Abstract
Purpose
The purpose of this study was to identify which swallowing task(s) yielded the worst performance during a standardized modified barium swallow study (MBSS) in order to optimize the detection of swallowing impairment.
Method
This secondary data analysis of adult MBSSs estimated the probability of each swallowing task yielding the derived Modified Barium Swallow Impairment Profile (MBSImP™©; Martin-Harris et al., 2008) Overall Impression (OI; worst) scores using generalized estimating equations. The range of probabilities across swallowing tasks was calculated to discern which swallowing task(s) yielded the worst performance.
Results
Large-volume, thin-liquid swallowing tasks had the highest probabilities of yielding the OI scores for oral containment and airway protection. The cookie swallowing task was most likely to yield OI scores for oral clearance. Several swallowing tasks had nearly equal probabilities (≤ .20) of yielding the OI score.
Conclusions
The MBSS must represent impairment while requiring boluses that challenge the swallowing system. No single swallowing task had a sufficiently high probability to yield the identification of the worst score for each physiological component. Omission of swallowing tasks will likely fail to capture the most severe impairment for physiological components critical for safe and efficient swallowing. Results provide further support for standardized, well-tested protocols during MBSS.
A modified barium swallow study (MBSS) is a commonly used instrumental examination for the assessment of oropharyngeal dysphagia that uses videofluoroscopic imaging to detail the nature and severity of swallowing impairment and to identify physiological targets of swallowing treatments (Dodds, 1989; Logemann, 1997, 1998; Martin-Harris & Jones, 2008; Martin-Harris, Logemann, McMahon, Schleicher, & Sandidge, 2000). MBSSs typically include administration of a variety of swallowing tasks known to influence swallowing physiology and airway protection. An MBSS swallowing task typically has three main characteristics: (a) a specific bolus consistency, (b) volume, and (c) presentation method. Each characteristic of the swallowing task is independent and the effect of manipulating these characteristics must be understood to optimize diagnosis and aid in treatment planning.
Because standardized health care practices, including diagnostic procedures and interventions, have been shown to optimize patient safety and outcomes, attempts have been made to standardize the MBSS to facilitate transparency in data acquisition and reproducibility of diagnostic information across clinics and laboratories (Agency for Healthcare Research and Quality, 2003; Ciucci, Jones, Malandraki, & Hutcheson, 2016; Clave & Shaker, 2015). Doing so has allowed for the consistent identification of physiological impairment(s) and the development of treatment plans known to effect positive change in swallowing physiology (Jaffer, Ng, Au, & Steele, 2015; Logemann, 1987; Martin-Harris et al., 2008; Palmer, Kuhlemeier, Tippett, & Lynch, 1993).
The MBSS is a relatively short procedure, averaging approximately 15 min, and every attempt should be made to minimize radiation exposure (e.g., ≤ 3 min) while maximizing clinical yield (Bonilha, Humphries, et al., 2013; Crawley, Savage, & Oakley, 2004; Morishima, Chida, & Watanabe, 2016). Within this context, the primary goal of the MBSS is to identify swallowing impairment and to assess physiological adaptation and compensation strategies that will improve swallowing. As such, the study must include boluses that facilitate these goals.
The primary objective of an MBSS is to actively identify the nature and type of swallowing impairment that places the patient at risk for airway invasion. The achievement of this goal requires visual interpretation of swallowing physiology across varied bolus consistencies and swallowing tasks that sufficiently challenge the swallowing mechanism. The identification of impairment requires offline, frame-by-frame, and slow motion analysis of swallows across all swallowing tasks. The process is time intensive, and attempts to improve efficiency would be welcomed by practicing clinicians. The purpose of this research project was to identify whether a particular swallowing task or tasks would yield the worst performance when using a standardized MBSS method that is intended to optimize the detection of swallowing impairment. We aimed to achieve this purpose by exploring the following research questions: (a) Which swallowing task(s) were most likely to yield the worst performance for each physiological component? (b) Did all swallowing tasks have equal probabilities of yielding the worst performance for each physiological component? As such, we selected a tested protocol and method, the Modified Barium Swallow Impairment Profile (MBSImP™©; Martin-Harris et al., 2008), that incorporates extreme scoring as one approach for capturing physiological swallowing impairment. The answers to these research questions could potentially provide clinicians with a strategy to focus their attention during MBSS assessment in order to optimize the identification of physiological swallowing impairment.
Methods
Participants
This study represents a secondary data analysis of a prospective collection of 345 adult MBSSs that were consecutively conducted in response to physician referral for dysphagia assessment at the Medical University of South Carolina in Charleston, South Carolina, and Saint Joseph's Hospital in Atlanta, Georgia, from 2005 through 2008. Twenty-two MBSSs were removed from this data set because the majority of scores were missing from the records. Twenty-five additional MBSSs were excluded because they represented repeat MBSSs. The institutional review boards at the Medical University of South Carolina and St. Joseph's Hospital approved this study.
