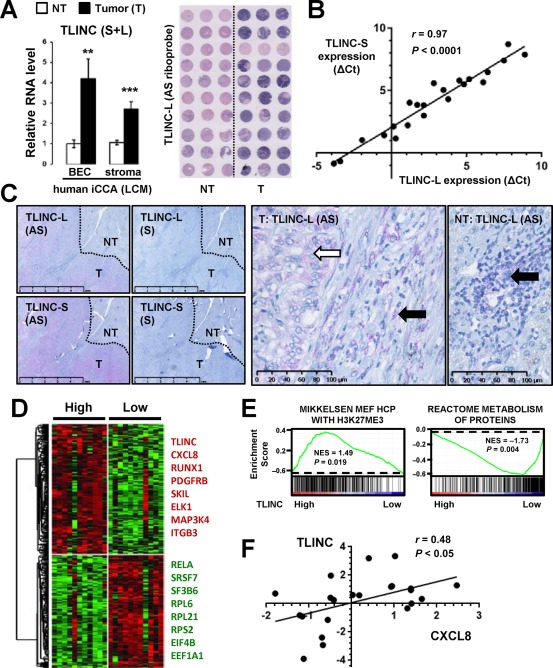
Clinical relevance of TLINC expression in human resected iCCA. (A) Analysis of TLINC expression in biliary epithelial cells and stroma isolated by laser capture microdissection from nontumor (white bars) and tumor (black bars) tissues (n = 10 patients, left panel). Data represent mean ± SD. ISH on human iCCA tissue microarrays using TLINC‐L antisense riboprobes demonstrating higher expression in the tumor than in the nontumoral tissues (tissue microarray, right panel). (B) Correlation between TLINC‐L and TLINC‐S expression in human iCCA resected tumors, as evaluated by Q‐RT‐PCR (n = 22 patients). (C) ISH analysis of TLINC‐L and TLINC‐S expression using specific antisense and sense riboprobes. In the tumor part, TLINC was expressed in both epithelial cells (white arrow) and in the stroma (black arrow). In the nontumor part, TLINC was only expressed in remodeled portal areas with signs of ductular reaction. Scale bars: 5 mm (left panels) and 100 µm (right panels). (D) Expression of TLINC identifies human iCCA with specific gene expression profiles. Median expression of TLINC was used to define high and low expression groups (n = 22 patients). (E) Example of signatures significantly enriched in the gene expression profiles of iCCA with high versus low TLINC expression, as evaluated by GSEA. (F) Correlation between the expression of TLINC and CXCL8 in 22 human iCCAs. Abbreviations: (AS), antisense; BEC, biliary epithelial cells; EEF1A1, eukaryotic translation elongation factor 1 alpha 1; EIF4B, eukaryotic translation initiation factor 4B; ELK1, ELK1, ETS transcription factor; ITGB3, integrin subunit beta 3; HCP, high‐CpG‐density promoters; MAP3K4, mitogen‐activated protein kinase kinase kinase 4; MEF, mouse embryonic fibroblast; NF‐kB subunit; NES, normalized enrichment score; NT, nontumor; PDGFRB, platelet derived growth factor receptor beta; RELA, RELA proto‐oncogene; RPL6, ribosomal protein L6; RPL21, ribosomal protein L21; RPS2, ribosomal protein S2; RUNX1, runt related transcription factor 1; (S), sense; SF3B6, splicing factor 3b subunit 6; SKIL, SKI like proto‐oncogene; SRSF7, serine and arginine rich splicing factor 7; T, tumor.
