Abstract
Hydroxyurea induces fetal hemoglobin, improves laboratory parameters, and ameliorates clinical complications of sickle cell anemia (SCA), but its long-term efficacy and safety in this patient population remain incompletely defined. Although generally considered non-DNA reactive, an important safety concern is that hydroxyurea may indirectly cause genotoxic damage. To better address this safety issue of hydroxyurea in patients with SCA, we measured the production of micronuclei (MN) in red blood cells (RBC) as a marker of genotoxicity.
Blood samples were collected from children with SCA enrolled in the Hydroxyurea Study of Long-term Effects (ClinicalTrials.gov NCT00305175). Flow cytometry quantified circulating MN-containing erythrocyte sub-populations before and during hydroxyurea exposure. The frequency of micronucleated reticulocytes (MN-CD71+) and micronucleated mature erythrocytes (MN-RBC) were then tested for associations with laboratory and clinical data. In cross-sectional analysis of 293 blood samples from 105 children with SCA and a median of 2 years of hydroxyurea therapy, exposure to hydroxyurea was associated with significantly increased frequencies of MN-CD71+ and MN-RBC compared to baseline. The increases were evident by 3 months of therapy, and did not escalate further with up to 12 years of continuous drug exposure. In prospective longitudinal analysis, substantial inter-individual variation in the effect of hydroxyurea on %MN-CD71+ was observed that was associated with the expected laboratory effects of hydroxyurea.
In conclusion, clinically relevant exposure to hydroxyurea is associated with increased MN production consistent with erythroblast genotoxicity but with substantial inter-patient variability. Associations between increased %MN-CD71+ and laboratory benefits suggest that hydroxyurea effects on MN production may be related to individual patient sensitivity to hydroxyurea within the bone marrow.
Keywords: Sickle Cell Anemia, Hydroxyurea, Micronuclei, Genotoxicity
1. Introduction
Sickle cell anemia (SCA) is a common genetic disorder caused by a mutation in the β-globin chain of hemoglobin, resulting in the production of abnormal sickle hemoglobin (HbS) tetramers. Current estimates suggest approximately 100,000 individuals in the US are suffering from SCA, with further millions worldwide. The clinical manifestations of SCA include hemolytic anemia, acute vaso-occlusive events, and chronic end organ damage, which collectively result in substantial morbidity and mortality [1–3].
One of the principal therapies for SCA is the pharmacological agent hydroxyurea, which is being increasingly used for the treatment of severely affected children and adults [4, 5]. Initially introduced as an antineoplastic compound in the 1960s, hydroxyurea has proven laboratory efficacy for SCA, including the induction of fetal hemoglobin (HbF) while also lowering white blood cell count (WBC) and the rate of hemolysis [6, 7]. Hydroxyurea also has clinical efficacy with decreased number of vaso-occlusive events, acute chest syndrome, transfusions, and hospitalizations [4, 7]. However, the long-term efficacy and safety of hydroxyurea in treating patients with SCA, especially children, remain incompletely defined.
As a ribonucleotide reductase inhibitor, one long-term safety concern is hydroxyurea’s genotoxic potential via disruption of deoxyribonucleotide synthesis. Indeed, hydroxyurea’s drug label indicates that it causes mutation and clastogenic effects in several in vitro systems, as well as cytogenetic damage in exposed mice, although the data used to prepare hydroxyurea’s package insert are not available for assessment [8, 9]. These and other preclinical data have led to a drug label which cautions hydroxyurea is an unequivocal genotoxic agent and a presumable trans-species carcinogen. Even so, the genotoxic potential of hydroxyurea in patients exposed to clinically relevant doses has not been thoroughly studied. In two studies examining the pharmacokinetic profile of hydroxyurea in children with SCA given a single hydroxyurea dose of 20mg/kg, the mean maximum serum concentrations were 260 ± 76 µM and 349 ± 106 µM, respectively [10, 11]. These pharmacokinetic studies showed that hydroxyurea was rapidly absorbed and that patients have serum levels greater than 100 µM for more than 4 hrs post drug administration. Hydroxyurea concentrations greater than 100 µM have been shown to increase chromosome aberrations and breaks in vitro, which reinforces concerns about the genotoxic effects in patients with SCA treated with hydroxyurea [12, 13]. A better understanding of hydroxyurea genotoxicity in vivo would allow a more informed discussion of the risks and benefits of hydroxyurea therapy for young patients with SCA.
