Abstract
Liver progenitor cells (LPCs)/ductular reactions (DRs) are associated with inflammation and implicated in the pathogenesis of chronic liver diseases. However, how inflammation regulates LPCs/DRs remains largely unknown. Identification of inflammatory processes that involve LPC activation and expansion represent a key step in understanding the pathogenesis of liver diseases. In the current study, we found that diverse types of chronic liver diseases are associated with elevation of infiltrated interleukin (IL)‐17‐positive (+) cells and cytokeratin 19 (CK19)+ LPCs, and both cell types colocalized and their numbers positively correlated with each other. The role of IL‐17 in the induction of LPCs was examined in a mouse model fed a choline‐deficient and ethionine‐supplemented (CDE) diet. Feeding of wild‐type mice with the CDE diet markedly elevated CK19+Ki67+ proliferating LPCs and hepatic inflammation. Disruption of the IL‐17 gene or IL‐27 receptor, alpha subunit (WSX‐1) gene abolished CDE diet‐induced LPC expansion and inflammation. In vitro treatment with IL‐17 promoted proliferation of bipotential murine oval liver cells (a liver progenitor cell line) and markedly up‐regulated IL‐27 expression in macrophages. Treatment with IL‐27 favored the differentiation of bipotential murine oval liver cells and freshly isolated LPCs into hepatocytes. Conclusion: The current data provide evidence for a collaborative role between IL‐17 and IL‐27 in promoting LPC expansion and differentiation, respectively, thereby contributing to liver regeneration. (Hepatology Communications 2018;2:329‐343)
Abbreviations
- Alb
albumin
- BMOL
bipotential murine oval liver
- CDE
choline‐deficient and ethionine‐supplemented
- CK19
cytokeratin 19
- DR
ductular reaction
- HNF
hepatocyte nuclear factor
- IL
interleukin
- LPC
liver progenitor cell
- Mcp1
monocyte chemoattractant protein 1
- MELD
Model for End‐Stage Liver Disease
- mRNA
messenger RNA
- TAT
tyrosine aminotransferase
- Th
T helper
- TNF
tumor necrosis factor
- WSX‐1
interleukin‐27 receptor, alpha subunit
- WT
wild‐type
Introduction
After liver injury, normally quiescent hepatocytes are capable of self‐renewal by entering the cell cycle until restoring the liver parenchyma and initial functions. However, when the liver is subjected to severe or chronic injury, hepatocyte‐driven liver regeneration is altered or insufficient, and an alternative regenerative process involving the liver progenitor cell (LPC) compartment is then engaged.1 In virtually all human liver diseases, LPC proliferation is frequently observed within proliferative ductular cells and is referred to as ductular reaction (DR), with an important histologic and mechanistic heterogeneity.2, 3 DR is defined as the proliferation of apparent ductules that accompany leukocyte infiltration in response to liver injury.4 In humans, the expansion of biliary‐like cells or LPCs is associated with severity of chronic liver disease, regardless of the etiology.5, 6, 7 While LPCs are reported as key cells promoting liver regeneration, in certain circumstances their presence is also correlated with progressive fibrogenesis8, 9 and could contribute to hepatocellular carcinoma initiation.10 Therefore, determination of the mechanisms leading to LPC activation and controlling their expansion represent a key step in understanding liver pathogenesis development and may help to propose novel therapeutic strategies.
The origin of LPCs is still subject to debate. However, most recent publications converge toward the likelihood of LPC emergence from a stem/progenitor cell niche located in the portal region around the canals of Hering. LPCs can differentiate toward functional hepatocytes and mature cholangiocytes in vitro. Numerous murine lineage‐tracing models have suggested that LPCs do not contribute to hepatocyte regeneration in several experimental models of liver injury, including 2/3 partial hepatectomy, bile duct ligation, carbon tetrachloride intoxication, and a 3,5‐diethoxycarbonyl‐1,4‐dihydrocollidine diet. However, after severe hepatocyte loss, biliary‐like or liver progenitor cells can differentiate toward functional hepatocytes and mature cholangiocytes in vivo in zebrafish and in mouse models.11, 12, 13 Furthermore, in another murine model using a choline‐deficient and ethionine‐supplemented diet (CDE), Español‐Suñer et al.14 and Rodrigo‐Torres et al.15 found that LPCs contribute to hepatic regeneration with up to 2% of newly generated hepatocytes arising from LPCs. It has recently been demonstrated that differentiated cells from such progenitors yield functional hepatocytes characterized by hepatocyte‐specific marker expressions, such as hepatocyte nuclear factor (HNF)4α.16 A contribution of LPCs to the restoration of the parenchymal architecture and liver function has been assumed in humans, and a recent study reported long‐term expansion of LPCs from human liver and their conversion into functional hepatocytes in vitro and with transplantation in vivo.17
Activation of the LPC compartment is a complex process that is not fully understood. The LPC response can be divided into four steps: activation, proliferation, migration, and differentiation.18 The induction and progression of the LPC‐driven regenerative process is highly influenced by the microenvironment and the cytokines released by immune cells during inflammation.19 For instance, a recent study reported the association between portal inflammation and DR in nonalcoholic fatty liver disease.20 Both innate and adaptive immune cells recruited during the inflammatory process are critical for the modulation of LPC‐driven liver regeneration, as demonstrated by numerous studies using the CDE model. Van Hul et al.21 have reported that macrophage depletion by clodronate injections attenuates fibrogenesis and LPC parenchymal invasion. Furthermore, it has been shown that in mice lacking T cells, the LPC response was drastically weakened and mice succumbed to acute liver failure.22 The LPC compartment is also highly activated during T‐cell‐mediated hepatitis induced by concanavalin A.23 Numerous cytokines constitute key links between inflammation and LPC proliferation, such as tumor necrosis factor (TNF)‐α, TNF‐like weak inducer of apoptosis (TWEAK), interferon‐gamma (IFN‐γ), interleukin‐6 (IL‐6), IL‐22, and lymphotoxin β.24, 25, 26, 27, 28
Among the key players in modulating liver inflammation, T helper (Th)17 lymphocytes have been implicated in several types of liver diseases through the effects of IL‐17A (IL‐17) and IL‐22.29, 30 While IL‐22 has been reported as hepatoprotective,31 antifibrotic,32 and promoting liver regeneration from LPCs,28 the potential role of IL‐17, notably in regeneration, has not been fully investigated. IL‐17 is a proinflammatory cytokine known to contribute to the crosstalk between innate and adaptive immunity. Recently, we and others reported direct and indirect profibrogenic and proinflammatory effects of IL‐17 by stimulating both myofibroblasts and macrophages.33, 34 Furthermore, it has been shown that IL‐17‐producing gamma delta T (γδT) cells were recruited during hepatocyte‐driven liver regeneration induced by partial hepatectomy.35 The authors showed that IL‐17‐induced IL‐6 production by macrophages and dendritic cells, favored hepatocyte proliferation, and could also be involved in LPC‐driven liver regeneration. To achieve hepatic regeneration from LPCs, those cells need to proliferate but also to undergo cell differentiation into mature cells. Interestingly, several groups demonstrated that IL‐27, a cytokine mainly produced by macrophages, has been shown to directly favor stem/progenitor cell differentiation in different organs.36, 37, 38, 39 IL‐27 is a pleiotropic cytokine belonging to the IL‐12 family that signals through its heterodimeric receptor composed of gp130 and IL‐27 receptor alpha (WSX‐1) subunits, mainly expressed by immune and epithelial cells, including hepatocytes.40 We therefore hypothesized that communication between adaptive and innate immune cells through IL‐17 and IL‐27 production, respectively, could contribute to the achievement of liver regeneration from LPCs.
