Abstract
Autoimmune hepatitis (AIH) and primary biliary cholangitis (PBC) are two major immune‐mediated chronic liver diseases. Overlap syndrome (OS) is diagnosed if patients have features of both AIH and PBC; however, there is no consensus on the definition or diagnostic criteria for OS. Here, we report a new scoring classification for OS and evaluate its usefulness. This new scoring classification was developed by modifying the International Autoimmune Hepatitis Group classification by selecting histologic features of AIH and PBC along with modifications of biochemical and immunologic characteristics. We evaluated 272 patients with chronic liver disease, including 105 with AIH, 102 with PBC, and 65 with OS. The best performance for the diagnosis of OS was noted among patients with an overlap score of ≥21 who had a sensitivity of 98.5%, a specificity of 92.8%, a positive predictive value of 81.0%, and a negative predictive value of 99.5%. By using a cut‐off score of 21, 64 (98.5%) patients were diagnosed with OS as opposed to 9 (8.8%) and 6 (5.7%) with PBC and AIH, respectively. All patients with OS had an aggregate score of >19, whereas most patients with PBC or AIH scored <19, making this a safe discriminatory cut‐off point against OS. Conclusion: The new scoring system for the diagnosis of OS has a high sensitivity and specificity for scores ≥21, while a score <19 suggests a diagnosis other than OS. This classification can identify patients and diagnose OS with a reasonable amount of accuracy and may be superior to current OS scoring systems in detecting mild forms of OS. (Hepatology Communications 2018;2:245‐253)
Abbreviations
- AIH
autoimmune hepatitis
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AMA
anti‐mitochondrial antibody
- ANA
anti‐nuclear antibody
- ASMA
anti‐smooth muscle antibody
- AST
serum aspartate aminotransferase
- CI
confidence interval
- IAIHG
International Autoimmune Hepatitis Group
- NPV
negative predictive value
- OS
overlap syndrome
- PBC
primary biliary cholangitis
- PPV
positive predictive value
- PSC
primary sclerosing cholangitis
- ULN
upper limit of normal
Introduction
Autoimmune hepatitis (AIH) and primary biliary cholangitis (PBC) are two major immune‐mediated chronic liver diseases that can be differentiated using clinical, biochemical, serologic, and histologic findings.1, 2 However, a group of patients may have characteristics of both either simultaneously or consecutively. Patients with AIH may have features of PBC, including anti‐mitochondrial antibodies (AMAs), elevated serum alkaline phosphatase (ALP), and bile duct injury in histologic findings. Conversely, patients with PBC may have features of AIH, including anti‐smooth muscle antibodies (ASMAs); anti‐nuclear antibodies (ANAs); elevated serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and immunoglobulin G; and histologic findings of interface hepatitis. These conditions are difficult to classify and are commonly designated as overlap syndrome (OS).3 There are other OSs, such as AIH‐primary sclerosing cholangitis (PSC) and PBC‐PSC OS; however, these are not as common as PBC‐AIH OS. In this manuscript, OS refers to PBC‐AIH OS.
The diagnosis of OS is usually difficult and problematic as internationally agreed criteria are lacking and a variety of definitions have been applied.3 The “Paris criteria” is the most commonly used tool for diagnosing OS.4, 5 This requires the presence of at least two of three key criteria for the diagnosis of PBC and AIH. Thus, for PBC the criteria are as follows: 1) ALP >2 times upper limit of normal (ULN) or g‐glutamyl‐transpeptidase >5 times ULN; 2) presence of AMAs; and 3) liver biopsy specimen showing florid bile duct lesions. For AIH, the criteria are as follows: 1) ALT levels >5 times ULN; 2) serum immunoglobulin G levels >2 times ULN or positive ASMAs; and 3) liver biopsy showing moderate or severe periportal or periseptal lymphocytic piecemeal necrosis. The 2009 European Association for the Study of the Liver guidelines on the management of cholestatic liver diseases endorsed the Paris criteria but stressed that the histologic evidence of moderate to severe lymphocytic piecemeal necrosis (interface hepatitis) was mandatory for the diagnosis of OS. The sensitivity and specificity of the Paris criteria for OS were reported to be 92% and 97%, respectively5; however, patients with less severe forms of AIH‐PBC OS may not be captured by the Paris criteria.6, 7, 8, 9, 10 In addition, in the natural evolution of PBC, 100% of untreated patients develop interface hepatitis in 4 years, and a significant number of patients have positive ANAs and/or ASMAs at the time of diagnosis.11 This suggests that meeting diagnostic criteria for PBC and having significant interface hepatitis with ANAs and/or ASMAs are insufficient to define OS.