Detection and measurement of micronuclei (MN) in blood lymphocyte or reticulocyte populations is now a widely studied marker of genotoxicity [14–18]. During normal erythropoiesis, red blood cells (RBC) extrude their nucleus as they develop into functional reticulocytes. Occasionally, membrane bound DNA resulting from double-strand breaks or else lagging whole chromosomes remains in the cell after erythrocyte maturation and these inclusion bodies are known as MN. During normal erythropoiesis, MN are formed at low frequency, and MN-containing erythrocytes are removed quickly and efficiently from peripheral blood circulation by a healthy spleen in most mammalian species, including man. The frequency of MN-containing mature RBC (MN-RBC) in human blood circulation is therefore largely dictated by splenic filtration function. However, in the context of asplenia, MN-RBC persists within the circulation, and their frequency represents a useful reporter of chromosomal damage (14). On the other hand, the frequency of circulating MN-containing young reticulocytes (MN-CD71+) appears to be indicative of MN production, irrespective of splenic filtration function. This is why MN-CD71+ is emerging as a useful cross-species cytogenetic damage endpoint [14–16, 19, 20].
In a previous small cross-sectional study, we showed that patients with SCA have increased basal MN production while also having decreased MN clearance due to their diminished splenic filtrative function [21]. We also showed that hydroxyurea exposure further increased MN-CD71+ production in SCA patients, but the magnitude, long-term effect and etiology of hydroxyurea-induced changes were not investigated [21]. To better address this long-term safety issue of hydroxyurea, we present data on MN production in a large cross-sectional study as well as in a prospective longitudinal analysis of children with SCA on hydroxyurea therapy.
2. Methods
2.1 Subject recruitment
Children with SCA receiving medical care at St. Jude Children’s Research Hospital (SJCRH) were offered hydroxyurea therapy for clinical severity, typically recurrent acute vaso-occlusive events. Hydroxyurea dosing and escalation to a maximum tolerated dose (MTD) were performed as previously described [5]. After written informed consent, children with SCA on hydroxyurea therapy were enrolled in the Hydroxyurea Study of Long-term Effects (HUSTLE, ClinicalTrials.gov NCT00305175) and venous blood samples were periodically collected. A total of 105 subjects recruited to this study had at least 1 MN measurement, including 37 subjects with serial measurements at baseline and at follow-up time points up to 2 years of hydroxyurea exposure. Age, gender, height, weight, body surface area, body mass index and hydroxyurea therapy adherence were assessed at every visit where there was MN measurement.
2.2 Standard laboratory testing
Each of the 105 subjects had standard clinical laboratory tests performed at the SJCRH diagnostic testing center. These included standard testing of hematological parameters and chemistries including hemoglobin (Hgb), fetal hemoglobin (HbF), hematocrit (Hct), alanine aminotransferase (ALT), absolute neutrophil count (ANC), aspartate aminotransferase (AST), bilirubin, lactate dehydrogenase (LDH), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), platelet count, red blood cell (RBC) count, absolute reticulocyte count (ARC) and white blood cell (WBC) count.
2.3 MN detection and measurement
Within 24 hours of collection, 10 µL whole blood was added to 200 µL of heparinized phosphate-buffered salt solution and then quickly and forcefully injected into 15-mL polypropylene tubes (VWR, Bristol, CT, USA) containing 2 mL ultra-cold (−85°C) methanol. Fixed samples were stored at −85°C until shipment on dry ice to Litron Laboratories (Rochester, NY). MN detection and measurement was performed at Litron as previously described [21]. Briefly, fixed whole blood cells were pelleted and incubated in an RNase/antibody solution containing anti-CD71-FITC (to detect young reticulocytes), anti-CD61-PE (to exclude platelets), and RNase in buffer solution supplemented with 1% fetal bovine serum (FBS). Following successive incubations at 4°C and 37°C, the stained cells were washed with 5 mL buffer with 1% FBS and finally resuspended in propidium iodide (1.25 µg/mL) solution to stain DNA. Samples were then stored at 4°C until same-day analysis by flow cytometry. Data acquisition proceeded until at least 106 total erythrocytes and at least 2 × 104 CD71+ reticulocytes per sample were acquired.
2.4 Statistical methods
The Wilcoxon Signed Rank test was used to test the median differences between MTD and baseline laboratory parameters. Associations between continuous variables were measured using Spearman’s Correlation coefficient (rs). Tests with p value< 0.05 are considered significance and no adjustment has been made for multiple comparisons.
3. Results
3.1 Long term effect of hydroxyurea exposure on MN production
Flow cytometry was used to detect the following sub-populations of circulating erythrocytes in each sample: 1) CD71+ reticulocytes among total erythrocytes, which identifies the youngest erythroid cells in circulation; 2) MN-CD71+, which are young erythrocytes containing MN and are an index of recent cytogenetic damage; and 3) MN-RBC, which are older erythrocytes (CD71−) containing MN that have not removed by splenic filtration. The frequency of CD71+ reticulocytes were determined as a percentage of the total number of erythrocytes in each sample while the sub-populations MN-CD71+ and MN-RBC are described as a percentage of young CD71+ and older CD71− erythrocytes containing MN, respectively.