In biopsies obtained from patients with various types of liver diseases, IL‐17+ cells were identified in close association with liver ductular cells, and their infiltration positively correlated with the degree of DR in our study. To address the role of IL‐17 and IL‐27 in LPC activation, proliferation, and differentiation into mature hepatocytes, IL‐17‐deficient (IL‐17−/−) and WSX‐1‐deficient (WSX‐1−/−) mice were fed a CDE diet, and murine liver progenitor cells were used. Our results showed complementary roles of IL‐17 and IL‐27 in achieving the regenerative process of the liver by inducing LPC proliferation and by favoring differentiation, respectively.
Materials and Methods
HUMAN SAMPLES
Forty‐three liver samples from patients with diverse chronic liver diseases were analyzed. The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki. As required by French legislation, the study was approved by the local ethics committee Ile de France I (Institutional Review Board 2017‐A01215‐48). Blocks of formalin‐fixed paraffin‐embedded samples from explanted livers were obtained from the Department of Pathology of Henri Mondor University Hospital (Creteil, France). For the assessment of cytokeratin 19 (CK19)+ and IL‐17+ cell density, a training set of 20 slides was first reviewed by two evaluators, including a pathologist specialized in liver diseases. The whole cohort was then analyzed independently by the two evaluators using a semiquantitative score, and patients were dichotomized into high versus low density of stained cells for each labeling.
ANIMALS
We used 6‐8‐week‐old male mice on a C57BL/6 background in this study. IL‐17−/− mice were generously provided by Professor Yoichiro Iwakura (Japan). WSX‐1−/− mice were purchased from the Jackson Laboratory. Mice were fed a control (choline‐sufficient) or choline‐deficient diet (MP Biomedicals, Illkirch, France), and drinking water was supplemented with DL‐ethionine (0.15% weight/volume) (Sigma‐Aldrich, Lyon, France) in the CDE‐fed group. Animals were killed at indicated time points, blood was collected for serum extraction, and the liver was either fixed in buffered formalin or snap frozen in liquid nitrogen. Experiments were performed on at least four animals per group and per time point. All animals were housed and fed ad libitum in a pathogen‐free animal facility and used in accordance with protocols approved by the French ethical committee (COMETH, Authorization N°12‐079) and under the supervision of authorized investigators.
STATISTICAL ANALYSIS
Results are expressed as mean ± SEM, and statistical significance was determined by a two‐tailed Student t test or one‐ or two‐way analysis of variance as appropriate, using PRISM 4.0 software. Data were considered significantly different for P < 0.05. Contingency between CK19 and IL‐17 staining and Model for End‐Stage Liver Disease (MELD) scores were assessed by Fisher's exact test and Child‐Pugh scores by the chi‐square test.
Results
DR CORRELATES WITH IL‐17‐PRODUCING CELL INFILTRATION IN HUMAN DISEASED LIVERS
DR with LPCs is frequently observed in several types of liver diseases and is often associated with the inflammatory process.3, 20 In addition, IL‐17+ cells were found in the livers of patients with chronic liver diseases.29, 30 To determine whether the proliferative LPCs correlate with the number of infiltrating IL‐17‐producing cells, a cohort of 43 patients with chronic liver diseases from various etiologies was analyzed. Most of the patients presented with mild inflammatory activity (86.1%, METAVIR score A1‐A2) with severe fibrosis (93.0% METAVIR F4) (Supporting Table S1). On paraffin‐embedded liver tissues from these patients, we revealed an infiltration of IL‐17‐producing cells surrounding CK19+ LPCs with a close interaction, regardless of the etiology (Fig. 1). A score was defined semiquantitatively, and patients were classified into two groups with a low and high degree of DR (Fig. 2A). Similarly, IL‐17+ cells infiltrating the livers were scored and classified into two groups with a low and high degree of IL‐17‐expressing cells. A positive correlation was found between the densities of these two cell types (P < 0.001) (Fig. 2B), with a majority of CK19high patients having a high density of infiltrating IL‐17+ cells in the liver. In addition, patients with low CK19 and IL‐17 scores were predominantly Child‐Pugh class A with a MELD score ≤15, whereas most patients who were CK19high IL‐17high were Child‐Pugh class B or C with a MELD score >15 (Fig. 2C,D; Supporting Table S1). Collectively, these data showed that increased IL‐17+ cell infiltration is associated with CK19+ LPC accumulation in human DR and with a less optimistic prognosis. These clinical observations led us to hypothesize that IL‐17 could promote LPC accumulation in diseased livers.
Figure 1.
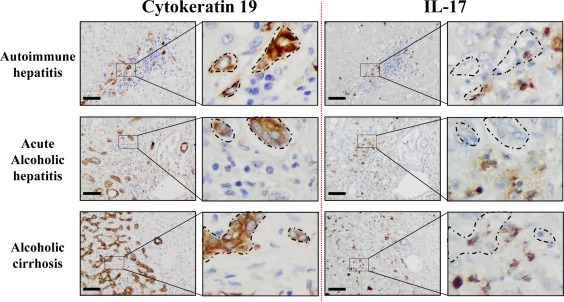
IL‐17‐expressing cells and CK19+ cells are localized in similar areas in diseased livers. Representative CK19 and IL‐17 (brown color) immunostaining on human serial liver sections from diverse etiologies with an enlargement magnification field. Areas where CK19+ cells accumulate are delimited with dotted lines on serial sections to highlight their proximity with IL‐17+‐infiltrating cells. Scale bar, 100 µm.
Figure 2.
