Abstract
Background/Objectives
Coronary artery disease (CAD) is responsible for significant morbidity and mortality. Inflammatory, pro-thrombotic and structural factors contribute to the etiology of CAD. This study sought to determine the relationship of plasma endothelin-1 (pET-1), a potent vasoconstrictor, mitogen and modulator of cardiac inflammation, to clinical characteristics and outcomes of CAD patients.
Methods
Blood samples were collected from 336 patients with underlying chest pain or recent myocardial infarction (MI), prior to coronary catheterization. pET-1 was correlated with clinical characteristics and outcomes following catheterization and at 30-day follow-up.
Results
pET-1 was higher in recent MI patients than in patients with CAD (coronary occlusion≥50%) or without CAD (<50%) (Mean±sem (pg/ml): 2.12±0.13, 1.51±0.10, 1.21±0.06; 95% confidence interval (1.85–2.38, 1.31–1.72, 1.07–1.32; respectively, P<.0001). Patients with ST elevation MI (STEMI) had higher pET-1 than non-STEMI (P=.008). pET-1 was associated with heart failure (HF) and low left ventricular ejection fraction (LVEF) and was highest in MI patients presented with acute HF. At 30-day follow up, pET-1 was not associated with the change in LVEF. In multivariate analysis, pET-1 was positively associated with age, smoking, HF, CAD status, and need for revascularization by coronary artery bypass surgery (CABG). pET-1 was negatively correlated with LVEF and preoperative statin use.
Conclusions
pET-1 is associated with recent MI, HF, age, smoking, CABG, and low LVEF. Preoperative statin use was associated with lower pET-1. pET-1 may serve as a risk marker and a potential therapeutic target in CAD patients.
Keywords: Endothelin-1, Myocardial infarction, Coronary artery disease
1. Introduction
Coronary artery disease (CAD) contributes to patient morbidity and negatively impacts health-related quality of life. Many CAD patients eventually require hospitalization for acute coronary syndrome (ACS) [1].
The mechanisms underlying development of coronary atherosclerosis and myocardial infarction (MI) are multiple and complex. Endothelial dysfunction, platelet activation and activation of inflammatory pathways may promote vascular obstruction and cardiac ischemia. Endothelin-1 (ET-1), a potent vasoconstrictor, mitogen, and pro-inflammatory mediator produced in response to hypoxia or wall stress, may aggravate myocardial ischemia [2]. We have shown that cardiac ET-1 mRNA and protein are increased in atrial fibrillation patients with underlying cardiac diseases such as HF [3]. Plasma ET-1 (pET-1) is elevated in patients with CAD relative to healthy subjects [4]. Components of the ET-1 system, including ET-1 peptide, ETA and ETB receptors are increased in the coronary arteries of infarcted hearts at early stages following percutaneous coronary intervention (PCI) [5].
Several studies have reported that elevated pET-1 levels are associated with reperfusion injury, microvascular obstruction, and long-term mortality following PCI in ST-segment elevated MI (STEMI) patients [6,7]. Plasma ET-1 levels increase within a few hours of STEMI and remain elevated following PCI [6,8]. However, it is unclear if this relationship is true for other clinical manifestations of CAD such as those with non-STEMI. ET-1 has been shown to increase coronary inflammation and to co-localize with atherosclerotic plaque in human coronary arteries [9] suggesting that it might promote plaque formation. Few studies have compared levels of ET-1 in CAD patients relative to non-CAD and MI patients.
This study sought to assess the relationship of pET-1 to clinical manifestations of CAD, left ventricular (LV) function, and coronary artery intervention following coronary angiography. We tested the hypothesis that pET-1 levels are increased in CAD patients and are associated with increased risk of MI, LV dysfunction and need for coronary artery reperfusion intervention.
2. Methods
2.1. Patient selection
Patients in this study presented with angina or recent MI to King Abdullah University Hospital (KAUH). Most patients were referred from other hospitals due to underlying chest pain that required catheterization for proper diagnosis, and, if indicated, reperfusion by PCI or coronary artery bypass surgery (CABG).
Clinical, demographic and laboratory parameters (lipid profile and other routine laboratory parameters) were prospectively obtained from patients and their medical records at KAUH. Left ventricular ejection fraction (LVEF), left atrial size, need for revascularization, and use of medications prior to hospitalization were also evaluated at baseline and for 152 patients at 30-day follow-up after coronary angiography.
Study inclusion criteria included a clinical history of current/recurring angina symptoms or recent MI (within one week). A detailed history and physical examination were obtained from all patients, and relevant laboratory tests were used to document the presence of CAD or MI.
Cardiac biomarkers assays (troponin I, troponin T, creatine kinase (CK) and/or CK-MB) were used to confirm acute MI. A diagnosis of acute MI was established according to the WHO [10] and AHA/ACC criteria [11] with a history of chest pain lasting >20min, characteristic ECG changes, and presence of elevated plasma cardiac enzyme levels. Acute STEMI was documented by elevated ST segment/Q-wave on the ECG while non-STEMI was considered present when no elevated ST segment/Q-waves developed on the ECG in the presence of cardiac enzyme elevation. Angina was defined as chest pain at rest or during exertion with slight or marked limitation of ordinary physical activity without enzyme leak (stable and unstable angina) [12].
Exclusion criteria included patients with recent infection or trauma, patients with heart failure (HF) who presented with normal coronaries (coronary artery stenosis<50%), patients with history of CAD who underwent previous PCI or CABG but presented with current normal coronaries (<50%), and patients who suffered an MI within the month before their current angina or MI.
2.2. Echocardiographic and coronary angiographic analyses
Echocardiographic studies were performed using a two-dimensional imaging system (ALT HD1 6000 ht, 2–4MHz probe, Philips Medical Systems Inc., Bothell, WA, USA) to evaluate LVEF and LA size (diameter) at admission and at 30-day follow-up. Left atrial (LA) size was indexed to body surface area. CAD was established by coronary angiography as the presence of at least 50% stenosis in one or more of the main coronary arteries. Angiographic measurements were performed by a single, experienced cardiologist (M.J.) at our institution.