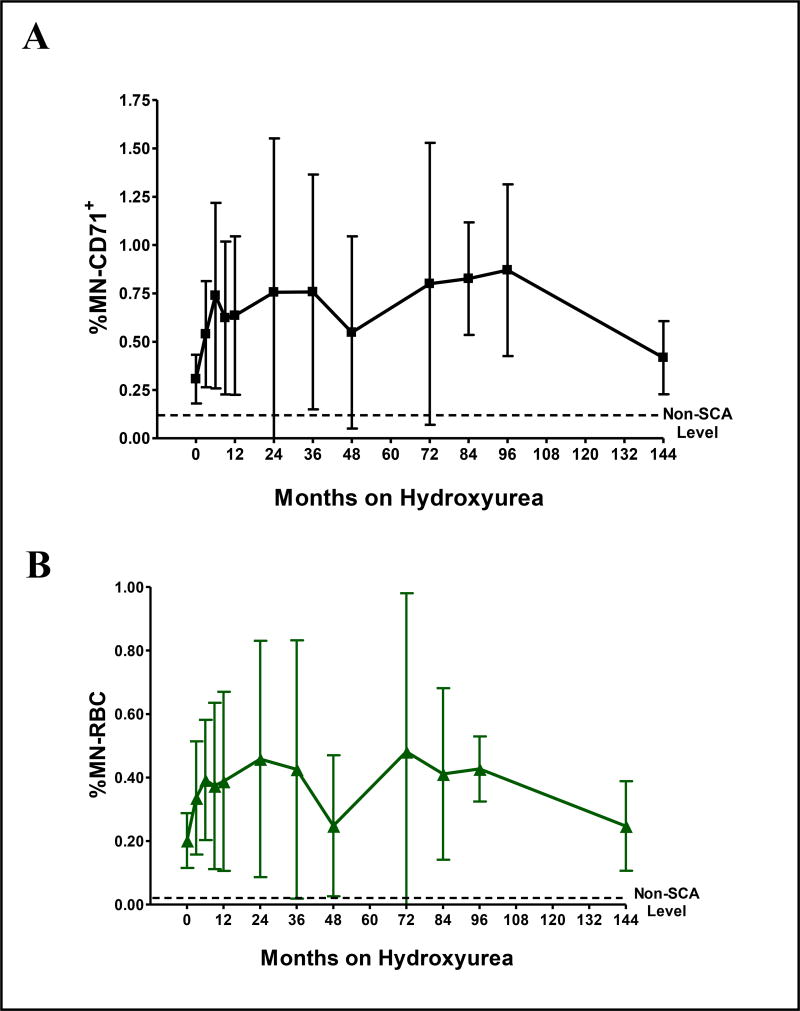
In the initial cross-sectional analysis of 293 samples from 105 children with SCA and a median of 2 years hydroxyurea therapy (range 3 months – 12 years), hydroxyurea exposure was associated with significantly higher numbers of circulating MN-containing erythrocytes. Compared to all baseline samples, hydroxyurea treatment predictably reduced the number of reticulocytes (mean fold reduction 0.53 ± 0.44, p < 0.001) while increasing the levels of MN in both reticulocytes (%MN-CD71+, mean fold increase 1.80 ± 0.91, p<0.05) and mature RBC (%MN-RBC, mean fold increase 1.89 ± 1.39, p<0.01). The effects were evident by 6–9 months of hydroxyurea treatment when most patients had reached their maximum tolerated dose (MTD), and did not significantly escalate further with up to 12 years of continued drug exposure (Figure 1). However, there was a large degree of inter-patient variability in the effect of hydroxyurea on MN production, suggesting variability in individual sensitivity to the drug.
Figure 1. Cross-sectional analysis of the effect of hydroxyurea on MN production and clearance.
A shows the effect of hydroxyurea on %MN-CD71+, reflecting MN production. B shows the effect of hydroxyurea on %MN-RBC levels (clearance). All patients were selected from the HUSTLE study. This included patients enrolled already on hydroxyurea therapy as well as patients beginning hydroxyurea therapy. The number of patients at each time point are: 0 (n=58), 3 months (n=45), 6 months (n=44), 9 months (n=41), 1 year (n=27), 2 years (n=18), 3 years (n=16), 4 years (n=11), 6 years (n=6), 7 years (n=6), 8 years (n=4) and 12 years (n=7). The mean and standard deviation are given for each time point. The non-SCA level is the frequency of the respective MN containing erythrocyte subpopulation in ethnic matched control subjects with no SCA.