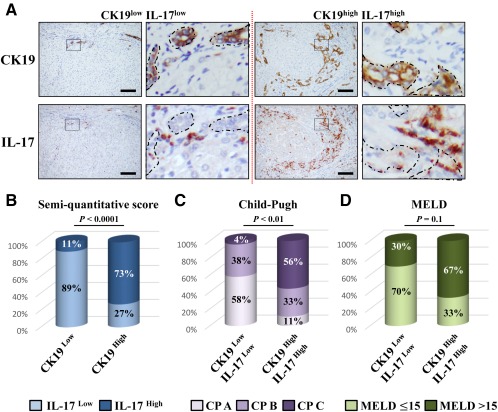
Ductular reaction correlates with IL‐17‐producing cell infiltration in human diseased livers. (A) Representative liver sections from patients with CK19low IL‐17low (left) and CK19high IL‐17high (right) immunolabeling. Scale bar, 100 µm. (B) Relative CK19 and IL‐17 quantification was realized. (C) Child‐Pugh score class A (5 to 6), B (7 to 9), and C (10 to 15) in CK19low IL‐17low and CK19high IL‐17high patients. (D) Percentage of patients with a MELD score ≤15 or >15 in both groups. Abbreviation: CP, Child‐Pugh.
DISRUPTION OF THE IL‐17 GENE IMPAIRS LPC ACTIVATION IN REGENERATING LIVER
To determine the role of IL‐17 in LPC‐compartment activation in regenerating livers, wild‐type (WT) and IL‐17−/− mice were subjected to the CDE diet for 3, 10, or 21 days. In this model, expression of several inflammatory genes inducing Th17 cell differentiation (IL‐6, transforming growth factor β) and specific markers, including IL‐23 receptor (IL‐23R) and retinoic acid receptor‐related orphan receptor alpha (ROR‐α) but not RORγt, were up‐regulated as early as 3 days and were maintained along with the diet in WT animals (Supporting Fig. S1). LPC accumulation was evaluated on liver tissue sections of both WT and IL‐17−/− CDE‐fed mice with CK19 immunostaining (Fig. 3A). WT mice showed a significant increase in CK19+ cells from day 3, with a progressive accumulation along with the CDE diet. In contrast, IL‐17 deficiency was sufficient to significantly prevent LPC accumulation as early as 3 days after the CDE diet (Fig. 3A). This result has been confirmed in another model of DR in a cholestatic environment induced by bile duct ligation and section (Supporting Fig. S2). Along the same line of evidence, messenger RNA (mRNA) expression of LPC response‐associated markers (alpha‐fetoprotein [AFP], M2‐pyruvate kinase), with the exception of the hematopoietic Thy‐1 cell surface antigen (Thy1) marker, were induced in the liver from WT mice in the CDE model, reaching a peak at day 3; such inductions were markedly reduced in IL‐17−/− mice (Fig. 3B). Double‐stained CK19+Ki67+ cells revealed proliferative LPCs reaching 15% of the total CK19+ counted cells in WT mice, whereas no proliferating LPCs were detected in IL‐17−/− mice after 21 days of the CDE diet (Fig. 3C,D). This reduced‐LPC activation in IL‐17−/− mice was not attributable to a difference in liver injury as serum transaminases (alanine aminotransferase, aspartate aminotransferase) and alkaline phosphatase activities were similarly increased in WT and IL‐17−/− animals at earlier time points (Fig. 3E). No difference was observed on histologic analysis on hematoxylin and eosin‐stained liver tissue sections (Supporting Fig. S3). In addition, food intake was assessed in both groups and did not show any difference (Supporting Fig. S4). Finally, despite a slight increase in fibrogenesis‐related gene expressions in livers of WT CDE‐fed mice, no obvious increase in sirius red staining was observed in those mice (Supporting Fig. S5A‐C). Taken together, these data showed that IL‐17 deficiency is associated with reduced LPC accumulation.
Figure 3.
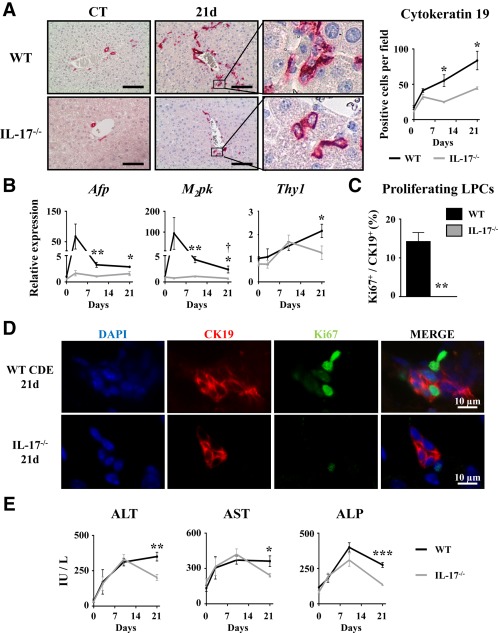
Disruption of the IL‐17 gene impairs liver progenitor cell activation in regenerating liver. Wild‐type and IL‐17−/− mice were fed a CDE diet and killed at the indicated time points. (A) Liver tissue sections were stained for CK19, and positive cell number quantification was realized. (B) Hepatic mRNA expression of LPC‐associated genes Afp, M2pk, and Thy1 was analyzed by qRT‐PCR and expressed as fold change over control diet‐fed WT mice. (C,D) Liver tissue sections were immunolabeled with antibodies directed against CK19 (red) and Ki67 (green), and the percentage of proliferating CK19+ cells was determined. (E) Serum ALT, AST, and ALP activities were measured. *P < 0.05, **P < 0.01, ***P < 0.005, WT versus IL‐17−/− mice; each group n = 4‐7 animals. Data represent mean ± SEM. Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, control; d, day; DAPI, 4′,6‐diamidino‐2‐phenylindole; M2pk, type 2 muscle pyruvate kinase; qRT‐PCR, quantitative reverse‐transcription polymerase chain reaction.
IL‐17 DEFICIENCY REPRESSES LIVER INFLAMMATION, INCLUDING IL‐27 PRODUCTION
It is well established that the liver inflammatory response triggered by macrophage recruitment and activation tightly controls LPC expansion. To further determine whether defective LPC accumulation, identified in IL‐17−/− animals, could result from the reduced liver inflammatory response, we evaluated macrophage recruitment and the expression of their secreted inflammatory mediators. While monocyte chemoattractant protein 1 (Mcp1) and F4/80 mRNA expressions were induced with a peak reached at 3 days in WT, such induction was not observed in IL‐17−/− mice (Fig. 4A). Furthermore, F4/80 immunostaining in WT mice showed a 3‐fold increase in macrophage cell numbers infiltrating the livers 3 days after the CDE diet; such infiltration was significantly lower in IL‐17−/− mice (Fig. 4B). Expressions of several macrophage‐associated inflammatory cytokines were also assessed; in WT animals under the CDE diet, the data revealed an up‐regulated hepatic expression of Tnfα, Il6, and of Epstein‐Barr virus‐induced 3 (Ebi3) and Il27p28, two subunits constituting the heterodimeric IL‐27 cytokine (Fig. 4C). In contrast, the expressions of those genes were not up‐regulated in mice lacking IL‐17 expression (Fig. 4C). In addition, treatment of RAW264.7 macrophages with recombinant IL‐17 significantly induced proinflammatory chemokine/cytokine mRNA expressions, including Mcp1, Tnfα, Il6, and Ebi3 and Il27p28 (Fig. 4D). Altogether, these data showed a key role of IL‐17 in triggering the well‐described hepatic inflammatory response necessary for LPC activation (e.g., Mcp1, Tnfα, Il6) and revealed an induced expression of IL‐27 with a putative role in LPC‐driven liver regeneration.