Procedures
Speech-language pathologists trained in the MBSImP scoring methodology who met reliability criteria on the physiological components (scoring ≥ 80% accuracy on reliability testing) completed scoring for 11 swallowing tasks (defined below) included in the standardized protocol (Martin-Harris et al., 2008). The 17 physiological components, representing oral, pharyngeal, and esophageal domains of swallowing, were scored on a rank order scale from no impairment to maximum impairment. The scale for components varies from low (0) to high (2, 3, or 4) for each swallowing task because initial testing showed that clinicians could not visually discriminate the same levels of behavioral variations for each component with acceptable reliability (Martin-Harris et al., 2008). The 11 swallowing tasks included administration of varying bolus consistencies, bolus volumes, and presentation methods of standardized ready-to-use barium contrast (VARIBAR® barium sulfate 40% weight/volume; Bracco Diagnostics, Inc., Monroe Township, NJ) in sagittal and anterior/posterior (A/P) viewing planes. The scored swallowing tasks included (a) thin barium (one 5-ml amount via teaspoon with clinician cue for oral hold, one cup sip with clinician cue for oral hold, and one presentation of self-administered sequential swallows from cup), (b) nectar barium (one trial of 5 ml via teaspoon with clinician cue for oral hold, one cup sip with clinician cue for oral hold, and one presentation of self-administered sequential swallows from cup), (c) thin-honey barium (one trial of 5 ml via teaspoon with clinician cue for oral hold), (d) pudding barium (one trial of 5 ml via teaspoon), (e) a solid (one-half portion of a Lorna Doone shortbread cookie coated with 3 ml of pudding barium), (f) nectar barium (one trial of 5 ml via teaspoon with clinician cue for oral hold presented in A/P view), and (g) pudding (one trial of 5 ml via teaspoon presented in A/P view; Martin-Harris, 2015; Martin-Harris et al., 2008; Northern Speech Services, Inc., 2016).
According to the MBSImP protocol, physiological components (see Table 1) are scored only for the swallowing tasks that yield assessment of that particular component (see Table 2; Martin-Harris et al., 2008). Only five swallowing tasks are scored for the physiological component of tongue control during bolus hold (Component 2). This physiological component tests whether or not the patient can maintain a liquid bolus in the oral cavity by sealing the margins of the oral tongue to the hard and soft palate, a requisite skill for successful achievement of airway protection strategies used in swallowing treatment (Clark, Henson, Barber, Stierwalt, & Sherrill, 2003). Holding a bolus is not applicable to several swallowing tasks, including sequential swallowing of liquids or swallowing of a cohesive bolus such as pudding or masticated cookie. In the same way, only one swallowing task, the cookie swallowing task, is scored for bolus preparation/mastication (Component 3). The physiological components of pharyngeal contraction (Component 13), derived by observations of combined pharyngeal shortening, compression, and stripping (Jones, Kramer, & Donner, 1985), and esophageal clearance in the upright position (Component 17) are scored only for swallowing tasks presented and visualized in the A/P view (5 ml nectar and 5 ml pudding). No other physiological components are scored for the A/P view swallowing tasks.
Table 1.
Modified Barium Swallow Impairment Profile (MBSImP) physiological components.
| Number | Physiological component |
|---|---|
| 1 | Lip closure |
| 2 | Tongue control during bolus hold |
| 3 | Bolus preparation/mastication |
| 4 | Bolus transport/lingual motion |
| 5 | Oral residue |
| 6 | Initiation of pharyngeal swallow |
| 7 | Soft palate elevation |
| 8 | Laryngeal elevation |
| 9 | Anterior hyoid excursion |
| 10 | Epiglottic movement |
| 11 | Laryngeal vestibular closure |
| 12 | Pharyngeal stripping wave |
| 13 | Pharyngeal contraction (A/P view) |
| 14 | Pharyngoesophageal segment opening |
| 15 | Tongue base retraction |
| 16 | Pharyngeal residue |
| 17 | Esophageal clearance upright position (A/P view) |
Note. A/P = anterior/posterior.
Table 2.
Modified Barium Swallow Impairment Profile (MBSImP) administration and scoring protocol.
| Swallowing task |
Physiological components scored | ||
|---|---|---|---|
| Barium consistency | Bolus volume | Presentation method | |
| Thin | 5 ml | Via teaspoon with clinician cue for oral hold (lateral view) | none |
| 5 ml | Via teaspoon with clinician cue for oral hold (lateral view) | 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16 | |
| Sip | From cup with clinician cue for oral hold (lateral view) | 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16 | |
| Sequential swallows | Self-administered from cup (lateral view) | 1, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16 | |
| Nectar | 5 ml | Via teaspoon with clinician cue for oral hold (lateral view) | 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16 |
| Sip | From cup with clinician cue for oral hold (lateral view) | 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16 | |
| Sequential swallows | Self-administered from cup (lateral view) | 1, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16 | |
| Thin-Honey | 5 ml | Via teaspoon with clinician cue for oral hold (lateral view) | 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16 |
| Pudding | 5 ml | Via teaspoon (lateral view) | 1, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16 |
| Solid | ½ portion of Lorna Doone shortbread cookie | Coated with 3 ml of pudding barium (lateral view) | 3 |
| Nectar | 5 ml | Via teaspoon with clinician cue for oral hold (A/P view) | 13, 17 |
| Pudding | 5 ml | Via teaspoon (A/P view) | 13, 17 |
Note. A/P = anterior/posterior.
Data Collection and Analysis
Two methods can be used for MBSImP scoring: swallow-by-swallow (SbS) and overall impression (OI) score, which represents the worst or extreme impairment. SbS scoring assigns an MBSImP score for all applicable physiological components for each swallowing task given during the protocol, which results in a total of 127 possible SbS scores for a single MBSS study. The OI scoring method represents the worst performance (highest score on an ordinal scale) across all the swallowing tasks and is used to capture impairment for each physiological component, resulting in 17 possible OI scores for a single MBSS study.