The International Autoimmune Hepatitis Group (IAIHG) scoring system for AIH is another widely used diagnostic criterion for the diagnosis of OS, although it was not intended for such use and has not proven to be an efficient tool for this purpose.3 The diagnostic criteria of AIH were initially introduced by the IAIHG in 1993 and were later revised in 1999.12, 13 The revised IAIHG classification was able to identify 19% of AIH overlap when applied to 137 patients diagnosed with PBC.14 However, the revised IAIHG classification was only 50% sensitive in diagnosing OS but had 92.3%‐100% specificity in diagnosing patients with AIH.15 The IAIHG suggests that patients with autoimmune liver disease should be categorized according to the predominating feature and that patients with overlapping features should not be considered as distinct diagnostic entities.
There is a need for new diagnostic criteria that can detect patients with both typical and mild OS with high sensitivity and specificity. This is crucial as studies have shown that patients with OS can have a worse clinical outcome compared to patients with PBC alone, with earlier onset of portal hypertension and the need for liver transplantation.16 A descriptive numerical scoring system would be useful in the diagnosis of OS where the diagnosis is based on the cumulative features of various manifestations. Here, we describe and evaluate our new scoring classification based on patients with OS in our liver center.
Patients and Methods
Patients with AIH, PBC, and OS who were being treated at the Saint Louis University Liver Center and had complete clinical, laboratory, and histologic data were included in this retrospective study. The AIH group consisted of patients who met criteria of the revised AIH score for probable or definite AIH (revised pretreatment IAIHG score ≥10). The biochemical profile was that of elevated transaminases and an increased globulin fraction; immunologic testing was characterized by a positive ANA and/or ASMA; the histologic features typically showed parenchymal inflammation in the form of interface hepatitis, lymphoplasmacytic infiltrate, and hepatic rosettes. The PBC group consisted of biopsy‐proven patients with a cholestatic pattern of liver dysfunction. Eighty‐seven out of 102 (85.3%) patients were AMA positive. Liver histology in PBC typically shows ductal injury with florid ductal lesions and loss of small bile ducts with portal inflammatory cell infiltration; well‐formed granulomas were noted in a portion of the cases. OS was diagnosed on the basis of either histologic assessment (presence of bile duct damage/florid ductal sessions and interface hepatitis with lymphoplasmacytic infiltrate in the same biopsy specimen) or serologic and biochemical data (presence of both AMAs and other autoantibodies and presence of both cholestatic enzymes and transaminase elevation).
Data collected from medical records included sex and age of diagnosis, serologic studies at diagnosis (including AMA, ANA, and ASMA), and laboratory results (including ALT, AST, ALP, and globulin). Liver biopsy was performed in all patients, and liver tissue specimens were examined by two hepatopathologists (G.C. and J.L.).
The overlap classification table (Table 1) was designed in a way to help isolate the OS group from the others, keeping in mind the nature of the biochemical, immunologic, and histologic presentation of patients with AIH and PBC. To create the overlap scoring system, the clinical features of AIH, PBC, and OS were first evaluated and then each item was selected to discriminate OS from the other two disorders. This classification was developed by modifying the IAIHG classification by selecting the histologic features favorable for AIH and adding histologic features favorable for PBC along with modifications of biochemical, immunologic, and other features. OS occupies a middle part of the spectrum of characteristics shared by AIH on one end and PBC on the other. All patients selected for the study were evaluated using this new overlap scoring classification and also the IAIHG scoring classification.
Table 1.
Proposed Scoring Classification for Overlap Syndrome
| Component | Result | Score |
|---|---|---|
| Biochemical category | ||
| AST or ALT above ULN | >2 | +3 |
| 1.5‐2 | +2 | |
| 1‐1.5 | +1 | |
| <1 | 0 | |
| ALP above ULN | >1 | +2 |
| 0.75‐1 | +1 | |
| <0.75 | 0 | |
| Serum globulin above ULN | >1.5 | +2 |
| 1‐1.5 | +1 | |
| <1 | 0 | |
| Immunologic category | ||
| ANA, ASMA, or LKM1 | >1:80 | +3 |
| 1:80 | +2 | |
| 1:40 | +1 | |
| <1:40 | 0 | |
| or | ||
| Anti‐SLA, pANCA | Positive | +2 |
| AMA | Positive | +3 |
| Histologic category | ||
| Interface hepatitis | +3 | |
| Lymphoplasmacytic | +1 | |
| Hepatic rosettes | +1 | |
| Biliarydamage | ||
| Granulomas | +3 | |
| Florid ductal lesion | +1 | |
| Ductular proliferation | +1 | |
| Bile duct loss | +1 | |
| Others category | ||
| Viral markers | Positive | –3 |
| Negative | +3 | |
| Drugs | Yes | –4 |
| No | +1 | |
| Alcohol | <25 g/day | +2 |
| >60 g/day | –2 | |
| Interpretation of scores | Definitive | ≥21 |
| Probable | 19 or 20 | |
| Rejected | <19 |
Abbreviations: anti‐SLA, antibody to soluble liver antigen; LKM1, antibody to liver/kidney microsomes type 1; pANCA, perinuclear anti‐neutrophil cytoplasmic antibody.