TLINC EXPRESSION DEFINES SPECIFIC SUBSETS OF HUMAN iCCA
Gene expression profiling was performed on 22 resected human iCCA, and a specific signature was defined on the basis of TLINC expression (Fig. 6D). High expression of TLINC correlated with a high expression of TGFβ target genes and inflammation‐associated genes, as previously observed in cell lines (Fig. 6D; Supporting Fig. S5). In addition, GSEA demonstrated that up‐regulated genes in high‐TLINC‐expressing tumors were significantly associated with the H3K27Me3 repressive epigenetic mark signature, while down‐regulated genes were linked to protein metabolism and included numerous genes encoding ribosomal components (Fig. 6D,E; Supporting Fig. S6). Among the chemokines induced in HuCCT1 cells directly overexpressing TLINC (Fig. 5B) and possibly contributing to a migration phenotype, IL8 was found to be significantly correlated with TLINC expression in human iCCA tumors (Fig. 6F).
EXPERIMENTAL EVIDENCE OF TLINC CIRCULAR ISOFORMS
A genome‐wide bioinformatic analysis of RNA‐sequencing data previously reported a 204‐nucleotide circular isoform of LINC00340/TLINC, probably derived from exon E7. This isoform was one of the most highly expressed circular RNAs (circRNAs) in H1‐human embryonic stem cells.21 To evaluate the putative expression of such circular isoforms in iCCA, we designed specific sets of convergent and divergent primers from exons E7 and E8 of TLINC (Fig. 7A). ACTB was used as a housekeeping gene control that was highly expressed and without prior evidence of a circular isoform. Interestingly, by combining RNase R treatment and RT‐PCR experiments, we provide evidence of the existence of several RNase R‐resistant circular TLINC isoforms not only in iCCA cells treated with TGFβ (Fig. 7B) but also in resected human iCCA tumors (Fig. 7C).
Figure 7.
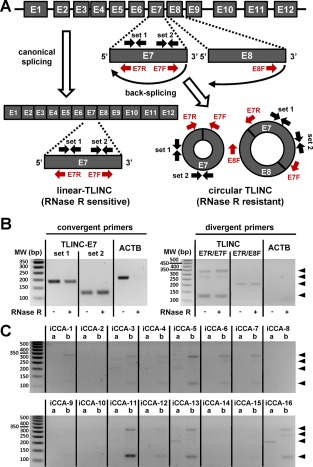
Experimental evidence of TLINC circular isoforms. (A) Schematic representation of TLINC RNA processing into linear RNA (canonical splicing) or circular RNA (back‐splicing). Several sets of convergent and divergent PCR primers were designed to detect specific linear and circular isoforms. (B) RNA was extracted from Huh28 cells after 16 hours of treatment with 1 ng/mL TGFβ. RNase R treatment was performed prior to RT‐PCR analysis. Convergent primers (left panel) detected specific TLINC‐E7 transcripts in both RNase R untreated and treated samples. For ACTB (a housekeeping gene highly expressed), no amplification was observed after RNase R treatment. By using a specific set of divergent primers located on exons E7 and E8, several TLINC circular isoforms were highlighted (black arrowheads). (C) RNAs were extracted from 16 human iCCA tumors. RT‐PCR analysis was performed using a specific set of divergent primers located on exons E7 and E8, as indicated at the top of the panels (a, E7R/E8F; b, E7R/E7F). Black arrowheads highlight TLINC circular isoforms. Abbreviations: bp, base pair; MW, molecular weight.
Discussion
The molecular mechanisms involved in iCCA carcinogenesis are complex and involve multiple interactions between tumor cells and their microenvironment.1 We previously demonstrated that active TGFβ signaling is an important feature in the iCCA microenvironment6; however, a deep characterization of the impact of TGFβ on tumor cells at the transcriptome level was lacking in iCCA. In this study, we first validated suitable experimental cellular models to study TGFβ signaling in iCCA cells. Particularly, we provided strong experimental evidence that Huh28 cells exhibit an endogenous activation of TGFβ, which correlates with a mesenchymal phenotype. This cell line may thus be relevant to test TGFβ inhibitors for therapeutic purposes, as was suggested in liver cancers.22 In addition, we provided the first TGFβ transcriptomic signature in iCCA cells, which allowed us to identify novel TGFβ transcriptional targets, including coding and noncoding genes, both in vitro and in resected human iCCA.