Statistical Analysis
For each of the 17 physiological components, the OI score was defined as a patient's SbSmax score (worst performance) across all swallowing tasks given. However, because the physiological component of bolus preparation/mastication (Component 3) uses only a single swallowing task, the corresponding OI score and the swallowing task score are equivalent. A patient who was presented with the full MBSImP protocol had a total of 17 OI scores derived from the SbSmax scores upon completion of the MBSS. Patients did not have 17 OI scores if (a) they were not given all swallowing tasks owing to safety concerns, (b) the MBSS image was unable to be assessed for technical reasons, or (c) there were incomplete records. The swallowing task data are provided at the level of the swallow. One data point per swallowing task per patient was collected for each component, with each data point either equal or not equal to the OI. An OI score of 0 indicates optimal performance on all swallowing tasks for a specific physiological component. To facilitate identification of swallowing tasks giving rise to physiological component OI scores, we constructed binary indicator variables equal to 1 for swallowing tasks with the maximum nonzero scores, and 0 otherwise. For example, if a patient's SbS scores for the physiological component of lip closure (Component 1) were 0, 1, 2, 0, 0, 2, 0, 0, and 0 for the nine non-A/P tasks (excluding the first thin 5 ml swallowing task), the SbSmax, or derived OI score, is 2 and the corresponding binary variables are 0, 0, 1, 0, 0, 1, 0, 0, and 0, indicating that the third and sixth swallowing tasks yield the OI score. As illustrated, there may be multiple instances of the indicator variable equaling 1 if more than one swallowing task score matched the derived OI score. These are aggregated over the sample to estimate the probabilities. The probabilities calculated are the proportions of 1s from each participant for each swallowing task by each physiological component. We estimated the probability that each swallowing task yielded the OI score using generalized estimating equations with a logit link function and the binary indicator variable as the outcome variable (Ziegler & Vens, 2010). We also calculated the range (maximum minus minimum) of probabilities across swallowing tasks for each physiological component to discern whether one swallowing task had sufficiently higher probability of yielding the worst performance. Probability ranges ≤ .20 were considered to be narrow, indicating nearly equal likelihood of the swallowing tasks yielding the OI score. Separate regression models were fit for each of the physiological components, excluding bolus preparation/mastication (Component 3) for which the OI score always occurs for the cookie swallowing task. Analyses for this project were completed using SAS software version 9.3 (SAS Institute Inc., Cary, NC).
Results
Our final sample included 298 MBSSs, with the majority of the subjects being male (58%) and White (79%). The mean age was 65.6 years (range 18–98 years). This was a heterogeneous representation of patients who were recruited in almost equal numbers between our two sites. The majority of the medical diagnoses relating to the patients' swallowing disorders were pulmonary conditions (23%), head and neck cancer (20%), and neurological disorders (16%). Diagnoses such as traumatic brain injury, vocal fold paresis, diabetes mellitus, lupus, and gastrointestinal concerns were categorized as “other” because of the small numbers (see Table 3). Less than 7% of the total data were missing across all physiological components. Data were considered to be missing from the data set if (a) swallowing tasks were withheld for safety concerns (< 3%), (b) patient positioning limited or obscured MBSS view (< 3%), and (c) the data were unrecoverable secondary to the retrospective design of this study (1%).
Table 3.
Demographics and clinical characteristics of patient population.
| Parameter | n | % |
|---|---|---|
| Diagnosis | ||
| Pulmonary | 68 | 23.1 |
| Head and neck cancer | 58 | 19.7 |
| Neurology | 47 | 15.9 |
| Gastroenterology | 38 | 12.9 |
| Other | 31 | 10.5 |
| Cardiothoracic | 26 | 8.8 |
| Neurosurgery | 10 | 3.4 |
| Oncology (other than head and neck cancer) | 7 | 2.4 |
| Endocrine | 6 | 2.0 |
| Orthopedics | 2 | 0.7 |
| Renal | 1 | 0.3 |
| Vascular | 1 | 0.3 |
| Unknown or Unreported | 3 | |
| Sex | ||
| Male | 173 | 58.2 |
| Female | 124 | 41.8 |
| Unreported | 1 | |
| Race | ||
| White | 231 | 79.1 |
| Black or African American | 59 | 20.2 |
| Asian | 2 | 0.7 |
| Unknown or Unreported | 6 | |
| Hospital | ||
| Medical University of South Carolina | 170 | 57.0 |
| Saint Joseph's Hospital | 128 | 43.0 |
| Age in years, mean (range) | 65.6 (18–98) | |
Swallowing Tasks Most Likely to Yield the Worst Performance
The swallowing task most likely to yield the worst performance across each of the physiological components was the thin-liquid, sequential swallowing task. This swallowing task had the highest probability of yielding the OI (ranging from .22 to .63) for nine physiological components: lip closure (Component 1), initiation of pharyngeal swallow (Component 6), soft palate elevation (Component 7), laryngeal elevation (Component 8), anterior hyoid excursion (Component 9), epiglottic movement (Component 10), laryngeal vestibular closure (Component 11), pharyngeal stripping wave (Component 12), and pharyngoesophageal segment opening (Component 14). The thin-liquid, cup-sip swallowing task had the highest probability of yielding the OI for one of the physiological components, tongue control during bolus hold (Component 2). Together, these large-volume, thin-liquid swallowing tasks had the highest likelihood of yielding the OI score for 10 physiological components (see Table 4), all of which relate to oral containment or airway protection.