Accordingly, the overlap classification was developed by grouping the characteristics into four major categories: biochemical, immunologic, histologic, and others. Pretreatment scores were given to each of these features depending on their representation in their respective categories.
In the biochemical category, aminotransferases (AST or ALT), globulin, and ALP were included. Scoring was graded on the degree of elevation of these liver tests above the ULN for that particular test. This classification differs from the revised IAIHG classification where the ratio of ALP to AST (or ALT) was taken into account as it was meant to distinguish between patients with AIH or PBC. The rationale of using globulin elevation is to favor the AIH group, whereas ALP elevation is to favor the PBC group. AST/ALT elevation is included to favor mostly the AIH group, although some patients with PBC also scored favorably. Keeping these three liver tests in the biochemical category is important for isolating patients with OS who will score favorably in the AST/ALT, globulin, and ALP categories as opposed to patients with AIH or PBC who will score selectively in their respective categories.
The immunologic category consists of ANA, ASMA, or F‐actin antibody/anti‐liver kidney microsomal antibody titers, where >1:80, 1:80, 1:40, and <1:40 score +3, +2, +1, and 0, respectively. Positivity for anti‐soluble liver antigen, anti‐liver cytosol, or perinuclear anti‐neutrophil cytoplasmic antibody is given +2 points. Between these two sets of autoimmune tests, the highest scored group is considered to balance the scoring between patients with AIH or PBC. Patients with a positive AMA will score +3.
The histologic category consists of two subsets. The first subset of histology is consistent with hepatocellular damage, which includes features of interface hepatitis (periportal and periseptal), plasmacytic features (lymphoplasmacytic necroinflammatory infiltrate), and formation of hepatic rosettes, representing scores of +3, +1, and +1, respectively. The second subset consists of histology with biliary damage, which includes presence of portal epithelioid granulomas, florid ductal lesions, evidence of bile duct loss, and ductular proliferation, representing scores of +3, +1, +1, and +1, respectively. In the first subset, the highest score (+3) is given to interface hepatitis as it is crucial and is one of the hallmark histologic features essential for the diagnosis of AIH. In the second subset, a high score (+3) is given to the presence of granulomas, which is unique for the diagnosis of PBC.
The initial pretreatment liver biopsies were considered in the patient groups. In some patients there was evidence of progression to OS from initially diagnosed AIH over a period of time since their presentation to our institution. These patients were considered patients with OS for our study. Their histologic scoring included the addition of features of hepatocellular damage during their initial presentation with AIH along with new features of biliary damage as was evident during their transformation to OS.
The others category consists of three subsets. The first subset includes viral markers where patients with seropositivity for markers of current infection with hepatitis A, hepatitis B (hepatitis B surface antigen), and hepatitis C viruses score –3, whereas patients with negative viral markers score +3. The second subset, which includes intake of drugs causing an elevation in liver tests, results in a score of –4, whereas no such history results in a score of +1. The third subset includes the level of ingestion of alcohol; patients with a history of alcohol ingestion of <25 g/day and >60g/day score +2 and –2, respectively.
STATISTICAL ANALYSIS
Descriptive statistical analyses were stratified by disease entities: OS, PBC, and AIH. Differences between patients with each disease entity by their demographic, serologic, and histologic characteristics were examined using chi square and Fisher's exact tests for categorical variables and analysis of variance and Kruskal–Wallis tests for continuous variables, where applicable. A scatterplot diagram was used to plot scores from IAIHG and the overlap scoring system developed in this study to visualize distinct areas for the three disease entities. Receiver operating characteristic curve analysis was used to identify the best cut‐off value to properly discriminate against OS. Diagnostic performance of overlap scores was evaluated using the statistical measures of performance, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
All analyses were performed with SAS System version 9.4 (SAS Institute Inc., Cary, NC). All statistical tests were two‐tailed, and P < 0.05 was considered statistically significant. Institutional review board approval was granted from Saint Louis University School of Medicine.