2.3. Blood collection
Blood was collected from the femoral artery of all patients using K2EDTA tubes. Specimens were brought from the clinic to the laboratory immediately and centrifuged at 2500rpm for 15 min. Frozen plasma was stored at −80°C until analysis.
2.4. Plasma ET-1 measurement
Plasma ET-1 concentrations were determined using enzyme-linked immunoassays (R&D Systems, Inc., USA). Human plasma samples, controls and standards (all 75μL) were pipetted into wells coated with monoclonal rat anti-endothelin-1 antibody and incubated at room temperature on a horizontal shaker for 1h. Standards and samples were assayed in duplicate. After washing, 200μl of monoclonal mouse anti-endothelin-1 antibody conjugated to horseradish peroxidase was added, and incubated for 3h following washing; 200μl of the substrate was then added followed by stopping buffer. Absorbance values (450nm) were determined in an ELISA reader, using 540nm absorbance as a reference. The assay is specific for ET-1 with no significant cross reactivity for human ET-2, 3 or big ET-1. The assay is sensitive, with a mean lower detection limit of 0.087pg/ml, and reproducible, with an average intra-assay coefficient of variation of <4%, and inter-assay coefficient of variation <10%.
2.5. Statistical analysis
Data are expressed as mean±standard error, unless otherwise specified. Univariate and multivariate linear regression analyses were performed to evaluate the association of clinical and demographic variables with pET-1 levels. Because pET-1 levels were not normally distributed, a square root transformation was used in the tables of univariate and multivariate analyses, effectively normalizing the dataset. Normally distributed variables were analyzed using ANOVA. Non-normally distributed variables were analyzed using Mann–Whitney or Kruskal–Wallis tests with the use of Dunn’s post-test for pair-wise comparisons. A Chi-square test was used to compare frequencies across groups. Spearman’s correlation was used for non- normally distributed variables. Sample size was pre-estimated at 90% power and 0.5-effect size at 0.05 alpha level of significance. Effect size was estimated by piloting 30 patients. Univariate, multivariate and sample size analyses were performed using JMP 11. Figures were prepared using graph pad Prism 5. Differences are cited at 95% confidence interval (CI). Values of P<.05 were considered statistically significant.
3. Theory/Calculation
ET-1 is a potent vasoconstrictor, mitogen, and inflammatory factor that might promote endothelial dysfunction and oxidative damage predisposing to coronary artery stenosis and ischemia. It was unclear if plasma ET-1 is differentially expressed in patients with different clinical manifestations of CAD, or if ET-1 levels predict severity of CAD and need for coronary reperfusion. In this study, we hypothesized that plasma ET-1 levels are higher in MI patients than CAD and non-CAD patients, correlating with risk classification. In the setting of CAD, ET-1 is likely to have an important role in plaque formation and rupture. We propose that plasma ET-1 is associated with CAD severity and thus could be used as a predictor for need of coronary artery intervention. To evaluate our hypothesis, we compared plasma ET-1 in patients with MI relative to CAD and non-CAD patients and among those who underwent CABG, PCI and no intervention. Differential expression of plasma ET-1 in MI may also promote LV dysfunction and HF. We assessed the correlation of ET-1 with HF, baseline LVEF and the change in LVEF after 30-day follow up. This study highlights the significance of plasma ET-1 as a risk marker of MI and may lead to additional therapies for treatment of CAD patients.
4. Results
4.1. Study groups and patients characteristics
Plasma samples from 336 patients were selected for biochemical analysis of ET-1 protein and other lab measurements. Table 1 shows the three study groups stratified based on clinical symptoms, coronary angiographic findings, ECG variables and cardiac biomarker levels. Study groups included: 1) patients without significant CAD in whom ACS had been ruled out who presented with chest pain but with negative cardiac biomarker tests and normal main coronaries (CAD<50% stenosis, N=112), 2) patients presenting with angina associated with at least 50% stenosis in one or more of the main coronary arteries, but with negative cardiac injury biomarker tests (CAD≥50%, N=134); and 3) patients presenting with recent MI (within the last week) as documented by ECG, cardiac enzymes and coronary angiogram (MI, N=90). MI patients were further subdivided into two groups; STEMI (N=43) and Non-STEMI (N=47). Most MI patients received medical therapy before arrival at our hospital; 28 STEMI patients received thrombolytic agents, in addition to standard anticoagulant/antiplatelet therapy that was given to all MI patients. 42% of MI patients underwent early coronary angiography (<24h) while angiography was delayed (24h to 1 week) in 57%. 14 Patients with severe CAD were scheduled for CABG. Most study patients had significant cardiovascular disease; HT and diabetes were present in 226 and 131, of patients, respectively (Table 1). 34 patients presented with HF; 13 CAD and 4 MI patients with history of chronic HF, and 17 MI patients with acute HF. Of those presenting with acute HF, 5 patients had a previous history of chronic HF.
Table 1.