Figure 4.
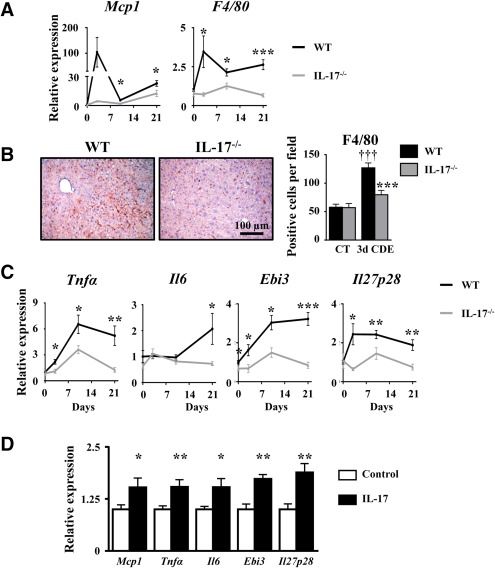
IL‐17 deficiency represses liver inflammation, including IL‐27 production. (A) mRNA expression of MCP1 and F4/80 were quantified by qRT‐PCR. (B) Liver macrophage infiltration was analyzed by F4/80 immunostaining and counting. (C) TNF‐α, IL‐6, and IL‐27 (Ebi3 and IL‐27p28 subunits) mRNA expressions were quantified by qRT‐PCR in WT and IL‐17−/− mice after 3, 10, or 21 days of the CDE diet. *P < 0.05, **P < 0.01, ***P < 0.005, WT versus IL‐17−/− mice. (D) Inflammatory gene expressions were quantified by qRT‐PCR in the IL‐17‐treated RAW264.7 macrophage cell line. Data represent mean ± SEM. Abbreviations: CT, control; d, day; qRT‐PCR, quantitative reverse‐transcription polymerase chain reaction.
WSX‐1 DEFICIENCY REPRESSES LPC‐DRIVEN LIVER REGENERATION
IL‐27 is a cytokine with a well‐known modulatory function in progenitor cell‐mediated tissue repair in other organs.41, 42 To address the potential role of the IL‐27–WSX‐1 axis in LPC accumulation, WT and WSX‐1−/− mice were subjected to CDE‐diet feeding. CK19 immunostaining on liver tissue sections showed a strong inhibition of LPC accumulation in WSX‐1−/− animals when compared with their WT counterpart from day 3 after the CDE diet (Fig. 5A). In agreement with these CK19 immunostaining data, mRNA expressions of LPC activation‐related genes (Afp, Thy1) were not induced in WSX‐1−/− mice (Fig. 5B). Interestingly, proliferating CK19+ cells identified by CK19 and Ki67 double staining revealed that 12% of CK19+ LPCs were proliferating 21 days after the CDE diet in WT mice, while only 2.5% of proliferating CK19+ cells were counted in WSX‐1−/− mice (Fig. 5C,D). This strongly suggests an essential involvement of the IL‐27–WSX‐1 axis in the LPC activation process.
Figure 5.
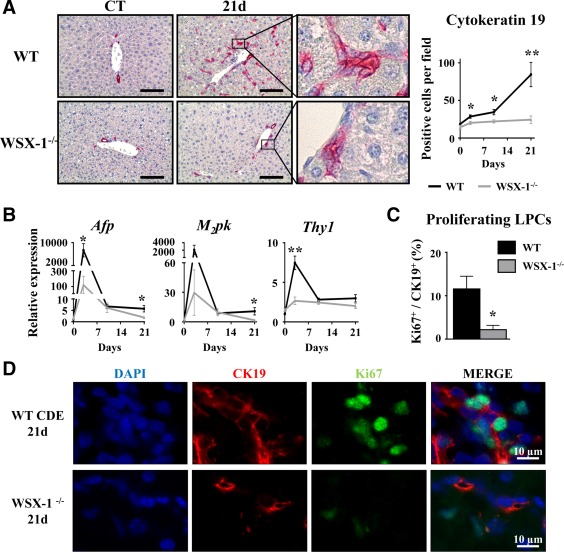
WSX‐1‐deficiency represses LPC‐driven liver regeneration. Wild‐type and WSX‐1−/− mice were fed a CDE diet, and samples were collected at the indicated time points. (A) CK19+ cells were stained and counted. Scale bar, 100 µm. (B) Hepatic mRNA expressions of LPC‐associated genes were measured by qRT‐PCR. (C,D) CK19 (red) and Ki67 (green) staining and counting after 21 days of the CDE diet. *P < 0.05, **P < 0.01, WT versus WSX‐1−/− mice; each group n = 4‐7 animals. Data represent mean ± SEM. Abbreviations: CT, control; d, day; DAPI, 4′,6‐diamidino‐2‐phenylindole; M2pk, type 2 muscle pyruvate kinase; qRT‐PCR, quantitative reverse‐transcription polymerase chain reaction.
CDE DIET‐INDUCED LIVER INFLAMMATION IS REDUCED IN WSX‐1−/− MICE
A disrupted IL‐27 signaling pathway is associated with reduced LPC accumulation after a CDE diet. To better characterize the mechanisms that could explain this observation, we evaluated liver injury and inflammation. Serum transaminases and hematoxylin and eosin staining on liver tissue sections showed similar injury in WT and WSX‐1−/− mice (Supporting Figs. S6A and S4B). In addition, food intake was assessed in both types of mice and did not show any difference (Supporting Fig. S6C). Liver macrophages infiltrating the liver after the CDE diet were quantified in WT and WSX‐1−/− mice. Mcp1 and F4/80 mRNA expressions were significantly induced and peaked 3 days after the CDE diet in WT animals but not in WSX‐1−/− mice (Fig. 6A). In addition, F4/80 immunostaining of liver samples confirmed a 3‐fold increase in the number of macrophages infiltrating the livers of CDE‐treated WT mice, but such increase was completely abolished in livers of CDE‐treated WSX‐1−/− animals (Fig. 6B). Analysis of inflammatory cytokines revealed an increase in mRNA expression of Tnfα, Il6, Ebi3, and Il27p28 in WT mice but not in WSX‐1−/− animals. Moreover, IL‐27‐treated RAW cells showed increased Il6, Tnfα, and Ebi3 but not Il27p28 mRNA expressions (Fig. 6D). Lastly, CDE‐induced Th1, Th2, and Th17 marker gene expressions were significantly lowered in WSX‐1−/− compared to WT mice (Supporting Fig. S7). This strongly suggests that the IL‐27–WSX‐1 axis is required to promote the inflammatory response required for supporting the LPC compartment.