3.2 Longitudinal prospective analysis of hydroxyurea exposure on MN production
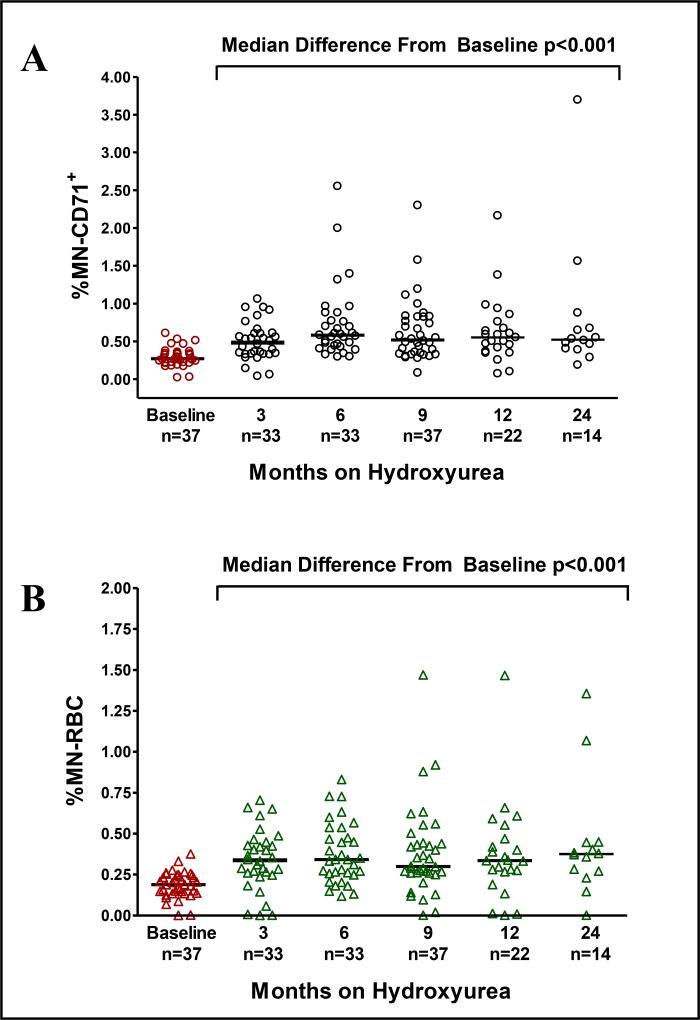
To better determine the effects of hydroxyurea before and during therapy, a longitudinal prospective study was performed to investigate the genotoxic effect associated with hydroxyurea exposure. Baseline studies followed by serial measurements over 2 years were performed on 37 patients. After 9 months on hydroxyurea therapy all subjects were on a stable MTD (average 25.1 mg/kg/day) with a clear clinical and laboratory benefit (Table 1). For this cohort of pediatric patients, the median increase from baseline to follow-up at MTD in hemoglobin concentration was 1.1 g/dL (min −2.1, max 4), increase in %HbF was 12.0% (min −0.6, max 33.6), along with decreased reticulocyte and WBC counts (all p<0.0001); all representing expected laboratory changes in response to hydroxyurea therapy [4, 22]. In this longitudinal prospectively measured cohort, substantial inter-individual variability was again observed in the levels of %MN-CD71+ and %MN-RBC (Figure 2). There were no significant associations between either MN subpopulation and gender, although the MN-RBC frequency while on hydroxyurea therapy increased slightly with age (rs=0.335, p=0.021). There was no association between MN production and hydroxyurea dose (rs=0.000, p=0.998).
Table 1. Effect of hydroxyurea on laboratory measurements in 37 children with sickle cell anemia.
MTD typically was established after 6–9 months of hydroxyurea therapy with dose escalation and titration. The median difference between MTD and baseline is given for each laboratory measurement. For this cohort of pediatric patients, the median increase from baseline to follow-up at MTD in hemoglobin concentration was 1.1 g/dL (min −2.1, max 4), increase in %HbF was 12.0% (min −0.6, max 33.6), along with decreased reticulocyte and WBC counts (all p<0.0001). NA implies not applicable.