Patients characteristics
| CAD<50% (n=112) | CAD≥50% (n=134) | MI (n=90) | P Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 52.0±1.02 | 60.2±0.93 | 55.3±1.13 | <0.0001* |
| Male sex, n (%) | 65 (58.0) | 98 (73.1) | 75 (83.3) | 0.0003* |
| Body Mass Index | 29.39±0.59 | 29.02±0.46 | 28.37±0.50 | 0.4298 |
| Hx of Hypertension, n (%) | 76 (68.4) | 105 (78.3) | 45 (50.0) | <0.0001* |
| Hx of Heart Failure, n (%) | 0 (0) | 13 (9.8) | 21 (23.3) | <0.0001* |
| Hx of Stroke, n (%) | 0 (0) | 5 (3.2) | 2 (2.2) | 0.1244 |
| Diabetes mellitus, n (%) | 31 (27.9) | 69 (52.6) | 31 (35.6) | 0.0003* |
| Previous CAD | 0 (0) | 47 (0.35) | 28 (31) | <0.0001 |
| Current smoking | 42 (37.8) | 53(39.0) | 54 (60) | 0.0024* |
| Lab tests | ||||
| Low Density Lipoprotein (LDL) | 3.34±0.12 | 2.93±0.10 | 3.23±0.13 | 0.0441* |
| High Density Lipoprotein (HDL) | 1.08±0.03 | 0.99±0.02 | 0.92±0.02 | 0.0031* |
| Triglyceride | 2.32±0.16 | 2.56±0.15 | 2.34±0.16 | 0.4697 |
| Total cholesterol (Tch) | 5.05±0.13 | 4.63±0.11 | 4.81±0.14 | 0.0570 |
| Fasting plasma glucose | 7.69±0.44 | 9.45±0.53 | 8.14±0.48 | 0.0277* |
| HBA1C | 6.84±0.30 | 7.49±0.23 | 7.10±0.34 | 0.2411 |
| Neutrophil/lymphocyte ratio | 2.43±0.14 | 2.61±0.17 | 4.66±0.91 | 0.0164* |
| Intervention | ||||
| PCI | 0 (0) | 93 (69.4) | 71 (78.8) | <0.0001* |
| Coronary artery bypass graft | 0 (0) | 7 (5.3) | 7 (7.8) | 0.0183* |
| Pre operative echocardiographic data | ||||
| Indexed LA diameter, cm/m2 | 1.95±0.04 | 2.04±0.04 | 2.01±0.03 | 0.0622 |
| Left ventricular ejection fraction (LVEF), % | 57.45±0.62 | 52.57±0.93 | 46.02±1.35 | <0.0001* |
| % Increase in LVEF at 30days | 1.32%±0.61 | −0.95%±2.22 | 8.35%±2.74 | 0.0166* |
| Pre operative use of medications | ||||
| ACE inhibitors | 30 (26.8) | 45 (34.6) | 20(22.2) | 0.1520 |
| ARBs | 17 (15.2) | 29 (21.9) | 7 (7.7) | 0.0185* |
| Diuretics | 17 (15.2) | 33 (25) | 11 (12.2) | 0.0342* |
| Beta blockers | 54 (48.2) | 77(58.3) | 35 (38.8) | 0.0195* |
| Statins | 50 (44.6) | 97 (73.4) | 36 (40.0) | <0.0001* |
| Aspirin | 79 (71.1) | 112 (83.5) | 44 (48.8) | <0.0001* |
Values are mean±SEM, unless indicated. CAD: coronary artery disease, Hx: history, HBA1C: glycosylated hemoglobin, PCI: percutaneous coronary intervention, LA: Left atria; % Increase in LVEF=((LVEF at 30days−LVEF at baseline)/LVEF at baseline)*100; ACE: angiotensin converting enzyme; ARBs: angiotensin-II receptor blockers. Unit for plasma LDL, HDL, glc etc is mmol/L.
P<.05 indicates a significant difference between groups.
4.2. Univariate predictors of plasma ET-1
Plasma ET-1 concentration (prior to catheterization) was evaluated as a function of clinical manifestation of CAD and comorbid cardiovascular conditions (Table 2). Group 2 & 3 patients (recent MI, CAD≥50%) had higher pET-1 than Group 1 patients (CAD<50%): (mean±sem (pg/mL): 1.76±0.08 vs. 1.2±0.06, P<.0001).
Table 2.
Univariate predictors of plasma ET-1
| Response=Square root of plasma ET-1 | ||||
|---|---|---|---|---|
|
| ||||
| Estimate (slope, B) | Confidence Interval (95%) | P value | Standardized coefficient (β) | |
| Age, years | 0.0064 | 0.0029–0.0098 | 0.0003* | 3.64 |
| Female Gender | −0.0973 | −0.1846 to −0.0100 | 0.0290* | −2.19 |
| BMI | −0.0022 | −0.0096–0.0050 | 0.5417 | −0.61 |
| CAD≥50% | 0.2077 | 0.1253–0.2901 | <0.0001* | 4.96 |
| CAD status | <0.0001* | |||
| Current smoking | 0.1682 | 0.0897–0.2466 | <0.0001* | 4.22 |
| Heart failure status | <0.0001* | |||
| Hypertension | −0.1192 | −0.2036 to −0.0347 | 0.0058* | −2.78 |
| Diabetes | −0.0400 | −0.1229–0.0427 | 0.3420 | −0.95 |
| HBA1C | 0.0021 | −0.020–0.0249 | 0.8523 | 0.19 |
| Intervention status | <0.0001* | |||
| Preoperative LVEF | −0.0169 | −0.0213 to −0.0126 | <0.0001* | −7.69 |
| Indexed LA diameter | 0.2093 | 0.0664–0.3522 | 0.0043* | 2.89 |
| LVEF at 30days | −0.0179 | −0.0244 to −0.0114 | <0.0001* | −5.45 |
| % Increase in LVEF after 30days | 0.0096 | 0.0053–0.0139 | <0.0001* | 4.41 |
| Degree of coronary stenosis | 0.0029 | 0.0019–0.0039 | <0.0001* | 5.96 |
| Neutrophil/Lymphocyte ratio | 0.0462 | 0.0290–0.0633 | <0.0001* | 5.32 |
| Preoperative use of aspirin | −0.1035 | −0.1903 to −0.0168 | 0.0195* | −2.35 |
| Preoperative use of statins | −0.1016 | −0.1813 to −0.0218 | 0.0127* | −2.51 |
| Pre operative use of beta blockers | −0.0980 | −0.1775 to −0.0186 | 0.0157* | −2.43 |
| Pre operative use of ACEi | −0.0466 | −0.1353–0.0421 | 0.3023 | −1.03 |
| Preoperative use of ARBs | −0.1111 | −0.2202 to −0.0019 | 0.0460* | −2.00 |
| Preoperative use of Diuretics | 0.0832 | −0.0202–0.1855 | 0.1145 | 1.58 |
CAD status coded as (non-CAD, CAD, MI, MI with acute HF). MI: myocardial infarction, heart failure (HF) coded as (acute HF, chronic HF, no HF), intervention coded as (CABG, PCI, and no intervention) LVEF: left ventricular ejection fraction, LA: left atria, % Increase in LVEF=((LVEF at 30days−LVEF at baseline)/LVEF at baseline)*100, β is the standardized coefficient (slope/std error).