Table 4.
Swallowing task probabilities by physiological component.
| Physiological component | Swallowing task |
Probability |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thin |
Nectar |
Semisolids |
A/P view |
Min | Max | Range | |||||||||
| 5 ml | CS | SS | 5 ml | CS | SS | H | P | C | N | P | |||||
| 1 | Lip closure | .11 | .19 | .25 | .12 | .16 | .20 | .14 | .16 | .24 | .11 | .25 | .15 | ||
| 2 | Tongue control during bolus hold | .29 | .38 | .23 | .31 | .26 | .23 | .38 | .15 | ||||||
| 4 | Bolus transport/lingual motion | .15 | .22 | .31 | .14 | .21 | .28 | .30 | .36 | .42 | .14 | .42 | .28 | ||
| 5 | Oral residue | .26 | .46 | .49 | .37 | .45 | .51 | .52 | .61 | .76 | .26 | .76 | .50 | ||
| 6 | Initiation of pharyngeal swallow | .32 | .44 | .59 | .22 | .33 | .46 | .19 | .19 | .33 | .19 | .59 | .40 | ||
| 7 | Soft palate elevation | .03 | .15 | .22 | .07 | .13 | .18 | .09 | .10 | .20 | .03 | .22 | .19 | ||
| 8 | Laryngeal elevation | .17 | .33 | .38 | .20 | .26 | .32 | .21 | .18 | .30 | .17 | .38 | .20 | ||
| 9 | Anterior hyoid excursion | .46 | .49 | .54 | .43 | .47 | .50 | .42 | .44 | .52 | .42 | .54 | .11 | ||
| 10 | Epiglottic movement | .27 | .28 | .34 | .25 | .29 | .30 | .22 | .20 | .28 | .20 | .34 | .14 | ||
| 11 | Laryngeal vestibular closure | .30 | .38 | .46 | .26 | .33 | .39 | .24 | .20 | .29 | .20 | .46 | .26 | ||
| 12 | Pharyngeal stripping wave | .38 | .42 | .47 | .37 | .42 | .44 | .39 | .37 | .43 | .37 | .47 | .11 | ||
| 13 | Pharyngeal contraction (A/P view) | .32 | .31 | .31 | .32 | .01 | |||||||||
| 14 | Pharyngoesophageal segment opening | .47 | .52 | .55 | .45 | .48 | .54 | .40 | .40 | .50 | .40 | .55 | .15 | ||
| 15 | Tongue base retraction | .30 | .48 | .56 | .43 | .54 | .65 | .53 | .48 | .58 | .30 | .65 | .35 | ||
| 16 | Pharyngeal residue | .37 | .59 | .63 | .51 | .57 | .66 | .60 | .55 | .66 | .37 | .66 | .30 | ||
| 17 | Esophageal clearance upright position (A/P view) | .59 | .72 | .59 | .72 | .13 | |||||||||
Note. Bold, italicized text indicates the swallowing task with the highest probability of producing the overall impression score for that physiological component. A/P = anterior/posterior; CS = cup sip; SS = sequential swallows; H = honey; P = pudding; C = cookie; N = nectar.
Two other swallowing tasks that also had high probabilities of yielding the worst performance: the cookie swallowing task (probabilities ranging from .20 to .76) and the nectar-thick, sequential swallowing task (probabilities ranging from .18 to .66). The cookie swallowing task was most likely to yield the OI score for three physiological components, all of which relate to oral clearance: bolus preparation/mastication (Component 3), bolus transport/lingual motion (Component 4), and oral residue (Component 5). The nectar-thick, sequential swallowing task was most likely to yield the worst performance for two physiological components, both which relate to pharyngeal clearance: tongue base retraction (Component 15) and pharyngeal residue (Component 16).
Probability of Swallowing Tasks Yielding Worst Performance for Each Physiological Component
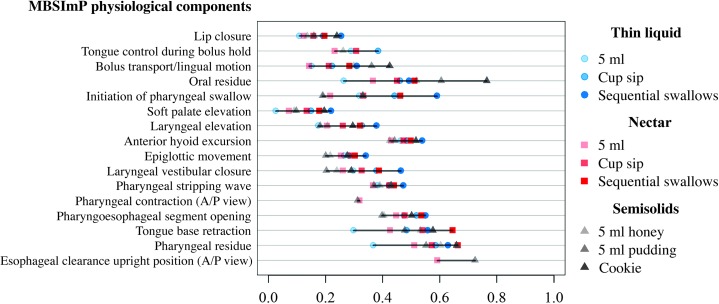
The specific estimated probabilities of each swallowing task yielding the OI score and the range of the estimated probabilities for each physiological component are summarized in Table 4. Figure 1 visually displays the quantitative information provided in Table 4, showing the contribution of each swallowing task to the OI score, along with the difference in probability ranges between swallowing tasks for each physiological component.
Figure 1.
Probability swallowing tasks yield overall impression (OI) score. A/P = anterior/posterior.
A narrow probability range observed between swallowing tasks within a physiological component indicates that all swallowing tasks are nearly equally likely to yield the OI score for that component. Narrow probability ranges (≤ .20) can be seen between swallowing tasks for 10 physiological components (Component number; range): lip closure (1; .15), tongue control during bolus hold (2; .15), soft palate elevation (7; .19), laryngeal elevation (8; .20), anterior hyoid excursion (9; .11), epiglottic movement (10; .14), pharyngeal stripping wave (12; .11), pharyngeal contraction (A/P view; 13; .01), pharyngoesophageal segment opening (14; .15), and esophageal clearance upright position (A/P view; 17; .13).