Results
DESCRIPTIVE STATISTICS
Descriptive statistics of the study participants are summarized in Table 2. Due to the skewed distribution of the continuous values, the median and interquartile range were used as measures of central tendency. Except for sex, statistically significant differences were evident between the three disease entities across all patient characteristics. Overall, patients with OS were older than their counterparts with AIH or PBC (P = 0.01). Higher levels of ALT/AST but lower levels of ALP were noted among patients with AIH compared to those with PBC or OS (P < 0.001); the lowest ALT/AST level was noted among patients with PBC, while the highest ALP level was seen in OS. The OS group had the highest serum globulin (4.1 g/dL), and patients with PBC had the lowest (3.5 g/dL) (P < 0.0001). Immunologically, patients with AIH expressed higher serum ANAs, ASMAs, or F‐actin (87.6%) compared to only 36.3% among patients with PBC (P < 0.0001). Conversely, 85.3% of patients with PBC had positive AMAs compared to only 2.9% of patients with AIH. The majority of patients with OS expressed high levels of AMAs (70.8%) and ANAs, ASMAs, or F‐actin (80.0%). Histologically, the majority of patients with OS had hepatocellular histology of interface hepatitis (90.8%) and lymphoplasmacytic infiltrate (92.3%). Furthermore, 81.5% of patients with OS had biliary duct infiltration/florid duct lesions. The AIH score of the OS group was higher than that of the PBC group but lower than the AIH group, with a significant difference between each group (P < 0.0001). However, the overlap score of the OS group was higher than both the PBC and AIH groups (P < 0.0001; Fig. 1), which indicates that OS could be a different entity from AIH or PBC.
Table 2.
Baseline Characteristics of Study Participants and Comparison of Demographic, Serologic, and Histologic Characteristics by Disease Entities
| Characteristicsa |
Overall Patients N = 272 n (%) or Median (IQR) |
Overlap Patients n = 65 n (%) or Median (IQR) |
PBC Patients n = 102 n (%) or Median (IQR) |
AIH Patients n = 105 n (%) or Median (IQR) |
P Value |
|---|---|---|---|---|---|
| Sex, n (%) | 0.53 | ||||
| Male | 37 (13.6) | 9 (13.8) | 11 (10.8) | 17 (16.2) | |
| Female | 235 (86.4) | 56 (86.2) | 91 (89.2) | 88 (83.8) | |
| Age, in yearsb | 53 (46‐60) | 55 (50‐61) | 52 (46‐59) | 51 (41‐59) | 0.01 |
| Serologyb | |||||
| ALT | 96.5 (53.0‐190.5) | 129 (67‐203) | 62 (42‐99) | 158 (67‐470) | <0.0001 |
| AST | 76.0 (44.0‐196.5) | 107 (59‐206) | 49 (39‐74) | 144 (55‐425) | <0.0001 |
| Globulin | 3.8 (3.3‐4.3) | 4.1 (3.7‐4.6) | 3.5 (3.1‐4.0) | 3.8 (3.3‐4.5) | <0.0001 |
| ALP | 163.5 (108.5‐245.0) | 209 (148‐287) | 182.5 (123‐298) | 117 (79‐183) | <0.0001 |
| Hepatocellular histology | |||||
| Interface hepatitis | 166 (61.0) | 59 (90.8) | 12 (11.8) | 95 (90.5) | <0.0001 |
| Lymphoplasmacytic infiltrate | 182 (66.9) | 60 (92.3) | 23 (22.5) | 99 (94.3) | <0.0001 |
| Rosettes | 62 (22.8) | 25 (38.5) | 2 (2.0) | 35 (33.3) | <0.0001 |
| Biliary histology | |||||
| Granulomas | 93 (34.2) | 38 (58.5) | 53 (52.0) | 2 (1.9) | <0.0001 |
| Duct infiltration/lesion | 149 (54.8) | 53 (81.5) | 90 (88.2) | 6 (5.7) | <0.0001 |
| Duct loss | 77 (28.3) | 37 (56.9) | 39 (38.2) | 1 (1.0) | <0.0001 |
| Duct proliferation | 75 (27.6) | 40 (61.5) | 29 (28.4) | 6 (5.7) | <0.0001 |
| Immunology | |||||
| ANA/ASMA | 181 (66.5) | 52 (80.0) | 37 (36.3) | 92 (87.6) | <0.0001 |
| AMA | 136 (50.0) | 46 (70.8) | 87 (85.3) | 3 (2.9) | <0.0001 |
| AIH scoreb | 11 (2‐16) | 12 (7‐14) | 0 (‐2.0‐3.8) | 17 (14‐19) | <0.0001 |
| Overlap scoreb | 18 (16‐21) | 24 (22‐26) | 17 (15‐19) | 17 (15‐19) | <0.0001 |
Categorical variables, such as sex, hepatocellular histology, biliary histology, and immunology were tested using χ2 and Fisher's exact tests. Continuous variables, such as age, AIH score, overlap score, and serology (ALT, AST, globulin, and ALP) were tested using analysis of variance and Kruskal‐Wallis tests.