Figure 6.
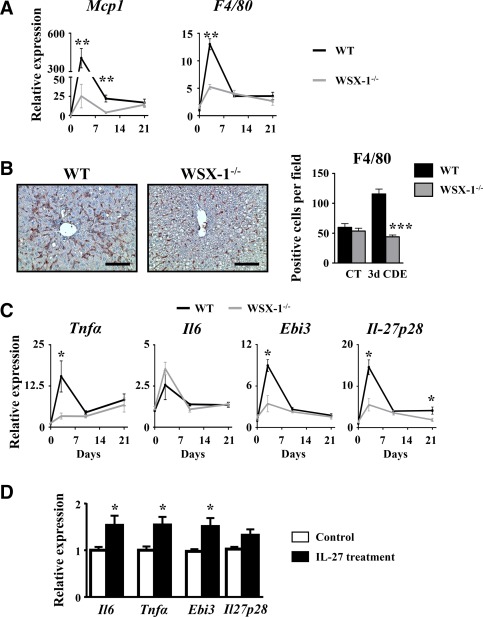
CDE diet‐induced liver inflammation is reduced in WSX‐1−/−. Wild‐type and WSX‐1−/− mice were subjected to the CDE model. (A) Hepatic mRNA expression of macrophage‐related genes was assessed by qRT‐PCR. (B) Immunostaining of F4/80 was performed on WT and WSX‐1−/− mice, and positive cells were counted after 3 days of the CDE diet. Scale bar, 100 µm. (C) Hepatic mRNA expressions of inflammation‐related genes were quantified by qRT‐PCR; each group n = 4‐7 animals. *P < 0.05, **P < 0.01, ***P < 0.005, WT versus WSX‐1−/− mice. (D) RAW264.7 cells were cultured in the presence of 50 ng/mL IL‐27, and mRNA expressions of IL‐6, TNF‐α, and IL‐27 subunits were analyzed by qRT‐PCR. *P < 0.05, control versus IL‐27‐treated cells. Data represent mean ± SEM. Abbreviations: CT, control; d, day; qRT‐PCR, quantitative reverse‐transcription polymerase chain reaction.
IL‐17 FAVORS LPC PROLIFERATION WHEREAS IL‐27 INDUCES EXPRESSION OF HEPATOCYTE DIFFERENTIATION MARKERS
As LPC accumulation was strongly altered in both IL‐17−/− and IL‐27−/− animals under the CDE diet, we further deciphered the direct role of IL‐17 and IL‐27 on LPCs in vitro. Bipotential murine oval liver (BMOL) cells, a well characterized LPC cell line, were cultured in the absence or presence of either IL‐27 or IL‐17. Cell viability and proliferation were evaluated by the 3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium assay and showed that IL‐17 potentiated BMOL cell growth along with a 5‐day culture while IL‐27 had no effect (Fig. 7A). To evaluate the potent role of IL‐17 and IL‐27 in LPC differentiation, hepatocytic cell differentiation marker mRNA expressions, including albumin (Alb), tyrosine aminotransferase (Tat), and Hnf4α were also assessed (Fig. 7B). Our data revealed that IL‐17 did not increase but instead decreased some of those markers, including Alb and Tat expressions. In contrast, BMOL treatment with IL‐27 increased Alb and Hnf4α mRNA expressions (Fig. 7B). Similarly, hepatocytic cell markers analyzed at protein levels by western blot showed an increased expression of Alb, TAT, and HNF4α in the presence of IL‐27 (Fig. 7C,D). IL‐27 did not potentiate the effects of the hepatocyte‐differentiation culture medium (Supporting Fig. S8). However, IL‐17 treatment in similar conditions reduced hepatocytic differentiating markers consistently with results obtained in normal culture conditions (Fig. 7B). To confirm the putative role of IL‐27 in differentiating LPCs into hepatocytes, freshly isolated LPCs from mouse livers after a 21‐day CDE diet were cultured in the absence or presence of IL‐27 or IL‐17. The purity of freshly isolated LPCs was assessed by flow cytometry and showed 94.8% epithelial cell adhesion molecule (EpCAM)+ CD45– isolated cells (Supporting Fig. S9). IL‐27 treatment strongly induced Alb, Tat, and Hnf4α mRNA expressions when compared to nontreated controls. IL‐17 treatment did not induce any of those hepatocytic markers but diminished Hnf4α mRNA expression (Fig. 7E). Similarly, HNF4α immunostaining on freshly isolated LPCs showed a weak basal level in control cells (Fig. 7F) while IL‐27 treatment significantly induced HNF4α expression. In contrast, IL‐17 treatment completely abolished HNF4α expression. Compared to BMOL cells that have been immortalized, freshly isolated LPCs do not show significant proliferation in vitro in our basal culture conditions. Therefore, no significant effect of IL‐17 or IL‐27 treatment was obtained on proliferation assays (data not shown). Taken together, these data demonstrate a direct role of IL‐17 in mediating LPC accumulation while IL‐27 plays a complementary role by favoring LPC differentiation toward a hepatocytic phenotype.
Figure 7.