Few studies have reported a deregulation of lncRNA in iCCA.23, 24 As an example, oncogenic metastasis‐associated lung adenocarcinoma transcript 1 (MALAT1) lncRNA was shown to promote proliferation and invasion of CCA cell lines by activating the phosphoinositide 3‐kinase/protein kinase B signaling pathway.24 Here, we provide the first report of lncRNA directly regulated by TGFβ in iCCA, supporting the previous description of TGFβ‐regulated lncRNA in other cancer types.11, 12 Notably, lncRNA activated by TGF‐β was shown to induce EMT and to promote HCC cell invasion through a TGFβ/miR‐200/zinc finger E‐box binding axis.12 Similarly, TGFβ‐induced lncRNA‐HIT was shown to promote EMT in breast cancer, notably by decreasing the expression of CDH1.11 By comparing the transcriptome profiles of HuCCT1 and Huh28 cells at the basal level and after TGFβ treatment, we identified a robust signature made of 42 nonredundant genes. This signature was validated by including well‐known TGFβ target genes (e.g., SERPINE1, IL11, CTGF, BCL2 modifying factor [BMF], SNAI1) reported in multiple cell types, including hepatocytes.25 Importantly, this approach highlighted several novel TGFβ‐induced lncRNA, including LINC00312, LINC00313, and LINC00340 (TLINC), possibly involved in TGFβ signaling. LINC00312 has been reported to inhibit the migration and invasion of bladder cancer cells by targeting miR‐197‐3p.26 High expression of LINC00313 has been associated with a poor prognosis and metastasis in lung cancer.27 Experimental data on TLINC were more complex given that several isoforms were reported to exhibit either tumor‐suppressive or tumor‐promoting features in various types of cancer.18, 19 Thus, in neuroblastoma, a short isoform was associated with tumor suppression by hampering cell growth and migration, and a lower expression of this short isoform was found in advanced tumors associated with a poor survival.18 Conversely, a long isoform of TLINC was reported in metastatic melanoma and associated with tumor progression.19 The differences in the basal expression and induction by TGFβ of TLINC isoforms in HuCCT1 and Huh28 cell lines suggest that the function of TLINC is highly dependent on the cellular context, as known for several TGFβ target genes.10
Although TLINC deregulation has been reported in cancer,18, 19 we provide the first evidence that TLINC is a common transcriptional target of TGFβ not only in liver cancer (iCCA and HCC) but also in other cancer types, including pancreatic cancer. Interestingly, we demonstrated that TLINC is also up‐regulated by TGFβ in cells from the tumor microenvironment, as evidenced by the analysis of its expression in activated hepatic stellate cells (LX2) and in human iCCA tumors by ISH and LCM of the stroma. These data suggest that TLINC may play a role in TGFβ‐induced remodeling of the tumor microenvironment. Supporting this hypothesis, our experimental data show that ectopic expression of TLINC directly impacts the expression of several inflammatory mediators, including chemokine IL8. Increased expression of IL8 has been reported in cancer and is associated with important changes within the tumor microenvironment, including promotion of angiogenesis, infiltration of neutrophils and tumor‐associated macrophages, and migration of cancer cells.28, 29 Interestingly, a functional link has been established between the expression of IL8 and nuclear paraspeckle assembly transcript 1 [NEAT1], another lncRNA that we found to be induced in cells transfected with the TLINC DNA construct (Fig. 5B). Indeed, it was demonstrated that NEAT1‐dependent relocation of a splicing factor proline/glutamine‐rich from the promoter region of the IL8 gene to paraspeckle led to the transcriptional activation of IL8.30 One can hypothesize that TLINC acts as a structural component of paraspeckles and that interaction with NEAT1 may result in IL8 expression. This hypothesis is in agreement with the partial nuclear location of TLINC. The enrichment of a gene signature