Values presented as median (IQR).
Abbreviations: IQR, interquartile range; n, number of patients.
Figure 1.
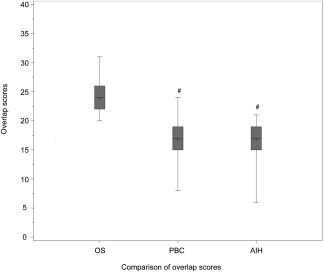
Comparison of overlap scores between PBC, AIH, and OS. # P < 0.001, comparison among three groups using analysis of variance and Kruskal–Wallis tests. Overlap scores for 3 groups, PBC 17 (15‐19), AIH 17 (15‐19), OS 24 (22‐26).
DIAGNOSTIC PERFORMANCE OF OVERLAP SCORE
Results of the diagnostic performance of the aggregate overlap score in relation to OS are presented in Table 3. Receiver operating characteristic analysis (Fig. 2) yielded a cut‐off value of 21 to be the best performance; this showed a sensitivity of 98.5% (95% confidence interval [CI], 91.7%, 99.9%), a specificity of 92.8% (95% CI, 88.3%, 95.9%), a PPV of 81.0% (95% CI, 72.4%, 87.4%), and a NPV of 99.5% (95% CI, 96.7%, 100.0%) for the diagnosis of OS.
Table 3.
Diagnostic Performance of Aggregate Overlap Scores in Relation to Overlap Syndrome
| Aggregate Scores | Overlap Syndrome |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
PPV % (95% CI) |
NPV % (95% CI) |
|
|---|---|---|---|---|---|---|
| Yes | No | |||||
| ≥22 | 51/65 | 6/207 | 78.5 (66.5‐87.9) | 97.1 (93.8‐98.9) | 89.5 (79.3‐95.0) | 93.5 (89.1‐96.3) |
| ≥21 | 64/65 | 15/207 | 98.5 (91.7‐99.9) | 92.8 (88.3‐95.9) | 81.0 (72.4‐87.4) | 99.5 (96.7‐100.0) |
| ≥20 | 65/65 | 41/207 | 100.0 (93.0‐100.0) | 80.2 (74.0‐85.3) | 61.3 (51.3‐70.5) | 100.0 (97.2‐100.0) |
| ≥19 | 65/65 | 64/207 | 100.0 (93.0‐100.0) | 69.1 (62.2‐75.2) | 50.4 (41.5‐59.2) | 100.0 (96.7‐100.0) |
Figure 2.
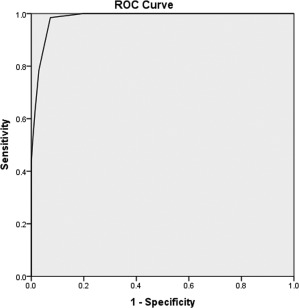
ROC curve for overlap score predicting the overlap patients. A cut‐off value of 21 provided the best balance of sensitivity (98.5%) and specificity (92.8%). Area under the ROC curve, 0.98 (P < 0.0001). Abbreviation: ROC, receiver operating characteristic.
An overlap score of ≥21 strongly favors the diagnosis of OS given that 64 (98.5%) patients with OS had an aggregate score of ≥21 as opposed to only 9 (8.8%) and 6 (5.7%) patients with PBC and AIH, respectively (Table 4). Furthermore, none of the patients with OS had an aggregate score <19 as opposed to most patients with PBC or AIH, who scored <19, making it a safe discriminatory cut‐off point against OS.
Table 4.
Incidence of Three Disease Entities According To Aggregate Overlap Scores
| Disease Entity | Aggregate Overlap Score | ||
|---|---|---|---|
|
Definite Overlap ≥21 n (%) |
Probable Overlap ≥19‐20 n (%) |
Rejected Overlap <19 n (%) |
|
| Primary biliary cirrhosis | 9 (8.8%) | 21 (20.6%) | 72 (70.6%) |
| Autoimmune hepatitis | 6 (5.7%) | 28 (26.7%) | 71 (67.6%) |
| Overlap syndrome | 64 (98.5%) | 1 (1.5%) | 0 (0.00%) |
Abbreviation: n, number of patients.