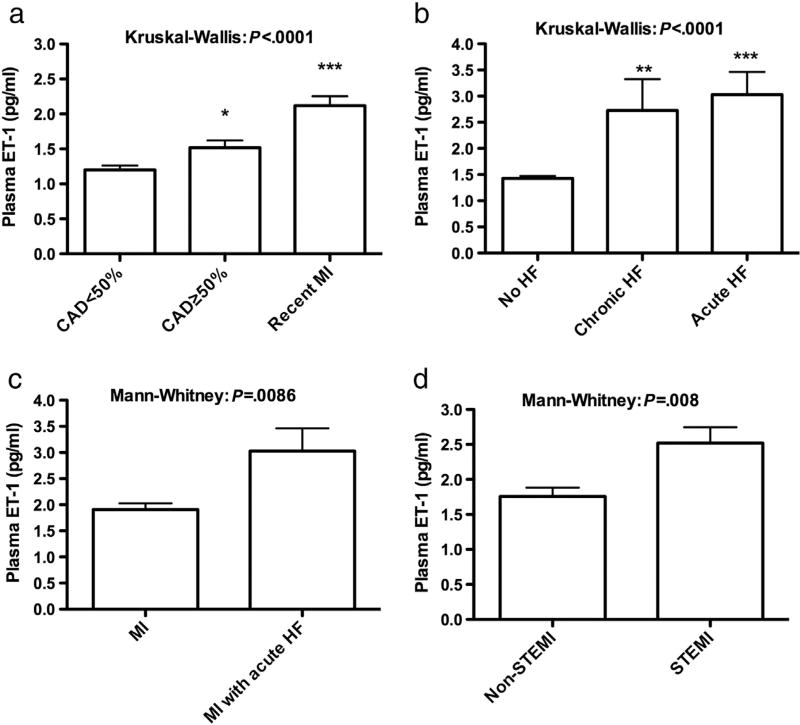
Plasma ET-1 levels in recent MI patients were>(CAD stenosis≥50%)>(CAD stenosis<50%); (2.12±0.13, 1.51±0.10, 1.20±0.06; 95% CI (1.85–2.38, 1.31–1.72, 1.07–1.32; respectively) P<.0001/Kruskal–Wallis/Dunn’s post-test (Fig. 1a). Plasma ET-1 was also associated with HF status (no HF=1.42±0.047, chronic HF=2.726±0.59, acute HF=3.028±0.43, 95% CI (1.33–1.52, 1.45–3.99, 2.11–3.98; respectively), P<.0001, Kruskal-Wallis) (Fig. 1b). Plasma ET-1 was higher in MI patients who presented with acute HF relative to MI patients without acute HF (3.028±0.43 vs. 1.9083±0.12, P=.0086) (Fig. 1c). Plasma ET-1 was higher in STEMI patients than non-STEMI (2.51±0.22 vs. 1.75±0.12, 95% CI (2.06–2.97 vs. 1.49–2.04), P=.008, Mann–Whitney test) (Fig. 1d). Plasma ET-1 was not different among MI patients who underwent early or delayed coronary angiography (2.15±0.18 vs. 2.11±0.19, 95% CI (1.77–2.52 vs. 1.72–2.50), P=.515, Mann–Whitney test) or among STEMI patients who received or did not receive thrombolytic agents (2.51±0.27 vs. 2.52±0.41, 95% CI (1.94–3.08 vs. 1.63–3.40), P=.798, Mann–Whitney test). With respect to interventions, pET-1 in patients scheduled for CABG was greater in patients who underwent PCI, and their pET-1 was greater than that in patients who received no intervention (2.65±0.74, 1.71±0.080, 1.33±0.05, 95% CI (1.05–4.26, 1.53–1.88, 1.22–1.45; respectively), P=.003, Kruskal–Wallis.
Figure 1.
Plasma ET-1 among patients with coronary artery disease. a) Plasma levels of ET-1 in patients with CAD<50%, CAD≥50%, and recent MI. * P<.01 CAD≥50% vs. CAD<50%, ***P<.0001 MI vs. CAD≥&<50%, Kruskal Wallis/Dunns post-test). b) Plasma ET-1 among patients with no heart failure (HF), chronic HF, and acute HF, **P<.001 chronic HF vs. no HF, ***P<.0001 acute HF vs. no HF (Kruskil Wallis/Dunns post-test). c) Plasma ET-1 among MI patients with/without acute HF. d) Plasma ET-1 in patients with STEMI and non-STEMI.
Plasma ET-1 was positively correlated with age (Spearman’s r=0.1987, P=.0002) and in a subset of patients (n=152) with neutrophils/lymphocytes ratio (Spearman’s r=0.2786, P=.0033). pET-1 was also positively correlated with the highest degree of stenosis in the main coronaries in all patients including MI patients with 100% occlusion (r=0.3204, P=<0.0001), and among CAD and non-CAD patients (r=0.2074, P=.0012). PET-1 was negatively correlated with LVEF (Spearman’s r=−0.327, P<.0001). Plasma ET-1 was also correlated with indexed left atrial size, smoking, age, use of statins, aspirin, angiotensin receptor blockers (ARBs), and beta blockers (Table 2). Plasma ET-1 was not associated with history of diabetes mellitus or use of angiotensin converting enzyme inhibitors (ACEi).