associated with repressive marks H3K27me3 in iCCA tumors characterized by a high expression of TLINC may also suggest a role of TLINC in epigenetic silencing, as was described for other lncRNAs, such as Kcnq1ot1 antisense.31 Similarly, enrichment of a gene signature associated with protein metabolism in iCCA with low TLINC expression together with the cytoplasmic expression of TLINC may suggest a role of TLINC in translation, as described for lncRNA growth arrest specific 5 (GAS5), which interacts with the translational machinery to inhibit c‐Myc translation.32 Another interesting aspect is the restricted expression of TLINC in remodeled portal areas with signs of ductular reaction in nontumor livers. These areas are usually associated with activation of the cancer stem cell compartment.33 Supporting a putative role of TLINC in cancer stem cell biology, TLINC has been reported to be highly expressed in human embryonic stem cells.21, 34 At the functional level, we showed that TLINC‐L overexpression in HuCCT1 cells resulted in increased cell migration. Although the underlying molecular mechanisms are still to be clarified, SNAI2 was found to be enriched in the gene expression profiles of TLINC‐L transfected cells, suggesting that the type of migration could be partly related to EMT. Other genes found to be tightly correlated with TLINC expression in human iCCA tumors may also contribute to the observed phenotypes. As an example, platelet‐derived growth factor receptor beta (PDGFRB) has been associated with the aggressive behavior of several types of tumors.35 Notably, PDGFRB has been shown to promote liver metastasis formation of mesenchymal‐like colorectal tumor cells.36
Finally, we provide experimental evidence of circular isoforms of TLINC that may represent putative, relevant, noninvasive biomarkers for iCCA. Indeed, recent data demonstrated that circRNAs are enriched in extracellular vesicles, including exosomes, compared to their linear counterparts.37, 38 The circular structure of circRNAs renders them more stable and resistant to degradation by exonucleases. Thus, pangenomic profiling of circRNA abundance is emerging in cancer, and several expression signatures have been reported, notably in HCC.39 Our study provides the first report of circRNA expression in iCCA. The function of circRNA–TLINC remains to be explored, but several experimental data demonstrated that circRNAs may act as sponges for miRNA and RNA binding proteins. With respect to the TGFβ pathway, circRNA_010567 has been recently shown to promote TGFβ‐induced myocardial fibrosis by directly suppressing miR‐141, which naturally targets TGFβ.40
In conclusion, our study identifies novel TGFβ transcriptional target genes in iCCA, including an lncRNA that may represent a promising biomarker for iCCA.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep4.1142/full.
Supporting Information Figures
Supporting Information Tables
Acknowledgment
We thank the core facilities of Biosit and Biogenouest (CRB Santé BB‐0033‐00056, GEH, H2P2, ImPACcell) and the French liver cancer biobanks network (BB‐0033‐00085), including Beaujon, Bordeaux, Mondor, Nantes, Paris‐Sud, and Rennes biobanks. C.C. thanks K.A. Cole (Children's Hospital of Philadelphia) and S. Sugano (University of Tokyo) for providing the CASC15 DNA constructs; S. Dréano (Institut de génétique et développement de Rennes [IGDR]) for DNA sequencing; and C. Père for reporter sensor cell line analysis. We thank D. Gilot, M.D. Galibert (IGDR, Rennes), B. Fromenty (NuMeCan, Rennes), and S. Diederichs (German Cancer Research Center [DKFZ], Heidelberg) for helpful discussions.
Potential conflict of interest: Nothing to report.
Supported by INSERM, University of Rennes 1, Institut National du Cancer (INCa, Cancéropôles Ile‐de‐France and Grand‐Ouest), Ligue contre le cancer (cd35, cd44, cd49), Novartis Oncology, and Association Française pour l'Etude du Foie, France (to L.S., C.C.); and by Deutsche Forschungsgemeinschaft DFG DO373/13‐1 (to S.D.).