For physiological components with wider probability ranges (> .20), any swallowing task may yield the OI, but some swallowing tasks are more likely to do so than others. The physiological components with wider probability ranges can be seen for (Component number; range): bolus transport (4; .28), oral residue (5; .50), initiation of pharyngeal swallow (6; .40), laryngeal vestibular closure (11; .26), tongue base retraction (15; .35), and pharyngeal residue (16; .30).
Discussion
It is well established that alterations in swallowing task characteristics such as bolus consistency (Clave et al., 2006; Leder, Judson, Sliwinski, & Madson, 2013; Momosaki, Abo, & Kobayashi, 2013; Newman, Vilardell, Clave, & Speyer, 2016), volume (Gumbley, Huckabee, Doeltgen, Witte, & Moran, 2008; Lawless, Bender, Oman, & Pelletier, 2003; Preiksaitis, Mayrand, Robins, & Diamant, 1992), and presentation methods (Daniels & Foundas, 2001; Daniels, Schroeder, DeGeorge, Corey, & Rosenbek, 2007; Dozier, Brodsky, Michel, Walters, & Martin-Harris, 2006; Murguia, Corey, & Daniels, 2009; Veiga, Fonseca, & Bianchini, 2014) yield changes in swallowing physiology and underpin why clinicians vary these characteristics during an MBSS to detect impairment and identify strategies to improve swallowing physiology. Varied practices in the application of these swallowing tasks and bolus characteristics likely lead to varied results (Ciucci et al., 2016). Our goal was to identify which swallowing task or tasks yielded the worst performance during a standardized MBSS in order to optimize the detection of swallowing impairment. Therefore, we chose the MBSImP OI scoring method toward this goal because the OI represents the most extreme impairment for each physiological component.
Our study showed that large-volume, thin-liquid swallowing tasks were most likely to yield the extreme impairment for the majority of the MBSImP physiological components, specifically those responsible for oral containment and airway protection. Because high scores on any one physiological component may not indicate functional swallowing problems, these swallowing tasks should be considered in the context of other and sometimes multiple physiological impairments that threaten airway protection and bolus clearance. Sequential, thin-liquid boluses have been shown to challenge the swallowing mechanism, as exemplified by adults without dysphagia who have been frequently observed to have the leading edge of the bolus in the distal pharynx with a partially elevated hyolaryngeal complex between swallows (Daniels et al., 2004). Sequential liquid swallows have also been associated with nonoptimal respiratory-swallowing patterning (Martin-Harris et al., 2015) in healthy adults (Lederle, Hoit, & Barkmeier-Kraemer, 2012; Preiksaitis et al., 1992) and hence pose a greater threat for airway invasion in patients with dysphagia. Our results for large-volume, liquid cup-sip tasks further support previous work showing that large-volume, liquid swallowing tasks yield the most extreme impairment for the physiological component of pharyngoesophageal segment opening (Leonard, Kendall, McKenzie, Gonçalves, & Walker, 2000). Excluding high-yield, large-volume swallowing tasks during an MBSS may result in failing to identify the greatest physiological impairment, or any impairment, and thus limit clinicians' diagnostic accuracy and the information available for subsequent treatment planning.
The complex coordination of bolus mastication and oral transport can be difficult for those with dysphagia (Palmer, Rudin, Lara, & Crompton, 1992; Saitoh et al., 2007), consequently leading to the frequent exclusion of solid boluses during the MBSS. However, it has been shown that swallowing tasks that include solid consistencies provide valuable information about the function of the oral mechanism, even in diagnostically challenging patient groups (Rogus-Pulia, Pierce, Mittal, Zecker, & Logemann, 2014). The results of this study revealed that solids should be included during the MBSS because they were most likely to yield information regarding extreme impairment for bolus transport/lingual motion (Component 4) and oral residue (Component 5).
When interpreting the results, it is important to recall that the cookie swallowing task is the only swallowing task scored for the physiological component of bolus preparation/mastication (Component 3), hence it is guaranteed to yield the OI score for this physiological component. In addition, the physiological components scored in the A/P view, pharyngeal constriction (Component 13) and esophageal clearance in upright position (Component 17), have only two swallowing tasks. Therefore, the probability for each of those swallowing tasks to yield the OI score is higher because any nonzero score will result in OI for one or both. In addition, the probability of each swallowing task yielding the OI score becomes less likely if more swallowing tasks are scored for a given physiological component. For the physiological component of tongue control during bolus hold (Component 2), eight swallowing tasks are scored. In comparison, the remaining physiological components have nine swallowing tasks to score. Thus, Figure 1 includes only those physiological components for which the same type and number of swallowing tasks are presented.
Narrow probability ranges that resulted between the swallowing tasks for the majority of the physiological components indicated that no single swallowing task revealed extreme impairment for those components. Wider probability ranges between the swallowing tasks for a given physiological component indicated that there was some separation between swallowing tasks. But because no single swallowing task probability was so conspicuously higher than others for any given physiological component, all swallowing tasks were considered necessary to detect the swallowing impairment. Therefore, unless clinical judgment determines a significant risk, our results do not support the omission of specific swallowing tasks during an MBSS.