| Parameter | Baseline | MTD | Difference (MTD - Baseline) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Min | Max | Median | Min | Max | Median | Min | Max | P-Value | |
| Age (years) | 10 | 2 | 17.9 | --- | --- | --- | --- | --- | --- | --- |
| Hydroxyurea dose (mg/kg/day) | --- | --- | --- | 25.0 | 13.9 | 32.4 | --- | --- | --- | --- |
| Height (cm) | 133.3 | 83.8 | 189.9 | 136.5 | 92.8 | 191 | 4.1 | 0 | 9 | < 0.0001 |
| Weight (kg) | 27.4 | 12.2 | 88.3 | 29.3 | 13.7 | 89 | 2.2 | 0 | 15.2 | < 0.0001 |
| Body mass index (kg/m2) | 16.5 | 13.3 | 29.2 | 16.4 | 13.7 | 30.5 | 0.5 | −1.5 | 3.3 | 0.0001 |
| Body surface area (m2) | 1.01 | 0.55 | 2.16 | 1.06 | 0.61 | 2.18 | 0.06 | 0.01 | 0.22 | < 0.0001 |
| Hemoglobin (g/dL) | 8.1 | 5.9 | 11.5 | 9.1 | 7.1 | 12.6 | 1.1 | −2.1 | 4 | < 0.0001 |
| Red blood cell count (×1012/L) | 2.7 | 1.8 | 3.8 | 2.5 | 1.9 | 3.7 | −0.1 | −1.6 | 0.7 | 0.0114 |
| % Fetal hemoglobin (%) | 5.9 | 0 | 27.1 | 19.1 | 2.6 | 39.3 | 12.0 | −0.6 | 33.6 | < 0.0001 |
| White blood cell count (×109/L) | 12.4 | 3.9 | 24.5 | 8.5 | 3.9 | 16.6 | −3.7 | −14.2 | 2.3 | < 0.0001 |
| Platelet count (×109/L) | 495 | 177 | 920 | 364 | 129 | 1329 | −102 | −625 | 691 | 0.0026 |
| Absolute neutrophil count (×106/L) | 5989 | 1519 | 14697 | 3510 | 1058 | 8062 | −1610 | −11857 | 1643 | < 0.0001 |
| Absolute reticulocyte count (×109/L) | 246 | 135 | 522 | 126 | 63 | 440 | −115 | −387 | 189 | < 0.0001 |
| Lactate dehydrogenase (U/L) | 616 | 324 | 1103 | 423 | 5 | 1103 | −173 | −501 | 367 | < 0.0001 |
| Bilirubin (mg/dL) | 2.7 | 0.8 | 13 | 1.45 | 0.6 | 13.9 | −0.8 | −4.2 | 0.9 | < 0.0001 |
| Alanine aminotransferases (U/L) | 21 | 9 | 79 | 20 | 9 | 96 | 0 | −59 | 46 | 1.0000 |
| Aspartate aminotransferases (U/L) | 51 | 12 | 87 | 42 | 20 | 83 | −11.5 | −35 | 40 | 0.0027 |
| Mean cell hemoglobin (pg) | 30.3 | 22.1 | 34.2 | 36.3 | 25.8 | 44.4 | 6.3 | −1.2 | 14.3 | < 0.0001 |
| Mean corpuscular volume (fL) | 86.2 | 67.2 | 99.2 | 104.2 | 73.9 | 128 | 17.3 | −0.9 | 42 | < 0.0001 |
| Mean Platelet Volume (fL) | 8.0 | 6.7 | 10.8 | 7.6 | 6.4 | 9.6 | −0.5 | −2.0 | 0.4 | < 0.0001 |
| Red cell distribution width (%) | 22.4 | 17.4 | 36.4 | 18.5 | 14.8 | 28.3 | −3.8 | −21 | 4.8 | < 0.0001 |
Figure 2. Prospective analysis of the effect of hydroxyurea on MN production and clearance.
Patients beginning hydroxyurea therapy had a baseline sample and serial samples collected at 3, 6, 9, 12 and 24 months while undergoing hydroxyurea treatment. After 3 months of hydroxyurea exposure, median %MN-CD71+ and %MN-RBC levels significantly increased compared to baseline values and did not further increase with extended exposure. The median levels are given for each time point.
When individual patient MN-CD71+ fold-increases were calculated from baseline to serial time points on hydroxyurea therapy, 16 of 37 children had < 2 fold increase within 9 months while 8 of 37 children had 2 to 3 fold increase and 13 of 37 children had >3 fold increase in %MN-CD71+ levels (mean fold increases of 1.29 ± 0.26, 2.44 ± 0.36 and 3.16 ± 0.98 respectively). MN-CD71+ induction was associated with increases in MN-RBC (rs=0.32, p=0.045) and reductions to reticulocyte frequency (rs=−0.45, p <0.006). When the levels of %MN-CD71+ in all 37 patients with SCA were compared to the laboratory effects of hydroxyurea, significant increases in %MN-CD71+ were positively associated with MTD values of HbF, mean corpuscular volume, and mean corpuscular hemoglobin but negatively associated with absolute neutrophil count and bilirubin levels (Table 2).