Three distinct areas were identified when scores were plotted from both the overlap scoring system designed in this study and the IAIHG (Fig. 3). Patients with OS occupied the top middle of the graph owing to their lower IAIHG scores and the highest overlap scores, while patients with AIH were clustered in the lower right of the graph owing to their high IAIHG scores and lower overlap scores.
Figure 3.
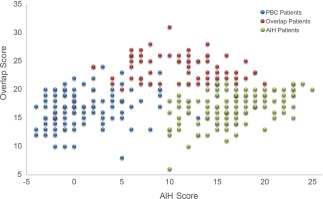
Scatterplot of overlap scores and AIH scores by patient group.
COMPARISON OF PARIS CRITERIA AND NEW SCORING SYSTEM
We also used Paris criteria to detect OS in our patients and compared the two diagnostic criteria regarding sensitivity, specificity, PPV, and NPV (Supporting Tables 1 and 2). The sensitivity of the Paris criteria in diagnosing OS was 58.46% (range, 45.56%‐70.56%), specificity was 99.52% (97.34%‐99.99%), PPV was 97.44% (86.52%‐99.94%), and NPV was 88.41% (83.59%‐92.22%). When compared with the Paris criteria, our new diagnostic criteria with a cut‐off score of 21 had better sensitivity and NPV but lower specificity and PPV.
Discussion
Our results suggest that a cut‐off score of 21 has the best balance of sensitivity and specificity in our new scoring classification. Our scoring classification is valuable and may be superior to current OS scoring systems, including the revised and the simplified AIH scoring systems and Paris criteria, for recognizing patients with OS.
The first case of OS was described in 1977 by Kloppel et al.17 The prevalence of PBC‐AIH overlap has been reported in 4.3%‐19% of patients with PBC8, 9, 18, 19, 20, 21 and 3%‐13% of patients with AIH.8, 9, 18, 19, 20, 22 The prevalence has a wide range because of the challenges involved with its diagnosis. Due to the lack of standardization and variations in the populations under study, the characteristics of these entities vary.3, 23 Controversy exists whether OS represents a distinct entity with peculiar histologic and clinical features or whether it is merely a presentation of two different diseases (PBC and AIH). Other names for this entity include “the hepatic form of PBC,” “secondary autoimmune hepatitis,” “autoimmune cholangitis,” “autoimmune sclerosing cholangitis,” or “combined hepatic/cholestatic syndrome” to describe patients with features of both AIH and PBC or PSC.24, 25 The overlapping features of AIH and PBC may be a unique autoimmune defect resulting in damage to bile ducts and hepatocytes.26
A variety of scoring classifications have been used to recognize and diagnose OS. A commonly used classification is based on the IAIHG scoring system for AIH diagnosis; this was introduced in 199312 and revised in 1999.13 The original IAIHG scoring system was the first tool designed to exclude cholestatic liver disease in patients with AIH6, 27; this criterion has been validated and is widely used in clinical practice for the diagnosis of AIH.14, 27, 28 The revised IAIHG classification isolated 19% of patients with overlapping AIH‐PBC compared to the original IAIHG criteria that isolated 62% of patients in a cohort of patients with PBC.14 The simplified diagnostic scoring system formulated in 2008 was a highly specific classification for diagnosing AIH with a specificity of 97% and 99% for scores of ≥6 and ≥7, respectively.29 Although this classification was proposed to isolate patients with AIH, applying this to a group of patients with PBC diagnosed 6% PBC‐AIH overlap compared to 12% using the revised IAIHG criteria, suggesting superior specificity.22 Chazouilleres et al.4 in 1998 suggested a Paris criteria classification that incorporated characteristics of both AIH and PBC to diagnose OS. A subsequent study validated the results showing 92% sensitivity and 94% specificity. However, application of this criterion to a large cohort of patients with PBC showed only 1% of patients satisfied the Paris criteria for OS when applied to a group of patients with PBC. Moreover, some patients could not be isolated as AIH‐PBC overlap when they presented with predominant features of AIH along with some cholestatic properties, such as AMAs, serum ALP levels <2‐fold ULN, and isolated features of bile duct injury or destruction.6, 9, 20, 30 The simplified AIH classification was seen to be less sensitive in recognizing OS but more specific in identifying patients who have the clinically more aggressive OS.22 The detection of a less severe form of OS is important as the treatment of OS differs from pure AIH or PBC. Patients who are diagnosed early may benefit from intervention to avoid severe consequences.15
With this background, a new overlap classification was proposed on the basis of the revised IAIHG classification by changing some of its variables. This overlap scoring system incorporated all the important aspects of biochemical, immunologic, and histologic presentation in patients with OS, and the net strength of the diagnosis of OS could be estimated by the quantitative scoring of the composite parameters. The validity of this scoring system was supported by the fact that 98.5% of patients with OS who obtained a score of ≥21 (achieving a sensitivity and specificity of 98.5% and 92.8%, respectively) were classified as “definite overlap.” A score of ≥19‐20 had 1.5% of patients with OS classified as “probable overlap,” and a score of ≤19, classified as “rejected overlap,” had no overlap patients, achieving a 100% NPV for our subset of overlap patients. These results suggest that this new overlap scoring classification appears to be valid for the set of patients selected for the study. It successfully separated the group of OS patients from patients with AIH or PBC. This classification could identify patients and diagnose OS with a reasonable amount of accuracy and could be used as an adjunct to the current clinical methods to correctly diagnose this entity.