Limitations
Although the MBSS provides invaluable information, the diagnostic conclusions drawn are highly dependent on the assessment protocol presented to the patient (Dodds, Logemann, & Stewart, 1990), the type of barium used (Dietsch, Solomon, Steele, & Pelletier, 2014; Steele, Molfenter, Peladeau-Pigeon, & Stokely, 2013; Stokely, Molfenter, & Steele, 2014), the instructions provided to the patient (Daniels et al., 2007; Nagy et al., 2013), and how the videofluoroscopic image is captured (Bonilha, Blair, et al., 2013). The MBSImP protocol was used to test the research questions addressed in this study because it allows for flexibility in administration, use of maneuvers and compensations, and evaluation of airway invasion. Although penetration-aspiration scale scores have been found to be significantly associated with OI scores and supplement physiological assessment, these scores alone are not sufficient for explaining all variation in swallowing performance (Martin-Harris et al., 2000, 2008). In addition, this standardized protocol does not include assessment of saliva swallows or repetition of swallowing tasks (Kendall, 2002), which could affect swallowing physiology. Therefore, the reader is reminded that the results of this study are based on findings from MBSSs conducted using the MBSImP approach and may not be similar when other MBSS administration protocols are used. These are all factors that could affect swallowing physiology. Furthermore, within-patient variability (Kendall, 2002; Molfenter, Leigh, & Steele, 2014; Power, Laasch, Kasthuri, Nicholson, & Hamdy, 2006) for particular swallowing tasks may not be sufficiently captured by our method because the MBSImP protocol eliminates repetition of swallowing tasks to minimize radiation exposure. Another limitation is that our sample was gathered from MBSSs obtained from a heterogeneous patient sample. Therefore, influences of specific patient diagnoses may affect the generalization of our findings. Future directions of this research include investigating how clinician knowledge of patient history, such as medical diagnosis and age, may affect assignment of the OI score and exploring whether swallowing tasks that yield the highest airway invasion measures relate to those yielding the most extreme physiological impairment.
Conclusions
The swallowing tasks that are most likely to yield the OI for the majority of the MBSImP physiological components were large-volume, thin-liquid swallowing tasks, particularly those responsible for oral containment and airway protection; the cookie swallowing task for physiological components of oral clearance; and nectar sequential swallows for two physiological components responsible for pharyngeal clearance. Although these swallowing tasks had the highest probabilities for identifying extreme impairment, they are not sufficient to stand alone as the only swallowing tasks presented during an MBSS because we found that multiple swallowing tasks have almost equal likelihood of yielding the OI score for most of the physiological components. By understanding how each swallowing task contributes to the impairment of each physiological component, clinicians can better evaluate the implications of omitting a particular swallowing task during an MBSS so that diagnostic accuracy and clinical yield are maximized. Standardized protocols should allow flexibility based on clinician intuition and patient characteristics because not all swallowing tasks can be safely tolerated by all patients. Clinical status and patient performance should always be considered as indicators of risk. Assumptions with no empirical basis should not be the reason for truncating MBSS examinations into low-yield tests by excluding swallowing tasks that have been proven to provide critical information necessary for the identification of physiological impairments and targets for swallowing therapy.
Acknowledgments
Grants K23DC005764 (awarded to B. Martin-Harris), 1K24DC12801 (awarded to B. Martin-Harris), and T32DC0014435 (awarded to J. Dubno) from The National Institute on Deafness and Other Communication Disorders and the Global Investigator Initiated Research Grant from Bracco Diagnostics, Inc., (awarded to the MUSC Health Evelyn Trammell Institute for Voice and Swallowing) supported this work. We thank J. Blair and J. Wilmskötter for their assistance with manuscript review. Portions of this work were presented as a scientific paper at the 24th Annual Meeting of the Dysphagia Research Society in Chicago, Illinois (March 2015) and accepted as an oral presentation at the 51st Annual Perry V. Halushka Student Research Day at the Medical University of South Carolina in Charleston, South Carolina (November 2016).
Funding Statement
Grants K23DC005764 (awarded to B. Martin-Harris), 1K24DC12801 (awarded to B. Martin-Harris), and T32DC0014435 (awarded to J. Dubno) from The National Institute on Deafness and Other Communication Disorders and the Global Investigator Initiated Research Grant from Bracco Diagnostics, Inc., (awarded to the MUSC Health Evelyn Trammell Institute for Voice and Swallowing) supported this work.