4.3. Baseline independent predictors of plasma ET-1
Multivariate analysis was performed to identify independent predictors of pET-1. To account for the increase in plasma ET-1 in MI with acute HF, CAD status was coded as CAD<50%, CAD≥50%, MI, MI with acute HF. By multivariate analysis, history of chronic HF and preoperative LVEF were both significantly associated with the square root of pET-1. As LVEF is co-linear with HF and CAD status, it was not presented in the multivariate model (Table 3). However, adjusted to variables presented in Table 3 but excluding CAD status, pET-1 was negatively correlated with LVEF (P<.0001). By step-wise analysis, diabetes, HT, and the use of ARBs/ACEi were not correlated with pET-1, and were excluded from the final model. Multivariate analysis showed that MI status with acute HF, MI without acute HF, age, current smoking, history of chronic HF, and need for revascularization by CABG were significantly associated with increased pET-1 (Table 3). Interestingly, this analysis revealed that patients on statins had lower pET-1 than patients not on statin (P=.0018). In a similar multivariate model, use of aspirin or beta blockers was also associated with lower pET-1. However, this relation was masked by CAD status and by statin use. Other medications were not associated with pET-1 and thus were excluded from the model. By step-wise analysis, indexed left atrial size was associated with pET-1 (P=.0005). As indexed left atrial size was co-linear with age, smoking, and HF, it was not included in the final model to avoid masking/underestimating the relation of pET-1 to these predictors.
Table 3.
Multivariate factors associated with baseline plasma ET-1 content
| Response=Square root of baseline plasma ET-1 | ||||
|---|---|---|---|---|
|
| ||||
| Estimate (slope, B) |
Confidence Interval (95%) |
P value | Standardized coefficient (β) |
|
| Age, years | 0.0080 | 0.0045–0.0114 | <0.0001* | 4.56 |
| Female Gender | −0.0033 | 0.0908–0.0842 | 0.9400 | −0.08 |
| BMI | 0.0049 | −0.0015–0.0115 | 0.1368 | 1.49 |
| MI with acute HF | 0.4414 | 0.2710–0.6171 | <0.0001* | 5.05 |
| MI without acute HF | 0.1772 | 0.0770–0.27774 | 0.0006* | 3.48 |
| CAD≥50% | 0.0393 | −0.0509–0.1296 | 0.3916 | 0.86 |
| History of chronic heart failure | 0.2098 | 0.0644–0.3553 | 0.0048* | 2.84 |
| Smoking | 0.1702 | 0.08941–0.2510 | <0.0001* | 4.14 |
| CABG | 0.2430 | 0.0601–0.4259 | 0.0094* | 2.61 |
| Use of statins | −0.0988 | −0.1728 to −0.0237 | 0.0099* | −2.59 |
CAD status was analyzed using CAD<50% as a reference, CABG: coronary artery bypass surgery. β is the standardized coefficient (slope/std error).
4.4. Independent predictors of baseline plasma ET-1 at 30-day follow up
A clinical follow-up evaluation was performed in 152 patients who were able to return to hospital at one month after catheterization (46 with MI, 72 with CAD≥50%, 34 with CAD<50%). This fraction of patients was comprehensive and the participants were not selected by the investigators. 10 patients who were scheduled for CABG underwent the surgery at our institution within a week after catheterization; none of these died before the 30-day follow-up. None of the included patients underwent PCI before the 30-day follow-up. At 1-month follow-up, three patients needed coronary reperfusion; one presented with recurrent MI (pET-1: 4.98pg/ml) and two presented with recurrent stenosis requiring PCI (pET-1: 0.92, 4.16pg/ml). Two patients who presented with recurrent angina underwent angiography without need for PCI (pET-1: 1.12, 1.20pg/ml). By multivariate analysis, baseline pET-1 was negatively associated with LVEF at 30 days (P<.0001, data not shown).
A multivariate analysis was also performed to evaluate the relation of baseline pET-1 to the % change in LVEF at 30-day follow-up. In the overall group, the increase in LVEF was small and non-significant (mean % increase in LVEF in all patients: 2.53%±1.37%, P=.107). However, the increase in LVEF was significant for MI patients relative to CAD≥50% and non-CAD patients (8.35%±2.7, −0.95%±2.22, 1.32% ±0.61; 95% CI (2.82–13.88 vs.−5.40–3.48 vs. 0.07–2.57; respectively, P=.016, Kruskall–Wallis). Use of medications post catheterizations was not associated with increase in LVEF at 1 month by either univariate or multivariate analysis (data not shown). By univariate analysis, pET-1 was positively associated with increase in LVEF at 30 days (r=0.34, P<.0001; Table 2), and this correlation was most evident among MI patients (r=0.46, P=.0017). However, by multivariate analysis adjusting to baseline LVEF and other factors, pET-1 was not associated with significant increase in LVEF at 1 month (P=.1587, Table 4).
Table 4.
Relation of baseline plasma ET-1 to the change in LVEF at 1-month follow-up
| Response=Square root of baseline plasma ET-1 | ||||
|---|---|---|---|---|
|
| ||||
| Estimate (slope, B) |
Confidence Interval (95%) |
P value | Standardized coefficient (β) |
|
| Age, years | 0.0078 | 0.0023–0.01325 | 0.0053* | 2.83 |
| Female Gender | 0.1650 | 0.0136–0.3164 | 0.033* | 2.16 |
| BMI | 0.0007 | −0.0105–0.0119 | 0.9007 | 0.12 |
| Baseline LVEF | −0.0173 | −0.0239 to −0.0107 | <0.0001* | −5.21 |
| % Increase in LVEF after 30days | 0.0029 | −0.0012–0.0071 | 0.1587 | 1.42 |
| Smoking | 0.3084 | 0.1729–0.4440 | <0.0001* | 4.50 |
LVEF: left ventricular ejection fraction. % Increase in LVEF=((LVEF at 30days−LVEF at baseline)/LVEF at baseline)*100. β is the standardized coefficient (slope/std error).