We also used the Paris criteria to diagnose OS in our patients, and we compared the sensitivity, specificity, PPV, and NPV of the two diagnostic criteria. With the Paris criteria, we were able to detect only 38 cases in 65 patients with OS, with a sensitivity of 58.46% but a high specificity of 99.52%. When compared to the Paris criteria, our new scoring system has better sensitivity (98.46%) but less specificity (92.75%). This is consistent with the findings of previous studies that the Paris criteria may miss less severe cases of OS. By using the new diagnostic criteria, a milder form of OS can be detected and treated early to avoid progression of OS.
In conclusion, our new scoring classification has a high sensitivity and specificity for the diagnosis of OS when the cut‐off score is 21; a score <19 rules out OS. This scoring system provides a useful mechanism by which OS could be diagnosed from a group of patients with AIH or PBC in a consistent fashion. Due to the selection of patients who strictly had AIH‐PBC OS, it is not possible to determine if this classification would favor other types of OS as well. Our study enrolled a limited number of patients from a single center, and more studies are necessary to further evaluate the validity of this scoring system in a prospective manner. A future study will incorporate a larger number of patients from different centers.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1148/full.
Supporting Information Tables
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005;353:1261‐1273. [DOI] [PubMed] [Google Scholar]
- 2. Krawitt EL. Autoimmune hepatitis. N Engl J Med 2006;354:54‐66. [DOI] [PubMed] [Google Scholar]
- 3. Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E; International Autoimmune Hepatitis Group . Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol 2011;54:374‐385. [DOI] [PubMed] [Google Scholar]
- 4. Chazouilleres O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis‐autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology 1998;28:296‐301. [DOI] [PubMed] [Google Scholar]
- 5. Kuiper EM, Zondervan PE, van Buuren HR. Paris criteria are effective in diagnosis of primary biliary cirrhosis and autoimmune hepatitis overlap syndrome. Clin Gastroenterol Hepatol 2010;8:530‐534. [DOI] [PubMed] [Google Scholar]
- 6. Czaja AJ. The overlap syndromes of autoimmune hepatitis. Dig Dis Sci 2013;58:326‐343. [DOI] [PubMed] [Google Scholar]
- 7. Czaja AJ. Diagnosis and management of the overlap syndromes of autoimmune hepatitis. Can J Gastroenterol 2013;27:417‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joshi S, Cauch‐Dudek K, Wanless IR, Lindor KD, Jorgensen R, Batts K, et al. Primary biliary cirrhosis with additional features of autoimmune hepatitis: response to therapy with ursodeoxycholic acid. Hepatology 2002;35:409‐413. [DOI] [PubMed] [Google Scholar]
- 9. Bonder A, Retana A, Winston DM, Leung J, Kaplan MM. Prevalence of primary biliary cirrhosis‐autoimmune hepatitis overlap syndrome. Clin Gastroenterol Hepatol 2011;9:609‐612. [DOI] [PubMed] [Google Scholar]
- 10. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145‐172. [DOI] [PubMed] [Google Scholar]
- 11. Christensen E, Crowe J, Doniach D, Popper H, Ranek L, Rodes J, et al. Clinical pattern and course of disease in primary biliary cirrhosis based on an analysis of 236 patients. Gastroenterology 1980;78:236‐246. [PubMed] [Google Scholar]
- 12. Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology 1993;18:998‐1005. [DOI] [PubMed] [Google Scholar]
- 13. Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999;31:929‐938. [DOI] [PubMed] [Google Scholar]
- 14. Talwalkar JA, Keach JC, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: an evaluation of a modified scoring system. Am J Gastroenterol 2002;97:1191‐1197. [DOI] [PubMed] [Google Scholar]