References
- Agency for Healthcare Research and Quality. (2003). AHRQ annual report on research and management, FY 2003. Retrieved from https://archive.ahrq.gov/about/annrpt03/annrpt03b.htm#Opportunities
- Bonilha H. S., Blair J., Carnes B., Huda W., Humphries K., McGrattan K., … Martin-Harris B. (2013). Preliminary investigation of the effect of pulse rate on judgments of swallowing impairment and treatment recommendations. Dysphagia, 28, 528–538. https://doi.org/10.1007/s00455-013-9463-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha H. S., Humphries K., Blair J., Hill E. G., McGrattan K., Carnes B., … Martin-Harris B. (2013). Radiation exposure time during MBSS: Influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia, 28, 77–85. https://doi.org/10.1007/s00455-012-9415-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci M., Jones C. A., Malandraki G. A., & Hutcheson K. A. (2016). Dysphagia practice in 2035: Beyond fluorography, thickener, and electrical stimulation. Seminars in Speech and Language, 37, 201–218. https://doi.org/10.1055/s-0036-1584155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. M., Henson P. A., Barber W. D., Stierwalt J. A. G., & Sherrill M. (2003). Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. American Journal of Speech-Language Pathology, 1, 40–50. [DOI] [PubMed] [Google Scholar]
- Clave P., de Kraa M., Arreola V., Girvent M., Farre R., Palomera E., & Serra-Prat M. (2006). The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Alimentary Pharmacology & Therapeutics, 24, 1385–1394. https://doi.org/10.1111/j.1365-2036.2006.03118.x [DOI] [PubMed] [Google Scholar]
- Clave P., & Shaker R. (2015). Dysphagia: Current reality and scope of the problem. Nature Reviews Gastroenterology & Hepatology, 12, 259–270. https://doi.org/10.1038/nrgastro.2015.49 [DOI] [PubMed] [Google Scholar]
- Crawley M., Savage P., & Oakley F. (2004). Patient and operator dose during fluoroscopic examination of swallow mechanism. British Journal of Radiology, 77, 654–656. [DOI] [PubMed] [Google Scholar]
- Daniels S. K., Corey D. M., Hadskey L. D., Legendre C., Priestly D. H., Rosenbek J. C., & Foundas A. L. (2004). Mechanism of sequential swallowing during straw drinking in healthy young and older adults. Journal of Speech, Language, and Hearing Research, 47, 33–45. [DOI] [PubMed] [Google Scholar]
- Daniels S. K., & Foundas A. L. (2001). Swallowing physiology of sequential straw drinking. Dysphagia, 16, 176–182. [DOI] [PubMed] [Google Scholar]
- Daniels S. K., Schroeder M. F., DeGeorge P. C., Corey D. M., & Rosenbek J. C. (2007). Effects of verbal cue on bolus flow during swallowing. American Journal of Speech-Language Pathology, 16, 140–147. https://doi.org/10.1044/1058-0360(2007/018) [DOI] [PubMed] [Google Scholar]
- Dietsch A. M., Solomon N. P., Steele C. M., & Pelletier C. A. (2014). The effect of barium on perceptions of taste intensity and palatability. Dysphagia, 29, 96–108. https://doi.org/10.1007/s00455-013-9487-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds W. J. (1989). The physiology of swallowing. Dysphagia, 3, 171–178. [DOI] [PubMed] [Google Scholar]
- Dodds W. J., Logemann J. A., & Stewart E. T. (1990). Radiologic assessment of abnormal oral and pharyngeal phases of swallowing. American Journal of Roentgenology, 154, 965–974. [DOI] [PubMed] [Google Scholar]
- Dozier T. S., Brodsky M. B., Michel Y., Walters B. C. Jr., & Martin-Harris B. (2006). Coordination of swallowing and respiration in normal sequential cup swallows. The Laryngoscope, 116, 1489–1493. https://doi.org/10.1097/01.mlg.0000227724.61801.b4 [DOI] [PubMed] [Google Scholar]
- Gumbley F., Huckabee M. L., Doeltgen S. H., Witte U., & Moran C. (2008). Effects of bolus volume on pharyngeal contact pressure during normal swallowing. Dysphagia, 23, 280–285. https://doi.org/10.1007/s00455-007-9137-9 [DOI] [PubMed] [Google Scholar]
- Jaffer N. M., Ng E., Au F. W., & Steele C. M. (2015). Fluoroscopic evaluation of oropharyngeal dysphagia: Anatomic, technical, and common etiologic factors. American Journal of Roentgenology, 204, 49–58. https://doi.org/10.2214/ajr.13.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Kramer S. S., & Donner M. W. (1985). Dynamic imaging of the pharynx. Gastrointestinal Radiology, 10, 213–224. [DOI] [PubMed] [Google Scholar]
- Kendall K. A. (2002). Oropharyngeal swallowing variability. The Laryngoscope, 112, 547–551. [DOI] [PubMed] [Google Scholar]
- Lawless H. T., Bender S., Oman C., & Pelletier C. (2003). Gender, age, vessel size, cup vs. straw sipping, and sequence effects on sip volume. Dysphagia, 18, 196–202. [DOI] [PubMed] [Google Scholar]
- Leder S. B., Judson B. L., Sliwinski E., & Madson L. (2013). Promoting safe swallowing when puree is swallowed without aspiration but thin liquid is aspirated: Nectar is enough. Dysphagia, 28, 58–62. https://doi.org/10.1007/s00455-012-9412-2 [DOI] [PubMed] [Google Scholar]
- Lederle A., Hoit J. D., & Barkmeier-Kraemer J. M. (2012). Effects of sequential swallowing on drive to breathe in young, healthy adults. Dysphagia, 27, 221–227. [DOI] [PubMed] [Google Scholar]
- Leonard R. J., Kendall K. A., McKenzie S., Gonçalves M. I., & Walker A. (2000). Structural displacements in normal swallowing: A videofluoroscopic study. Dysphagia, 15, 146–152. [DOI] [PubMed] [Google Scholar]
- Logemann J. A. (1987). Criteria for studies of treatment for oral-pharyngeal dysphagia. Dysphagia, 1, 193–199. [Google Scholar]