5. Discussion
Vascular endothelin-1 production is enhanced in the setting of venous congestion and endothelial dysfunction, even in healthy individuals, leading to neurohormonal, inflammatory and autonomic activation [13]. ET-1 is produced by endothelial cells of the coronary arteries, as well as by macrophages, endothelial cells, fibroblasts and cardiac myocytes [3,9]. Plasma ET-1 levels are higher in CAD patients than in healthy individuals [4]. Immunoreactive ET-1 staining is more abundant in atherosclerotic than in healthy coronary arteries, especially in macrophage- rich plaques [14].
Elevated pET-1 has been associated with worse clinical outcome and reperfusion injury following PCI in STEMI patients who presented within 12h of symptom onset [15]. However, it is unknown if pET-1 is different among patients with other clinical manifestations of CAD. Here, we documented elevated pET-1 in patients with recent MI who presented within a week following onset of symptoms relative to patients with/without CAD, correlating with risk classification. MI patients presenting with acute HF had higher pET-1 levels relative to those without acute LV failure, suggesting that ET-1 may contribute to ventricular failure during MI. We found that pET-1 levels were higher in STEMI than non-STEMI patients. STEMI patients have larger infarcts and higher mortality rates than non-STEMI patients [1].
A large body of experimental data implicates ET-1 as a key player involved in the pathophysiology of CAD at various stages of the disease, rather than acting merely as a passive marker. In a study evaluating the contribution of ET-1 to coronary artery tone, it was found that the ET-1 antagonist BQ-123 had a greater vasorelaxant effect in atherosclerotic than in normal coronary arteries, and even greater impact in regions of coronary artery stenosis; while ET-1 was estimated to account for 39% of vasotone in normal coronaries, it accounted for 74% in atherosclerotic arteries and 106% in areas of stenosis [16]. The association of pET-1 with CAD severity suggests that ET-1 in atherosclerosis coronaries actively contributes to regional ischemia. Here we show that pET-1 is also correlated with the degree of coronary stenosis, the circulating neutrophil/lymphocyte ratio, and the need for CABG, further supporting a role for ET-1 in the pathophysiology of CAD.
A significant increase in pET-1 was observed in MI patients relative to CAD and non-CAD patients. Whether pET-1 was present at the time of initial diagnosis and contributed to the etiology of MI, or it became manifest with time in predisposed MI patients cannot be determined from this study. Thus, ET-1 released by the diseased coronaries and the myocardium may result in intense and sustained coronary constriction resulting in increased afterload and reduced myocardial perfusion predisposing into myocardial injury and low LVEF [17–19].
Expression of ET-1 mRNA is enhanced both by ischemia, via HIF1α [20], and by increased wall stress [21], suggesting that plasma ET-1 levels might vary in perfused vs. non-perfused MI patients relative to the onset of symptoms. However, we found that pET-1 was similar among MI patients, regardless of the time of onset of symptoms relative to the sampling time, and irrespective of thrombolytic therapy use. This could be due to the inclusion of MI patients with acute HF, as acute ventricular failure has been shown to be associated with increased plasma ET-1 for several days post MI [22], whereas others suggested that pET-1 persists several days post MI [23]. This finding is limited by the small number and the heterogeneous clinical background of the MI patients in this study. To confirm this, larger studies are needed to measure serial plasma ET-1 levels over time in the same MI patients.
LVEF is both an important determinant of cardiac function and a predictor of morbidity and mortality [24]. Here we report that elevated pET-1 is associated with both chronic and acute HF, and with low LVEF at baseline and at 30-day follow-up, suggesting that elevated pET-1 might contribute to reduced LVEF probably by attenuating coronary artery blood flow. On the other hand, the increase in pET-1 might occur following reduction in LVEF as compensatory response to possibly maintain blood pressure in the face of reduced cardiac output. Plasma levels of pro-endothelin peptide (a precursor of ET-1) 3–5 days post-MI have been shown to predict the development of HF and mortality [25].
While ET-1 might be only another marker of disease activity and/or risk, the diversity of coronary conditions and relative levels of pET-1 suggest that ET-1 is likely involved in the basic mechanism of atherosclerosis. Previous studies suggested that ET-1 promotes progression of CAD and contributes to ventricular remodeling and fibrosis in the long-term post MI [17]. However, it is unclear from our study if pET-1 is associated with disease progression. The persistence of plasma ET-1 in patients who presented with in a week of MI and the higher pET-1 levels in STEMI patients who are at increased risk of aneurysm formation relative to the non-STEMI patients suggest that ET-1 may have a role post MI. We evaluated the prognostic significance of pET-1 shortly at 30-day follow-up and we observed an increase in LVEF in MI patients indicating an increase in muscle perfusion. Although plasma ET-1 was positively associated with the increase in LVEF among MI patients by univariate analysis, this relation disappeared adjusting to baseline LVEF [2]. This finding is limited by the small number of patients evaluated at follow-up. Further studies with larger sample size and longer duration are warranted.
It has recently been reported that use of an ET-1 antagonist during primary PCI in STEMI patients was safe and might improve LVEF and tissue perfusion [6,8]. Use of ET-1 antagonists might serve as a protective adjunctive therapy to reperfusion therapy in acute MI patients [26,27. The data presented here do not provide direct evidence supporting ET-1 antagonist treatment, but rather offer new insights regarding the significance of ET-1 in the etiology of CAD.
In preclinical studies, we reported that dietary fish oil reduced atrial and pET-1 in a canine cardiac surgery model [28], and that statin use was associated with lower abundance of atrial ET-1 in cardiac surgery patients [3]. Here, we show that statin use is also associated with lower levels of pET-1. Concordant with our finding, others have also found that statin use reduced pET-1 [29]. Recent changes in the AHA/ACC guidelines seem likely to increase statin use [30].
We also recently reported that left atrial ET-1 content is strongly associated with left atrial diameter and volume [3]. Here we report that pET-1 levels are also correlated with indexed left atrial size, suggesting that some pET-1 may have a cardiac origin.