Table 2. Hydroxyurea effect on the association of %MN-CD71+ and laboratory parameters.
The change in %MN-CD71+ was compared to 17 laboratory parameters using the Spearman correlation coefficient. Five parameters were significantly associated with the hydroxyurea effect on MN production.
| Laboratory Parameter | Correlation Coefficient | Significance |
|---|---|---|
| HbF | rs = 0.35 | p=0.0360 |
| Mean Corpuscular Volume | rs = 0.63 | p=0.0001 |
| Mean Corpuscular Hemoglobin | rs = 0.54 | p=0.0006 |
| Absolute Neutrophil Count | rs = −0.46 | p=0.0054 |
| Bilirubin | rs = −0.44 | p=0.0076 |
4. Discussion
Hydroxyurea is an antineoplastic agent that has been used for many years to treat various conditions including malignancy, HIV and sickle cell anemia. In patients with SCA, hydroxyurea has been associated with decreased vaso-occlusive events and increased hemoglobin F levels [7, 23, 24]. Despite its frequent use in this population, the exact mechanisms by which hydroxyurea induces these clinical and laboratory responses remain incompletely understood. Hydroxyurea is known to inhibit ribonucleotide reductase activity thereby interrupting the cell cycle [25]. In addition to this cytotoxic effect, hydroxyurea also has a well established genotoxic activity in cell culture and rodent models [8, 9, 26]. However, there are conflicting reports regarding its DNA-damaging potential in exposed humans. Some studies have shown that hydroxyurea is genotoxic [26, 27] while other studies suggest that hydroxyurea has low mutagenicity in vivo [28]. Patients with SCA treated with clinical relevant doses of hydroxyurea (20mg/kg) have hydroxyurea serum levels greater than 100 µM for greater than 4 hours post drug administration [11]. This level of hydroxyurea exposure is known to cause genotoxicity in vitro, which emphasizes the need for research into the long-term safety of administering hydroxyurea. A better understanding of hydroxyurea genotoxicity in vivo would allow a more informed discussion of the risks and benefits of hydroxyurea therapy for young patients with SCA.
We previously used a quantitative flow cytometric technique to assess the in vivo genotoxic effects of hydroxyurea [21]. Children with SCA but with no hydroxyurea exposure were shown to have high basal MN production in young reticulocytes, a finding that may be related to their stimulated erythropoiesis status. In this follow-up to that small cross-sectional analysis, we have now established that hydroxyurea increases production of MN in vivo in both a large cross-sectional and in a longitudinal prospective study of pediatric patients with SCA. Hydroxyurea significantly reduced reticulocyte production in all patients but had a detectable genotoxic effect on MN production. However, it is important to stress that the increases in MN production with hydroxyurea was observed in reticulocytes, and reflects chromosomal damage that occurred in erythroblasts. Whether erythroid cells are particularly sensitive to hydroxyurea-induced genotoxicity, or whether these cells are reporting damage that is also occurring in other lineages, requires further study [15, 29].
The increases in %MN-RBC and MN-CD71+ are observed within 3 months of starting hydroxyurea therapy and there is substantial inter-patient variability in hydroxyurea-induced MN levels. When patients were followed prospectively from baseline to MTD, only 13 of 37 (35%) patients had greater than a 3 fold increase in their MN-CD71+ frequencies. When the %MN-CD71+ values of all these 37 patients were compared to the expected hydroxyurea effects on laboratory parameters, there was a significant correlation between MN-CD71+ and HbF, MCV, MCH, ANC and bilirubin levels. This suggests that hydroxyurea induces measurable genotoxicity that may be related to individual patient sensitivity and efficacy of hydroxyurea within the bone marrow. Interestingly, final hydroxyurea dose was not associated with %MN-CD71+ or %MN-RBC. Levels of %MN-RBC rose modestly with increased time on hydroxyurea therapy, a finding that may be related to decreased splenic function in older children with SCA, as previously observed [21].
In conclusion, hydroxyurea has a measurable genotoxic effect on MN production and is associated with individual patient response to hydroxyurea therapy. The full clinical importance of these findings is not clear at this time. None of the patients examined, even with 12 years hydroxyurea exposure, had any myelodysplastic syndrome or malignancy symptoms. These observations are encouraging, and reinforce the important point that the observed genotoxicity of uncertain significance reported herein must be weighed against the clear clinical benefits of hydroxyurea therapy [30]. However, these patients will be monitored further in order to continue assessing the significance of the erythroid lineage genotoxicity associated with clinically relevant exposure to hydroxyurea that is evident in a portion of this population.