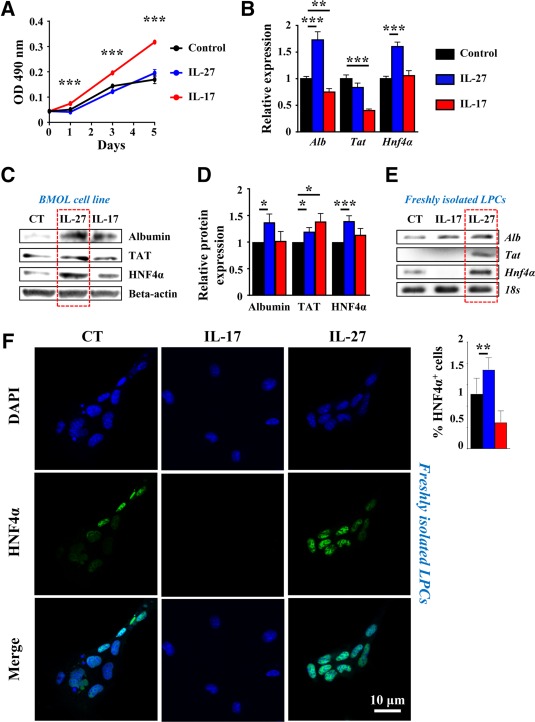
IL‐17 favors LPC proliferation whereas IL‐27 induces their differentiation. BMOL cells were treated with 50 ng/mL recombinant IL‐27 or IL‐17, and (A) proliferation was assessed by optical density using an MTS assay. Hepatocyte differentiation marker expression was analyzed by (B) qRT‐PCR after 6 hours or (C) by western blot after 24 hours of treatment. (D) Western blot quantification (n = 6‐8 independent experiments). Results are expressed as fold change over untreated cells. *P < 0.05, **P < 0.01, ***P < 0.005. (D,E) Primary LPCs were cultured in the presence of 50 ng/mL recombinant IL‐17 or IL‐27 for 24 hours. Hepatocyte differentiation marker expressions were analyzed by (E) qRT‐PCR and (F) immunocytochemistry using an anti‐HNF4α antibody. HNF4α+ cells were counted in each condition. Data represent mean ± SEM. Abbreviations: CT, control; DAPI, 4′,6‐diamidino‐2‐phenylindole; MTS, 3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium; OD, optical density; qRT‐PCR, quantitative reverse‐transcription polymerase chain reaction.
Discussion
A large spectrum of growth factors and cytokines has been reported to contribute to LPC niche activation, and some of these have been described as dispensable due to redundant functions. In the present study, we report a correlation between IL‐17‐producing cell recruitment and the severity of the DR, and we identify IL‐17 as a cytokine with a central role in triggering LPC compartment activation and proliferation. We also reveal that IL‐17 is responsible for macrophage‐induced IL‐27 expression that favors LPC differentiation into hepatocytes. We therefore highlight collaborative work between IL‐17 and IL‐27 that is required to properly achieve liver regeneration from progenitor cells (Fig. 8).
Figure 8.
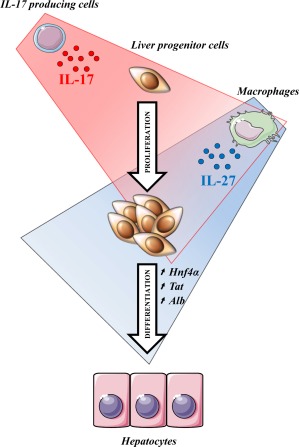
IL‐17 induces liver progenitor cell proliferation while IL‐27 favors their differentiation toward a hepatocytic phenotype. Taken together, these data provide evidence of a collaborative role of IL‐17 and IL‐27 in promoting liver regeneration. IL‐17 directly acts on LPCs to favor their proliferation. IL‐17 also induces macrophage IL‐27 production, which enhances LPC differentiation toward hepatocytes.
ASSOCIATION OF DR WITH INFLAMMATORY CELLS
In virtually all types of chronic liver diseases, biliary and liver progenitor cell accumulation, referred to as DR, is frequently observed.3 In humans, LPC accumulation is an important prognostic marker in advanced liver diseases and is often associated with a less optimistic outcome.43 In our study, comparison of CK19low with CK19high groups of patients revealed a higher Child‐Pugh and MELD score severity associated with increased LPC expansion. DR is accompanied by recruitment of immune cells nearby, and compelling findings in both animal and human studies emphasize the pivotal role of inflammatory cytokines in the LPC‐driven regenerative process.19, 20, 23, 24, 25
IL‐17 IN LPC ACTIVATION AND PROLIFERATION
Based on the clinical study of chronic liver diseases, regardless of etiology, we report a positive correlation between the degree of DR and the number of IL‐17‐producing cells infiltrating the liver. The proximity between IL‐17+ cells and LPCs led us to hypothesize that IL‐17 could be associated with LPC activation and proliferation. Moreover, increased IL‐17+ cells infiltrating the liver of CK19high patients aggravate their Child‐Pugh and MELD scores (Supporting Table S1). Mixed phenotypes CK19high IL‐17low and CK19low IL‐17high were found in few patients. The source of IL‐17‐producing cells reported in human livers is heterogeneous and mainly includes Th17 lymphocytes, γδ T cells, and neutrophils.20, 29, 30, 33 In mouse, we cannot exclude a different profiling of IL‐17‐producing cell types, depending on the experimental model, when compared to human liver diseases. However, in the murine model of CDE‐diet‐induced liver regeneration from LPCs, we revealed an induced expression of Th17‐associated genes as early as 3 days after a CDE diet in WT animals. Such induced expressions were maintained along with the protocol (Supporting Fig. S1), although IL‐17 levels in the serum or liver were not detectable by using currently available kits (data not shown). In keeping with results obtained in a previous work,22 our data strongly support the participation of Th17 cells in the hepatic production of IL‐17. Interestingly, we showed that in mice lacking IL‐17 expression and subjected to a CDE diet, the capacity of LPCs to accumulate is dramatically altered when compared to WT animals. Along the same lines of evidence, LPC treatment with IL‐17 promoted LPC expansion in vitro. Furthermore, we previously reported a role of IL‐17 in polarizing macrophages toward a proinflammatory M1 phenotype.34 In this study, we show that IL‐17 deficiency causes impairment of macrophage cell recruitment in CDE‐diet‐induced liver regeneration, resulting in reduced hepatic inflammation. This reduced inflammatory response may explain the reduced liver injury observed at later time points in IL‐17−/− animals. These results are consistent with previous reports showing that macrophage depletion by clodronate injections abrogates LPC accumulation and subsequent liver regeneration during a CDE diet.21 The results obtained in vivo clearly provide evidence that IL‐17 deficiency alters LPC expansion, which fits with in vitro data. However, neither IL‐17 deficiency (Fig. 3A) nor macrophage depletion44 were sufficient to completely abolish DR. This suggests that IL‐17 could contribute to LPC expansion by i) directly promoting LPC proliferation and ii) indirectly through M1‐macrophage‐induced production of required factors, e.g., TNF‐α and IL‐6, which support LPC accumulation.19, 45 IL‐17 has been detected in several types of chronic liver diseases29, 30 and was associated with increased liver injury and fibrosis33, 34 and hepatocellular carcinoma.46, 47 Our data highlight that a sustained IL‐17 inflammatory response lacking a differentiating process may be responsible for incomplete hepatic regeneration, with uncontrolled accumulation of progenitor cells susceptible to undergo genetic and epigenetic alterations and to initiate carcinogenic processes.