Common risk factors for developing CAD include diabetes and smoking, likely due to their impact on endothelial function [31]. Additional risk factors, such as advanced age may place patients with preexisting CAD at increased risk for MI [31]. In this study, elevated pET-1 levels were significantly associated with both advanced age and smoking, independent of CAD status. This overlap in plasma ET-1 among CAD patients and underlying risk factors may limit the diagnostic value of pET-1. Alternatively, the overlap may suggest that age and smoking dependent increases in ET-1 contribute to increased risk of CAD and MI making ET-1 a potential useful risk marker or therapeutic target in this population.
5.1. Study limitations
This study has several limitations. The inclusion of patients who underwent both early and delayed coronary angiography who presented within a week of MI and received medical therapies might have influenced pET-1 levels. However, pET-1 was similar among MI patients who underwent early or delayed catheterization, irrespective of the use of thrombolytic therapy. In addition, we cannot rule out the occurrence of transient coronary vasospasm or presence of microvascular obstruction (as in syndrome X) in patients with CAD<50% who presented with angina symptoms that could not be explained by normal main coronaries. However, relative to patients with CAD≥50% or MI, this group has the lowest risk of ACS. Future studies are warranted that compare pET-1 among patients with different manifestations of CAD with that of healthy individuals. While this study demonstrates a strong association of pET-1 with CAD status, it does not prove a causal role of ET-1 as a mediator of coronary atherosclerosis or MI.
5.2. Conclusions
ET-1 is a potent vasoconstrictor, inflammatory mediator and mitogen. Here, plasma ET-1 levels were associated with the severity of CAD and the need for CABG, suggesting that ET-1 may contribute to endothelial dysfunction, CAD severity, and risk of MI. The association of pET-1 with low LVEF and HF further suggests that elevated ET-1 may contribute to ventricular dysfunction. Targeted use of interventions that either reduce ET-1 production (e.g. statins or fish oil) or block its receptors (e.g. ET-1 antagonists) may limit MI vulnerability or related morbidity. This study may help with the selection of patients who are at greatest risk of MI, and calls for studies evaluating the potential benefit from using adjunctive treatments to reperfusion therapy that reduce pET-1.
Acknowledgments
Funding: This study was supported by a grant from deanship of research at Jordan University of Science and Technology, Irbid, Jordan.
Footnotes
Conflict of Interest: Fadia Mayyas, Mohammad Al-Jarrah, Khalid Ibrahim, Doaa Mfady, and David Van Wagoner declare no conflict of interest.
Human subjects/Informed consent statement: All patients in the study provided written informed consent, and the study protocol and all procedures were approved by the Institutional Review Board of KAUH and comply with Helsinki Declaration of 1975, as revised in 2000.
References
- 1.Fitchett DH, Theroux P, Brophy JM, Cantor WJ, Cox JL, Gupta M, Kertland H, Mehta SR, Welsh RC, Goodman SG. Assessment and management of acute coronary syndromes (ACS): a Canadian perspective on current guideline-recommended treatment—part 2: ST-segment elevation myocardial infarction. Can J Cardiol. 2011;27(Suppl. A):S402–12. doi: 10.1016/j.cjca.2011.08.107. [DOI] [PubMed] [Google Scholar]
- 2.Abraham D, Dashwood M. Endothelin—role in vascular disease. Rheumatology (Oxford) 2008;47(Suppl. 5):v23–4. doi: 10.1093/rheumatology/ken282. [DOI] [PubMed] [Google Scholar]
- 3.Mayyas F, Niebauer M, Zurick A, Barnard J, Gillinov AM, Chung MK, Van Wagoner DR. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol. 2010;3:369–79. doi: 10.1161/CIRCEP.109.924985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chai SB, Li XM, Pang YZ, Qi YF, Tang CS. Increased plasma levels of endothelin-1 and urotensin-II in patients with coronary heart disease. Heart Vessels. 2010;25:138–43. doi: 10.1007/s00380-009-1178-6. [DOI] [PubMed] [Google Scholar]
- 5.Shirai N, Naruko T, Ohsawa M, Ikura Y, Sugama Y, Hirayama M, Kitabayashi C, Ehara S, Inoue T, Itoh A, Haze K, Tanzawa K, Yoshiyama M, Yoshikawa J, Ueda M. Expression of endothelin-converting enzyme, endothelin-1 and endothelin receptors at the site of percutaneous coronary intervention in humans. J Hypertens. 2006;24:711–21. doi: 10.1097/01.hjh.0000217854.97369.8c. [DOI] [PubMed] [Google Scholar]
- 6.Freixa X, Heras M, Ortiz JT, Argiro S, Guasch E, Doltra A, Jimenez M, Betriu A, Masotti M. Usefulness of endothelin-1 assessment in acute myocardial infarction. Rev Esp Cardiol. 2011;64:105–10. doi: 10.1016/j.recesp.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Jain D, Schafer U, Dendorfer A, Kurz T, Lindemann C, Tolg R, Hartmann F, Katus HA, Richardt G. Neurohumoral activation in percutaneous coronary interventions: apropos of ten vasoactive substances during and immediately following coronary rotastenting. Indian Heart J. 2001;53:301–7. [PubMed] [Google Scholar]
- 8.Stewart DJ, Kubac G, Costello KB, Cernacek P. Increased plasma endothelin-1 in the early hours of acute myocardial infarction. J Am Coll Cardiol. 1991;18:38–43. doi: 10.1016/s0735-1097(10)80214-1. [DOI] [PubMed] [Google Scholar]
- 9.Fukuchi M, Giaid A. Expression of endothelin-1 and endothelin-converting enzyme-1 mRNAs and proteins in failing human hearts. J Cardiovasc Pharmacol. 1998;31(Suppl. 1):S421–3. doi: 10.1097/00005344-199800001-00120. [DOI] [PubMed] [Google Scholar]