REFERENCES
- 1. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS‐CCA). Nat Rev Gastroenterol Hepatol 2016;13:261‐280. [DOI] [PubMed] [Google Scholar]
- 2. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268‐1289. [DOI] [PubMed] [Google Scholar]
- 3. Moeini A, Sia D, Bardeesy N, Mazzaferro V, Llovet JM. Molecular pathogenesis and targeted therapies for intrahepatic cholangiocarcinoma. Clin Cancer Res 2016;22:291‐300. [DOI] [PubMed] [Google Scholar]
- 4. Sirica AE. The role of cancer‐associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;9:44‐54. [DOI] [PubMed] [Google Scholar]
- 5. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sulpice L, Rayar M, Desille M, Turlin B, Fautrel A, Boucher E, et al. Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology 2013;58:1992‐2000. [DOI] [PubMed] [Google Scholar]
- 7. Massague J. TGFbeta in cancer. Cell 2008;134:215‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell 2016;166:21‐45. [DOI] [PubMed] [Google Scholar]
- 9. Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 2010;10:415‐424. [DOI] [PubMed] [Google Scholar]
- 10. Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol 2012;13:616‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richards EJ, Zhang G, Li ZP, Permuth‐Wey J, Challa S, Li Y, et al. Long non‐coding RNAs (LncRNA) regulated by transforming growth factor (TGF) beta. LncRNA‐HIT‐mediated TGF‐induced epithelial to mesenchymal transition in mammary epithelia. J Biol Chem 2016;291:22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, et al. A long noncoding RNA activated by TGF‐beta promotes the invasion‐metastasis cascade in hepatocellular carcinoma. Cancer Cell 2014;25:666‐681. [DOI] [PubMed] [Google Scholar]
- 13. Klingenberg M, Matsuda A, Diederichs S, Patel T. Non‐coding RNA in hepatocellular carcinoma: mechanisms, biomarkers and therapeutic targets. J Hepatol 2017;67:603‐618. [DOI] [PubMed] [Google Scholar]
- 14. Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov 2011;1:391‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allain C, Angenard G, Clement B, Coulouarn C. Integrative genomic analysis identifies the core transcriptional hallmarks of human hepatocellular carcinoma. Cancer Res 2016;76:6374‐6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coulouarn C, Corlu A, Glaise D, Guenon I, Thorgeirsson SS, Clement B. Hepatocyte‐stellate cell cross‐talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res 2012;72:2533‐2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dubois‐Pot‐Schneider H, Fekir K, Coulouarn C, Glaise D, Aninat C, Jarnouen K, et al. Inflammatory cytokines promote the retrodifferentiation of tumor‐derived hepatocyte‐like cells to progenitor cells. Hepatology 2014;60:2077‐2090. [DOI] [PubMed] [Google Scholar]
- 18. Russell MR, Penikis A, Oldridge DA, Alvarez‐Dominguez JR, McDaniel L, Diamond M, et al. CASC15‐S is a tumor suppressor lncRNA at the 6p22 neuroblastoma susceptibility locus. Cancer Res 2015;75:3155‐3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lessard L, Liu M, Marzese DM, Wang H, Chong K, Kawas N, et al. The CASC15 long intergenic noncoding RNA locus is involved in melanoma progression and phenotype switching. J Invest Dermatol 2015;135:2464‐2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y, Siadaty MS, Berens ME, Hampton GM, Theodorescu D. Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N‐methyltransferase as novel regulators of cell migration. Oncogene 2008;27:6679‐6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell‐type specific features of circular RNA expression. PLoS Genet 2013;9:e1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giannelli G, Mikulits W, Dooley S, Fabregat I, Moustakas A, ten Dijke P, et al. The rationale for targeting TGF‐beta in chronic liver diseases. Eur J Clin Invest 2016;46:349‐361. [DOI] [PubMed] [Google Scholar]