LPC‐MEDIATED DIFFERENTIATION THROUGH THE IL‐27–WSX‐1 AXIS
In addition to its function in triggering LPC activation in regenerating livers, we demonstrated that IL‐17 also induced IL‐27 cytokine production by macrophages. IL‐27 is also described as an IFN‐γ‐like cytokine that favors hematopoietic and neural precursor differentiation through signal transducer and activator of transcription 141, 42; this is in addition to its proinflammatory48 or anti‐inflammatory49 role according to the pathogenesis. Numerous studies revealed antitumor properties of IL‐27 through the complex regulation of immune response, and this cytokine has also been reported to exert antiproliferative and anti‐angiogenic effects by directly acting on cancer cells.50 IL‐27 has also been shown to directly favor cardiac progenitor cell differentiation,37 expansion and differentiation of hematopoietic stem cells,38, 39 and could support retinal progenitor cell differentiation.36 In this model, we showed that disruption of IL‐27 receptor signaling also prevented LPC accumulation. Alternatively, we showed a direct role of IL‐27 on LPCs by favoring their differentiation into a hepatocytic phenotype in vitro without a direct mitogenic effect. Additional experiments, such as lineage tracing in several models, including CDE, or hepatocyte‐specific MDM2 proto‐oncogene (Mdm2)‐deficient mice, are required to conclude on the role of IL‐27 effects on LPC differentiation in vivo.11, 12, 13 Reduced LPC accumulation in WSX‐1−/− mice was associated with a significant decrease in recruitment of macrophages, which are reported as crucial actors in supporting LPC expansion.44 We also showed a direct effect of IL‐27 on promoting proinflammatory cytokine gene expressions in macrophages. These data suggest that IL‐27 may indirectly enhance LPC proliferation through favoring macrophage activation and cytokine production.
Taken together, in this present work we provide evidence of a dual role of IL‐17 in regenerating livers from progenitors. IL‐17 not only targets LPCs and stimulates their proliferation but also promotes IL‐27‐induced expression from macrophages, which contribute to LPC differentiation into a hepatocytic phenotype. These data shed light on the fact that both proliferative and differentiating processes of LPCs are essential to achieve liver regeneration. Our data also suggest that lack of a differentiating process may lead to immature LPC accumulation that is susceptible to cell transformation into cancer cells.
Author names in bold designate shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1145/full.
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8
Supplementary Figure 9
Supplementary Table 1
Supplementary math Table 1
Supplementary Material
Acknowledgment
We thank Professor Yoichiro Iwakura (Japan) for providing IL‐17‐deficient mice and Professor George Yeoh (Australia) for providing the BMOL cell line. We are grateful to Gilles Carpentier from the CRRET laboratory at the University of Paris‐Est for his kind help in confocal microscopy analysis.
Potential conflict of interest: Nothing to report.
Supported by the Institut National de la Santé et de la Recherche Médicale, by Université Paris‐Est‐Créteil grants for young investigators (to F.L.), from the Agence Nationale de la Recherche ANR‐09‐RPDOC‐016‐01 HEPATICEL (Retour Postdoctorants 2009‐2013 to F.L.), and the Institut Universitaire de France (Promotion 2016). A.G. was the recipient of a fellowship from the French Ministry of Education and Research (2011‐2014).
REFERENCES
- 1. Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology 2014;146:349‐356. [DOI] [PubMed] [Google Scholar]
- 2. Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch 2011;458:251‐259. [DOI] [PubMed] [Google Scholar]
- 3. Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology 2011;54:1853‐1863. [DOI] [PubMed] [Google Scholar]
- 4. Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis 2003;23:385‐396. [DOI] [PubMed] [Google Scholar]
- 5. Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology 2005;41:809‐818. [DOI] [PubMed] [Google Scholar]
- 6. Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander‐Tetri BA, Bhathal PS, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology 2007;133:80‐90. [DOI] [PubMed] [Google Scholar]
- 7. Sancho‐Bru P, Altamirano J, Rodrigo‐Torres D, Coll M, Millan C, Jose Lozano J, et al. Liver progenitor cell markers correlate with liver damage and predict short‐term mortality in patients with alcoholic hepatitis. Hepatology 2012;55:1931‐1941. [DOI] [PubMed] [Google Scholar]
- 8. Ruddell RG, Knight B, Tirnitz‐Parker JE, Akhurst B, Summerville L, Subramaniam VN, et al. Lymphotoxin‐beta receptor signaling regulates hepatic stellate cell function and wound healing in a murine model of chronic liver injury. Hepatology 2009;49:227‐239. [DOI] [PubMed] [Google Scholar]
- 9. Van Hul NK, Abarca‐Quinones J, Sempoux C, Horsmans Y, Leclercq IA. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE‐induced murine model of chronic liver injury. Hepatology 2009;49:1625‐1635. [DOI] [PubMed] [Google Scholar]
- 10. Wu K, Ding J, Chen C, Sun W, Ning BF, Wen W, et al. Hepatic transforming growth factor beta gives rise to tumor‐initiating cells and promotes liver cancer development. Hepatology 2012;56:2255‐2267. [DOI] [PubMed] [Google Scholar]
- 11. Choi TY, Ninov N, Stainier DY, Shin D. Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology 2014;146:776‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He J, Lu H, Zou Q, Luo L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology 2014;146:789‐800.e788. [DOI] [PubMed] [Google Scholar]
- 13. Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol 2015;17:971‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Español‐Suñer R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 2012;143:1564‐1575.e1567. [DOI] [PubMed] [Google Scholar]
- 15. Rodrigo‐Torres D, Affo S, Coll M, Morales‐Ibanez O, Millan C, Blaya D, et al. The biliary epithelium gives rise to liver progenitor cells. Hepatology 2014;60:1367‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, et al. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology 2009;49:920‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, et al. Long‐term culture of genome‐stable bipotent stem cells from adult human liver. Cell 2015;160:299‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erker L, Grompe M. Signaling networks in hepatic oval cell activation. Stem Cell Res 2007;1:90‐102. [DOI] [PubMed] [Google Scholar]
- 19. Knight B, Matthews VB, Akhurst B, Croager EJ, Klinken E, Abraham LJ, et al. Liver inflammation and cytokine production, but not acute phase protein synthesis, accompany the adult liver progenitor (oval) cell response to chronic liver injury. Immunol Cell Biol 2005;83:364‐374. [DOI] [PubMed] [Google Scholar]
- 20. Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 2014;59:1393‐1405. [DOI] [PubMed] [Google Scholar]