- Logemann J. A. (1997). Role of the modified barium swallow in management of patients with dysphagia. Otolaryngology–Head & Neck Surgery, 116, 335–338. [DOI] [PubMed] [Google Scholar]
- Logemann J. A. (1998). Evaluation and treatment of swallowing disorders. Austin, TX: ProEd. [Google Scholar]
- Martin-Harris B. (2015). Standardized training in swallowing physiology: Evidence-based assessment using the Modified Barium Swallow Impairment Profile (MBSImP™) approach. Gaylord, MI: Northern Speech Services. [Google Scholar]
- Martin-Harris B., Brodsky M. B., Michel Y., Castell D. O., Schleicher M., Sandidge J., … Blair J. (2008). MBS measurement tool for swallow impairment—MBSImp: Establishing a standard. Dysphagia, 23, 392–405. https://doi.org/10.1007/s00455-008-9185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Harris B., & Jones B. (2008). The videofluorographic swallowing study. Physical Medicine and Rehabilitation Clinics of North America, 19, 769–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Harris B., Logemann J. A., McMahon S., Schleicher M., & Sandidge J. (2000). Clinical utility of the modified barium swallow. Dysphagia, 15, 136–141. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B., McFarland D., Hill E. G., Strange C. B., Focht K. L., Wan Z., … McGrattan K. (2015). Respiratory-swallow training in patients with head and neck cancer. Archives of Physical Medicine and Rehabilitation, 96, 885–893. https://doi.org/10.1016/j.apmr.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter S. M., Leigh C., & Steele C. M. (2014). Event sequence variability in healthy swallowing: Building on previous findings. Dysphagia, 29, 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momosaki R., Abo M., & Kobayashi K. (2013). Swallowing analysis for semisolid food texture in poststroke dysphagic patients. Journal of Stroke and Cerebrovascular Diseases, 22, 267–270. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Morishima Y., Chida K., & Watanabe H. (2016). Estimation of the dose of radiation received by patient and physician during a videofluoroscopic swallowing study. Dysphagia, 31, 574–578. https://doi.org/10.1007/s00455-016-9718-6 [DOI] [PubMed] [Google Scholar]
- Murguia M., Corey D. M., & Daniels S. K. (2009). Comparison of sequential swallowing in patients with acute stroke and healthy adults. Archives of Physical Medicine and Rehabilitation, 90, 1860–1865. https://doi.org/10.1016/j.apmr.2009.05.014 [DOI] [PubMed] [Google Scholar]
- Nagy A., Leigh C., Hori S. F., Molfenter S. M., Shariff T., & Steele C. M. (2013). Timing differences between cued and noncued swallows in healthy young adults. Dysphagia, 28, 428–434. https://doi.org/10.1007/s00455-013-9456-y [DOI] [PubMed] [Google Scholar]
- Newman R., Vilardell N., Clave P., & Speyer R. (2016). Effect of bolus viscosity on the safety and efficacy of swallowing and the kinematics of the swallow response in patients with oropharyngeal dysphagia: White paper by the European Society for Swallowing Disorders (ESSD). Dysphagia, 31, 232–249. https://doi.org/10.1007/s00455-016-9696-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northern Speech Services, Inc. (2016). The MBSImP™ Guide. Retrieved from https://www.mbsimp.com/uploads/MBSImP-Guide.pdf
- Palmer J. B., Kuhlemeier K. V., Tippett D. C., & Lynch C. (1993). A protocol for the videofluorographic swallowing study. Dysphagia, 8, 209–214. [DOI] [PubMed] [Google Scholar]
- Palmer J. B., Rudin N. J., Lara G., & Crompton A. W. (1992). Coordination of mastication and swallowing. Dysphagia, 7, 187–200. [DOI] [PubMed] [Google Scholar]
- Power M., Laasch H.-U., Kasthuri R. S., Nicholson D. A., & Hamdy S. (2006). Videofluoroscopic assessment of dysphagia: A questionnaire survey of protocols, roles and responsibilities of radiology and speech and language therapy personnel. Radiography, 12, 26–30. [Google Scholar]
- Preiksaitis H. G., Mayrand S., Robins K., & Diamant N. E. (1992). Coordination of respiration and swallowing: Effect of bolus volume in normal adults. American Journal of Physiology, 263(3, Pt. 2), R624–R630. [DOI] [PubMed] [Google Scholar]
- Rogus-Pulia N. M., Pierce M. C., Mittal B. B., Zecker S. G., & Logemann J. A. (2014). Changes in swallowing physiology and patient perception of swallowing function following chemoradiation for head and neck cancer. Dysphagia, 29, 223–233. https://doi.org/10.1007/s00455-013-9500-y [DOI] [PubMed] [Google Scholar]
- Saitoh E., Shibata S., Matsuo K., Baba M., Fujii W., & Palmer J. B. (2007). Chewing and food consistency: Effects on bolus transport and swallow initiation. Dysphagia, 22, 100–107. [DOI] [PubMed] [Google Scholar]
- Steele C. M., Molfenter S. M., Peladeau-Pigeon M., & Stokely S. (2013). Challenges in preparing contrast media for videofluoroscopy. Dysphagia, 28, 464–467. https://doi.org/10.1007/s00455-013-9476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokely S. L., Molfenter S. M., & Steele C. M. (2014). Effects of barium concentration on oropharyngeal swallow timing measures. Dysphagia, 29, 78–82. https://doi.org/10.1007/s00455-013-9485-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga H. P., Fonseca H. V., & Bianchini E. M. (2014). Sequential swallowing of liquid in elderly adults: Cup or straw? Dysphagia, 29, 249–255. https://doi.org/10.1007/s00455-013-9503-8 [DOI] [PubMed] [Google Scholar]
- Ziegler A., & Vens M. (2010). Generalized estimating equations. Methods of Information in Medicine, 49, 421–425. [DOI] [PubMed] [Google Scholar]