- 23. Yang W, Li Y, Song X, Xu J, Xie J. Genome‐wide analysis of long noncoding RNA and mRNA co‐expression profile in intrahepatic cholangiocarcinoma tissue by RNA sequencing. Oncotarget 2017;8:26591‐26599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang C, Mao ZP, Wang L, Wu GH, Zhang FH, Wang DY, et al. Long non‐coding RNA MALAT1 promotes cholangiocarcinoma cell proliferation and invasion by activating PI3K/Akt pathway. Neoplasma 2017;64:725‐731. [DOI] [PubMed] [Google Scholar]
- 25. Coulouarn C, Factor VM, Thorgeirsson SS. Transforming growth factor‐beta gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology 2008;47:2059‐2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang YY, Wu ZY, Wang GC, Liu K, Niu XB, Gu S, et al. LINC00312 inhibits the migration and invasion of bladder cancer cells by targeting miR‐197‐3p. Tumour Biol 2016;37:14553‐14563. [DOI] [PubMed] [Google Scholar]
- 27. Li M, Qiu M, Xu Y, Mao Q, Wang J, Dong G, et al. Differentially expressed protein‐coding genes and long noncoding RNA in early‐stage lung cancer. Tumour Biol 2015;36:9969‐9978. [DOI] [PubMed] [Google Scholar]
- 28. Waugh DJ, Wilson C. The interleukin‐8 pathway in cancer. Clin Cancer Res 2008;14:6735‐6741. [DOI] [PubMed] [Google Scholar]
- 29. Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, et al. The CXCL8‐CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev 2016;31:61‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imamura K, Imamachi N, Akizuki G, Kumakura M, Kawaguchi A, Nagata K, et al. Long noncoding RNA NEAT1‐dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell 2014;53:393‐406. [DOI] [PubMed] [Google Scholar]
- 31. Pandey RR, Mondal T, Mohammad F, Enroth S, Redrup L, Komorowski J, et al. Kcnq1ot1 antisense noncoding RNA mediates lineage‐specific transcriptional silencing through chromatin‐level regulation. Mol Cell 2008;32:232‐246. [DOI] [PubMed] [Google Scholar]
- 32. Hu G, Lou Z, Gupta M. The long non‐coding RNA GAS5 cooperates with the eukaryotic translation initiation factor 4E to regulate c‐Myc translation. PLoS One 2014;9:e107016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marquardt JU, Andersen JB, Thorgeirsson SS. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer 2015;15:653‐667. [DOI] [PubMed] [Google Scholar]
- 34. Tang X, Hou M, Ding Y, Li Z, Ren L, Gao G. Systematically profiling and annotating long intergenic non‐coding RNAs in human embryonic stem cell. BMC Genomics 2013;14(Suppl. 5):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Demoulin JB, Essaghir A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev 2014;25:273‐283. [DOI] [PubMed] [Google Scholar]
- 36. Steller EJ, Raats DA, Koster J, Rutten B, Govaert KM, Emmink BL, et al. PDGFRB promotes liver metastasis formation of mesenchymal‐like colorectal tumor cells. Neoplasia 2013;15:204‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lasda E, Parker R. Circular RNAs co‐precipitate with extracellular vesicles: a possible mechanism for circRNA clearance. PLoS One 2016;11:e0148407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res 2015;25:981‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han D, Li J, Wang H, Su X, Hou J, Gu Y, et al. Circular RNA circMTO1 acts as the sponge of miR‐9 to suppress hepatocellular carcinoma progression. Hepatology 2017;66:1151‐1164. [DOI] [PubMed] [Google Scholar]
- 40. Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR‐141 by targeting TGF‐beta1. Biochem Biophys Res Commun 2017;487:769‐775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep4.1142/full.
Supporting Information Figures
Supporting Information Tables