Acknowledgments
This work was supported in part by R01-HL-090941 and U54-HL070590-07 from the National Heart, Lung, and Blood Institute (NHLBI) and by the American Lebanese Syrian Associated Charities (ALSAC). The authors would like to thank the St. Jude Children’s Research Hospital clinical staff for providing hydroxyurea management, and to all patients and families for study participation.
Abbreviations
- SCA
sickle cell anemia
- RBC
red blood cells
- MN-CD71+
micronucleated reticulocytes
- MN-RBC
micronucleated mature erythrocytes
- MTD
maximum tolerated dose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
S.L.A. and S.D.D. are employed by Litron Laboratories, a company that owns patents regarding flow cytometry-based scoring of micronucleated erythrocytes. Litron sells assay kits related to these patented methods that are designed for several preclinical animal models; note that human blood-based analysis as described herein are not yet approved, cleared, validated or intended for clinical diagnostic use.
References
- 1.Gill FM, Sleeper LA, Weiner SJ, Brown AK, Bellevue R, Grover R, Pegelow CH, Vichinsky E. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86:776–783. [PubMed] [Google Scholar]
- 2.Leikin SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatrics. 1989;84:500–508. [PubMed] [Google Scholar]
- 3.Ballas SK, Lieff S, Benjamin LJ, Dampier CD, Heeney MM, Hoppe C, Johnson CS, Rogers ZR, Smith-Whitley K, Wang WC, Telen MJ. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol. 2009 doi: 10.1002/ajh.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinney TR, Helms RW, O'branski EE, Ohene-Frempong K, Wang W, Daeschner C, Vichinsky E, Redding-Lallinger R, Gee B, Platt OS, Ware RE. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood. 1999;94:1550–1554. [PubMed] [Google Scholar]
- 5.Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Pediatr. Clin North Am. 2008;55:483–501. doi: 10.1016/j.pcl.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Ferster A, Vermylen C, Cornu G, Buyse M, Corazza F, Devalck C, Fondu P, Toppet M, Sariban E. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood. 1996;88:1960–1964. [PubMed] [Google Scholar]
- 7.Hankins JS, Ware RE, Rogers ZR, Wynn LW, Lane PA, Scott JP, Wang WC. Long-term hydroxyurea therapy for infants with sickle cell anemia: the HUSOFT extension study. Blood. 2005;106:2269–2275. doi: 10.1182/blood-2004-12-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bristol-Meyers-Squibb. Droxia. 2006 http://packageinserts.bms.com/pi/pi_droxia.pdf.
- 9.Bristol-Meyers-Squibb. Hydrea. 2006 http://packageinserts.bms.com/pi/pi_hydrea.pdf.
- 10.Rogers ZR, Thompson B, Ware RE, Wang WC, Iyer RV, Miller ST, Minniti C, Rana S, Barredo JC, Toledano S, Zimmerman SA, Casella JF, Files BA, Waclawiw MA, Gerber AR, Bonds DR. Pharmacokinetics of Hydroxyurea in Young Children with Sickle Cell Anemia: A Report from the BABY HUG Trial. ASH Annual Meeting Abstracts. 2005;106:3184. [Google Scholar]
- 11.Ware RE, He J, Mortier NA, Howard TA, Cheng C, Flanagan JM, Sparreboom A. Distinct Phenotypes of Hydroxyurea Absorption among Children with Sickle Cell Anemia. ASH Annual Meeting Abstracts. 2008;112:709. [Google Scholar]
- 12.Speit G, Schntz P. The effect of inhibited replication on DNA migration in the comet assay in relation to cytotoxicity and clastogenicity. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2008;655:22–27. doi: 10.1016/j.mrgentox.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto K, Nakajima Y, Matsumura S, Chatani F. An in vitro micronucleus assay with size-classified micronucleus counting to discriminate aneugens from clastogens. Toxicology in Vitro. 2010;24:208–216. doi: 10.1016/j.tiv.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Schlegel R, MacGregor JT, Everson RB. Assessment of cytogenetic damage by quantitation of micronuclei in human peripheral blood erythrocytes. Cancer Res. 1986;46:3717–3721. [PubMed] [Google Scholar]