- 10.Proposal for the multinational monitoring of trends and determinants in cardiovascular disease. Geneva: Cardiovascular Disease Unit of WHO World Health Organization criteria for the diagnosis of acute myocardial infarction; 1981. [Google Scholar]
- 11.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC. 2011 ACCF/AHA focused update incorporated Into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 12.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1) N Engl J Med. 1992;326:242–50. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 13.Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, Lejemtel TH, Bucciarelli L, Kebschull M, Papapanou P, Uriel N, Schmidt AM, Sabbah HN, Jorde UP. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J. 2014;35:448–54. doi: 10.1093/eurheartj/eht456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeiher AM, Goebel H, Schachinger V, Ihling C. Tissue endothelin-1 immunoreactivity in the active coronary atherosclerotic plaque. A clue to the mechanism of increased vasoreactivity of the culprit lesion in unstable angina. Circulation. 1995;91:941–7. doi: 10.1161/01.cir.91.4.941. [DOI] [PubMed] [Google Scholar]
- 15.Eitel I, Nowak M, Stehl C, Adams V, Fuernau G, Hildebrand L, Desch S, Schuler G, Thiele H. Endothelin-1 release in acute myocardial infarction as a predictor of long-term prognosis and no-reflow assessed by contrast-enhanced magnetic resonance imaging. Am Heart J. 2010;159:882–90. doi: 10.1016/j.ahj.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Kinlay S, Behrendt D, Wainstein M, Beltrame J, Fang JC, Creager MA, Selwyn AP, Ganz P. Role of endothelin-1 in the active constriction of human atherosclerotic coronary arteries. Circulation. 2001;104:1114–8. doi: 10.1161/hc3501.095707. [DOI] [PubMed] [Google Scholar]
- 17.Kolettis TM, Barton M, Langleben D, Matsumura Y. Endothelin in coronary artery disease and myocardial infarction. Cardiol Rev. 2013;21:249–56. doi: 10.1097/CRD.0b013e318283f65a. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Farre A, Caramelo C, Esteban A, Alberola ML, Millas I, Monton M, Casado S. Effects of aspirin on platelet-neutrophil interactions. Role of nitric oxide and endothelin-1. Circulation. 1995;91:2080–8. doi: 10.1161/01.cir.91.7.2080. [DOI] [PubMed] [Google Scholar]
- 19.Ozdemir R, Parlakpinar H, Polat A, Colak C, Ermis N, Acet A. Selective endothelin a (ETA) receptor antagonist (BQ-123) reduces both myocardial infarct size and oxidant injury. Toxicology. 2006;219:142–9. doi: 10.1016/j.tox.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2008;294:L309–18. doi: 10.1152/ajplung.00091.2007. [DOI] [PubMed] [Google Scholar]
- 21.Loennechen JP, Stoylen A, Beisvag V, Wisloff U, Ellingsen O. Regional expression of endothelin-1, ANP, IGF-1, and LV wall stress in the infarcted rat heart. Am J Physiol Heart Circ Physiol. 2001;280:H2902–10. doi: 10.1152/ajpheart.2001.280.6.H2902. [DOI] [PubMed] [Google Scholar]
- 22.Battistelli S, Billi M, Manasse G, Vittoria A, Roviello F, Forconi S. Behavior of circulating endothelin-1 in a group of patients with acute myocardial infarction. Angiology. 1999;50:629–38. doi: 10.1177/000331979905000803. [DOI] [PubMed] [Google Scholar]
- 23.Vojacek J, Kolar J, Lisy O, Hrabos V, Simek S, Jindra A, Jachymova M. Time course of endothelin-1 plasma level in patients with acute coronary syndromes. Cardiology. 1999;91:114–8. doi: 10.1159/000006890. [DOI] [PubMed] [Google Scholar]
- 24.King M, Kingery J, Casey B. Diagnosis and evaluation of heart failure. Am Fam Physician. 2012;85:1161–8. [PubMed] [Google Scholar]
- 25.Khan SQ, Dhillon O, Struck J, Quinn P, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL. C-terminal pro-endothelin-1 offers additional prognostic information in patients after acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) Study. Am Heart J. 2007;154:736–42. doi: 10.1016/j.ahj.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Leskela HV, Vuolteenaho O, Koivula MK, Taskinen P, Ruskoaho H, Peltonen T, Lehenkari P. Tezosentan inhibits uptake of proinflammatory endothelin-1 in stenotic aortic valves. J Heart Valve Dis. 2012;21:23–30. [PubMed] [Google Scholar]
- 27.Tamareille S, Terwelp M, Amirian J, Felli P, Zhang XQ, Barry WH, Smalling RW. Endothelin-1 release during the early phase of reperfusion is a mediator of myocardial reperfusion injury. Cardiology. 2013;125:242–9. doi: 10.1159/000350655. [DOI] [PubMed] [Google Scholar]
- 28.Mayyas F, Sakurai S, Ram R, Rennison JH, Hwang ES, Castel L, Lovano B, Brennan ML, Bibus D, Lands B, Barnard J, Chung MK, Van Wagoner DR. Dietary omega3 fatty acids modulate the substrate for post-operative atrial fibrillation in a canine cardiac surgery model. Cardiovasc Res. 2011;89:852–61. doi: 10.1093/cvr/cvq380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Minami E, Letterer RA, Lawler RL, McDonald GB, Levy WC. The effects of atorvastatin (10mg) on systemic inflammation in heart failure. Am J Cardiol. 2005;96:1699–704. doi: 10.1016/j.amjcard.2005.07.092. [DOI] [PubMed] [Google Scholar]
- 30.Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PW. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 31.Davidson MH. Cardiovascular risk factors in a patient with diabetes mellitus and coronary artery disease: therapeutic approaches to improve outcomes: perspectives of a preventive cardiologist. Am J Cardiol. 2013;110:43B–9B. doi: 10.1016/j.amjcard.2012.08.033. [DOI] [PubMed] [Google Scholar]