- 15. Papamichalis PA, Zachou K, Koukoulis GK, Veloni A, Karacosta EG, Kypri L, et al. The revised international autoimmune hepatitis score in chronic liver diseases including autoimmune hepatitis/overlap syndromes and autoimmune hepatitis with concurrent other liver disorders. J Autoimmune Dis 2007;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silveira MG, Talwalkar JA, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: long‐term outcomes. Am J Gastroenterol 2007;102:1244‐1250. [DOI] [PubMed] [Google Scholar]
- 17. Kloppel G, Seifert G, Lindner H, Dammermann R, Sack HJ, Berg PA. Histopathological features in mixed types of chronic aggressive hepatitis and primary biliary cirrhosis. Correlations of liver histology with mitochondrial antibodies of different specificity. Virchows Arch A Pathol Anat Histol 1977;373:143‐160. [DOI] [PubMed] [Google Scholar]
- 18. Poupon R, Chazouilleres O, Corpechot C, Chretien Y. Development of autoimmune hepatitis in patients with typical primary biliary cirrhosis. Hepatology 2006;44:85‐90. [DOI] [PubMed] [Google Scholar]
- 19. Tanaka A, Harada K, Ebinuma H, Komori A, Yokokawa J, Yoshizawa K, et al. Primary biliary cirrhosis ‐ autoimmune hepatitis overlap syndrome: a rationale for corticosteroids use based on a nation‐wide retrospective study in Japan. Hepatol Res 2011;41:877‐886. [DOI] [PubMed] [Google Scholar]
- 20. Czaja AJ. Frequency and nature of the variant syndromes of autoimmune liver disease. Hepatology 1998;28:360‐365. [DOI] [PubMed] [Google Scholar]
- 21. Gheorghe L, Iacob S, Gheorghe C, Iacob R, Simionov I, Vadan R, et al. Frequency and predictive factors for overlap syndrome between autoimmune hepatitis and primary cholestatic liver disease. Eur J Gastroenterol Hepatol 2004;16:585‐592. [DOI] [PubMed] [Google Scholar]
- 22. Neuhauser M, Bjornsson E, Treeprasertsuk S, Enders F, Silveira M, Talwalkar J, et al. Autoimmune hepatitis‐PBC overlap syndrome: a simplified scoring system may assist in the diagnosis. Am J Gastroenterol 2010;105:345‐353. [DOI] [PubMed] [Google Scholar]
- 23. Czaja AJ. Diagnosis and management of autoimmune hepatitis: current status and future directions. Gut Liver 2016;10:177‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muratori L, Cassani F, Pappas G, Guidi M, Mele L, Lorenza V, et al. The hepatitic/cholestatic “overlap” syndrome: an Italian experience. Autoimmunity 2002;35:565‐568. [DOI] [PubMed] [Google Scholar]
- 25. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol 2015;63:971‐1004. [DOI] [PubMed] [Google Scholar]
- 26. Terracciano LM, Patzina RA, Lehmann FS, Tornillo L, Cathomas G, Mhawech P, et al. A spectrum of histopathologic findings in autoimmune liver disease. Am J Clin Pathol 2000;114:705‐711. [DOI] [PubMed] [Google Scholar]
- 27. Czaja A, Carpenter HA. Validation of scoring system for diagnosis of autoimmune hepatitis. Dig Dis Sci 1996;41:305‐314. [DOI] [PubMed] [Google Scholar]
- 28. Toda G, Zeniya M, Watanabe F, Imawari M, Kiyosawa K, Nishioka M, et al. Present status of autoimmune hepatitis in Japan‐‐correlating the characteristics with international criteria in an area with a high rate of HCV infection. Japanese National Study Group of Autoimmune Hepatitis. J Hepatol 1997;26:1207‐1212. [DOI] [PubMed] [Google Scholar]
- 29. Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL, et al.; International Autoimmune Hepatitis Group . Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169‐176. [DOI] [PubMed] [Google Scholar]
- 30. Czaja AJ. Cholestatic phenotypes of autoimmune hepatitis. Clin Gastroenterol Hepatol 2014;12:1430‐1438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1148/full.
Supporting Information Tables