- 21. Van Hul N, Lanthier N, Espanol Suner R, Abarca Quinones J, van Rooijen N, Leclercq I. Kupffer cells influence parenchymal invasion and phenotypic orientation, but not the proliferation, of liver progenitor cells in a murine model of liver injury. Am J Pathol 2011;179:1839‐1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strick‐Marchand H, Masse GX, Weiss MC, Di Santo JP. Lymphocytes support oval cell‐dependent liver regeneration. J Immunol 2008;181:2764‐2771. [DOI] [PubMed] [Google Scholar]
- 23. Hines IN, Kremer M, Isayama F, Perry AW, Milton RJ, Black AL, et al. Impaired liver regeneration and increased oval cell numbers following T cell‐mediated hepatitis. Hepatology 2007;46:229‐241. [DOI] [PubMed] [Google Scholar]
- 24. Knight B, Lim R, Yeoh GC, Olynyk JK. Interferon‐gamma exacerbates liver damage, the hepatic progenitor cell response and fibrosis in a mouse model of chronic liver injury. J Hepatol 2007;47:826‐833. [DOI] [PubMed] [Google Scholar]
- 25. Nguyen LN, Furuya MH, Wolfraim LA, Nguyen AP, Holdren MS, Campbell JS, et al. Transforming growth factor‐beta differentially regulates oval cell and hepatocyte proliferation. Hepatology 2007;45:31‐41. [DOI] [PubMed] [Google Scholar]
- 26. Tirnitz‐Parker JE, Viebahn CS, Jakubowski A, Klopcic BR, Olynyk JK, Yeoh GC, et al. Tumor necrosis factor‐like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology 2010;52:291‐302. [DOI] [PubMed] [Google Scholar]
- 27. Subrata LS, Voon DC, Yeoh GC, Ulgiati D, Quail EA, Abraham LJ. TNF‐inducible expression of lymphotoxin‐beta in hepatic cells: an essential role for NF‐kappaB and Ets1 transcription factors. Cytokine 2012;60:498‐504. [DOI] [PubMed] [Google Scholar]
- 28. Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, et al. Interleukin‐22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology 2012;143:188‐198.e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hammerich L, Bangen JM, Govaere O, Zimmermann HW, Gassler N, Huss S, et al. Chemokine receptor CCR6‐dependent accumulation of gammadelta T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology 2014;59:630‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lafdil F, Miller AM, Ki SH, Gao B. Th17 cells and their associated cytokines in liver diseases. Cell Mol Immunol 2010;7:250‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL‐22) plays a protective role in T cell‐mediated murine hepatitis: IL‐22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 2004;39:1332‐1342. [DOI] [PubMed] [Google Scholar]
- 32. Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, et al. Interleukin‐22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012;56:1150‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin‐17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 2012;143:765‐776.e763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guillot A, Hamdaoui N, Bizy A, Zoltani K, Souktani R, Zafrani ES, et al. Cannabinoid receptor 2 counteracts interleukin‐17‐induced immune and fibrogenic responses in mouse liver. Hepatology 2014;59:296‐306. [DOI] [PubMed] [Google Scholar]
- 35. Rao R, Graffeo CS, Gulati R, Jamal M, Narayan S, Zambirinis CP, et al. Interleukin 17‐producing gammadeltaT cells promote hepatic regeneration in mice. Gastroenterology 2014;147:473‐484.e472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu Y, Balasubramaniam B, Copland DA, Liu J, Armitage MJ, Dick AD. Activated adult microglia influence retinal progenitor cell proliferation and differentiation toward recoverin‐expressing neuron‐like cells in a co‐culture model. Graefes Arch Clin Exp Ophthalmol 2015;253:1085‐1096. [DOI] [PubMed] [Google Scholar]
- 37. Tanaka T, Obana M, Mohri T, Ebara M, Otani Y, Maeda M, et al. Interleukin‐27 induces the endothelial differentiation in Sca‐1 + cardiac resident stem cells. Cytokine 2015;75:365‐372. [DOI] [PubMed] [Google Scholar]
- 38. Hoyt TR, Dobrinen E, Kochetkova I, Meissner N. B cells modulate systemic responses to Pneumocystis murina lung infection and protect on‐demand hematopoiesis via T cell‐independent innate mechanisms when type I interferon signaling is absent. Infect Immun 2015;83:743‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Furusawa J, Mizoguchi I, Chiba Y, Hisada M, Kobayashi F, Yoshida H, et al. Promotion of expansion and differentiation of hematopoietic stem cells by interleukin‐27 into myeloid progenitors to control infection in emergency myelopoiesis. PLoS Pathog 2016;12:e1005507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bender H, Wiesinger MY, Nordhoff C, Schoenherr C, Haan C, Ludwig S, et al. Interleukin‐27 displays interferon‐gamma‐like functions in human hepatoma cells and hepatocytes. Hepatology 2009;50:585‐591. [DOI] [PubMed] [Google Scholar]
- 41. Seita J, Asakawa M, Ooehara J, Takayanagi S, Morita Y, Watanabe N, et al. Interleukin‐27 directly induces differentiation in hematopoietic stem cells. Blood 2008;111:1903‐1912. [DOI] [PubMed] [Google Scholar]
- 42. Koch M, May U, Kuhns S, Drechsler H, Adam N, Hattermann K, et al. Interleukin 27 induces differentiation of neural C6‐precursor cells into astrocytes. Biochem Biophys Res Commun 2007;364:483‐487. [DOI] [PubMed] [Google Scholar]
- 43. Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol 1999;154:537‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elsegood CL, Chan CW, Degli‐Esposti MA, Wikstrom ME, Domenichini A, Lazarus K, et al. Kupffer cell‐monocyte communication is essential for initiating murine liver progenitor cell‐mediated liver regeneration. Hepatology 2015;62:1272‐1284. [DOI] [PubMed] [Google Scholar]
- 45. Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, et al. TWEAK induces liver progenitor cell proliferation. J Clin Invest 2005;115:2330‐2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, et al. High expression of IL‐17 and IL‐17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res 2013;32:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lukacs‐Kornek V, Lammert F. The progenitor cell dilemma: cellular and functional heterogeneity in assistance or escalation of liver injury. J Hepatol 2017;66:619‐630. [DOI] [PubMed] [Google Scholar]
- 48. Siebler J, Wirtz S, Frenzel C, Schuchmann M, Lohse AW, Galle PR, et al. Cutting edge: a key pathogenic role of IL‐27 in T cell‐ mediated hepatitis. J Immunol 2008;180:30‐33. [DOI] [PubMed] [Google Scholar]
- 49. Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, et al. Interleukin‐27 priming of T cells controls IL‐17 production in trans via induction of the ligand PD‐L1. Immunity 2012;36:1017‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hasegawa H, Mizoguchi I, Chiba Y, Ohashi M, Xu M, Yoshimoto T. Expanding diversity in molecular structures and functions of the IL‐6/IL‐12 heterodimeric cytokine family. Front Immunol 2016;7:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1145/full.
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8
Supplementary Figure 9
Supplementary Table 1
Supplementary math Table 1
Supplementary Material