- 15.Dertinger SD, Chen Y, Miller RK, Brewer KJ, Smudzin T, Torous DK, Hall NE, Olvany KA, Murante FG, Tometsko CR. Micronucleated CD71-positive reticulocytes: a blood-based endpoint of cytogenetic damage in humans. Mutat. Res. 2003;542:77–87. doi: 10.1016/j.mrgentox.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Dertinger SD, Miller RK, Brewer K, Smudzin T, Torous DK, Roberts DJ, Avlasevich SL, Bryce SM, Sugunan S, Chen Y. Automated human blood micronucleated reticulocyte measurements for rapid assessment of chromosomal damage. Mutat. Res. 2007;626:111–119. doi: 10.1016/j.mrgentox.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Telez M, Ortiz-Lastra E, Gonzalez AJ, Flores P, Huerta I, Ramerez JM, Barasoain M, Criado B, Arrieta I. Assessment of the genotoxicity of atenolol in human peripheral blood lymphocytes: Correlation between chromosomal fragility and content of micronuclei. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2010;695:46–54. doi: 10.1016/j.mrgentox.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Arsoy NS, Neuss S, Wessendorf S, Bommer M, Viardot A, Schutz P, Speit G. Micronuclei in peripheral blood from patients after cytostatic therapy mainly arise ex vivo from persistent damage. Mutagenesis. 2009;24:351–357. doi: 10.1093/mutage/gep015. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka A, Kodama Y, Fukuhara K, Honda S, Hayashi M, Sai K, Hasebe M, Fujiwara Y. A pilot study of evaluation of the antioxidative activity of resveratrol and its analogue in a 6-month feeding test in young adult mice. Food Chem. Toxicol. 2008;46:1125–1130. doi: 10.1016/j.fct.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Hotchkiss CE, Bishop ME, Dertinger SD, Slikker W, Jr, Moore MM, MacGregor JT. Flow cytometric analysis of micronuclei in peripheral blood reticulocytes IV: an index of chromosomal damage in the rhesus monkey (Macaca mulatta) Toxicol. Sci. 2008;102:352–358. doi: 10.1093/toxsci/kfn013. [DOI] [PubMed] [Google Scholar]
- 21.Harrod VL, Howard TA, Zimmerman SA, Dertinger SD, Ware RE. Quantitative analysis of Howell-Jolly bodies in children with sickle cell disease. Exp. Hematol. 2007;35:179–183. doi: 10.1016/j.exphem.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, Ware RE. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103:2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 23.Charache S, Barton FB, Moore RD, Terrin ML, Steinberg MH, Dover GJ, Ballas SK, McMahon RP, Castro O, Orringer EP. Hydroxyurea and Sickle Cell Anemia: Clinical Utility of a Myelosuppressive "Switching" Agent. Medicine. 1996;75 doi: 10.1097/00005792-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Ferster A, Tahriri P, Vermylen C, Sturbois G, Corazza F, Fondu P, Devalck C, Dresse MF, Feremans W, Hunninck K, Toppet M, Philippet P, Van GC, Sariban E. Five years of experience with hydroxyurea in children and young adults with sickle cell disease. Blood. 2001;97:3628–3632. doi: 10.1182/blood.v97.11.3628. [DOI] [PubMed] [Google Scholar]
- 25.Yarbro JW. Mechanism of action of hydroxyurea. Semin. Oncol. 1992;19:1–10. [PubMed] [Google Scholar]
- 26.Ziegler-Skylakakis K, Schwarz LR, Andrae U. Microsome- and hepatocyte-mediated mutagenicity of hydroxyurea and related aliphatic hydroxamic acids in V79 Chinese hamster cells. Mutat. Res. 1985;152:225–231. doi: 10.1016/0027-5107(85)90065-x. [DOI] [PubMed] [Google Scholar]
- 27.Friedrisch JR, Prá D, Maluf SW, Bittar CM, Mergener M, Pollo T, Kayser M, da Silva MAL, Henriques JAP, da Rocha Silla LM. DNA damage in blood leukocytes of individuals with sickle cell disease treated with hydroxyurea. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2008;649:213–220. doi: 10.1016/j.mrgentox.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Hanft VN, Fruchtman SR, Pickens CV, Rosse WF, Howard TA, Ware RE. Acquired DNA mutations associated with in vivo hydroxyurea exposure. Blood. 2000;95:3589–3593. [PubMed] [Google Scholar]
- 29.Witt KL, Cunningham CK, Patterson KB, Kissling GE, Dertinger SD, Livingston E, Bishop JB. Elevated frequencies of micronucleated erythrocytes in infants exposed to zidovudine in utero and postpartum to prevent mother-to-child transmission of HIV. Environ. Mol Mutagen. 2007;48:322–329. doi: 10.1002/em.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brawley OW, Cornelius LJ, Edwards LR, Gamble VN, Green BL, Inturrisi C, James AH, Laraque D, Mendez M, Montoya CJ, Pollock BH, Robinson L, Scholnik AP, Schori M. National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Ann. Intern. Med. 2008;148:932–938. doi: 10.7326/0003-4819-148-12-200806170-00220. [DOI] [PubMed] [Google Scholar]