A recent headline on theHeart.org was “Western Diet Increases MI Risk Worldwide.” In the past 30 years, it has become apparent that dietary fatty acids have a profound impact on the composition of plasma and cardiovascular tissue lipid pools, and as a result, on the risks of cardiovascular disease. Although significant progress has been made to reduce the incidence of death caused by coronary heart disease, it still afflicts ~450 000 patients per year in the United States, with many of these dying from cardiac arrhythmias.1 Atrial fibrillation (AF), the most common arrhythmia, afflicts more than 2.2 million Americans. It has been estimated that more than 12 million Americans will have AF by 2050 because of the aging of the population as well as the increasing incidence of diabetes and obesity, both risk factors for AF.1 Reasons underlying the increased prevalence of these acquired diseases are complex, involving societal changes in diet, lifestyle, and physical activity. Efforts to address these risk factors seem likely to reduce the burden of cardiac arrhythmia and cardiovascular disease. Although all are important, this review focuses on the relationship between dietary fatty acids and mechanisms of cardiac arrhythmogenesis.
Dietary Fatty Acids
What Fatty Acids Are Present in the Diet?
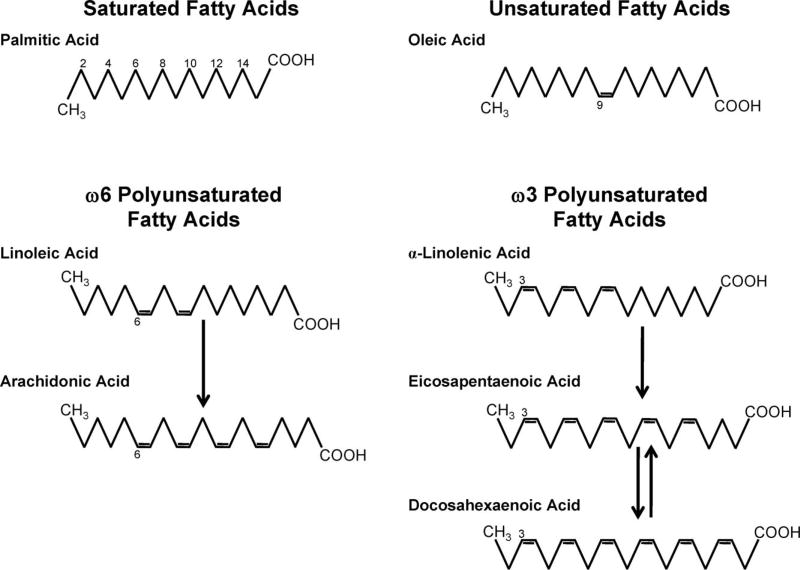
As shown in Figure 1, fatty acids consist of a straight chain of carbon atoms with a carboxylic end (COOH) and a methyl (CH3) or omega end and are classified based on the saturation of the carbon chain. Common saturated fatty acids, those with no double bonds, include palmitic acid (16:0) and stearic acid (18:0). Foods high in saturated fatty acids include dairy products, red meats, and tropical oils.2 Unsaturated fatty acids are further classified based on the number and location of double bonds. Monounsaturated fatty acids, such as oleic acid (18:1n9), have a single double bond, whereas polyunsaturated fatty acids (PUFA) have multiple double bonds. ω6 PUFA, such as linoleic acid (LA, 18:2n6) and arachidonic acid (AA, 20:4n6), have the first double bond located at the sixth carbon (when counting from the omega end) and are found readily in dietary sources such as vegetable oils, meat, eggs, and dairy. ω3 PUFA, such as α-linolenic acid (ALA, 18:3n3), eicosapentaenoic acid (EPA, 20:5n3), and docosahexaenoic acid (DHA, 22:6n3), have the first double bond located at the third carbon. Although ALA is found in flax seed and other plants, EPA and DHA are primarily found in fatty fish, such as salmon.2
Figure 1.
Chemical structure of saturated, monounsaturated, and polyunsaturated fatty acids. Fatty acids are straight chains of carbon atoms with a carboxylic end (COOH) and a methyl, or omega, end (CH3). Saturated fatty acids, such as palmitic acid (16:0), have no double bonds. Oleic acid (18:1n9), an 18-carbon monounsaturated fatty acid, has 1 double bond on the ninth carbon when counting from the omega end. ω6-polyunsaturated fatty acids, such as linoleic acid (18:2n6) and arachidonic acid (20:4n6), have the first double bond at the sixth carbon from the omega end. Similarly, ω3-polyunsaturated fatty acids, such as α-linolenic acid (18:3n3), eicosapentaenoic acid (20:5n3), and docosahexaenoic acid (22:6n3), have the first double bond at the third carbon from the omega end.
How Are Dietary Fatty Acids Used and Stored by the Body?
Dietary fatty acids are metabolized as fuel for oxidative phosphorylation, stored as triglycerides, or rapidly incorporated into plasma phospholipids, high-density lipoprotein particles, and cell membranes. Fatty acids seldom exist in a “free” form; nonesterified fatty acids are bound by plasma albumin. The mass of lipids incorporated into the various lipid pools limits the kinetics of turnover. Plasma triglyceride composition can be modified within days of a dietary modification, but changes in cardiac tissue lipid composition resulting from dietary changes require several weeks to reach steady state. In patients scheduled for cardiac surgery (with low dietary fish intake), a 1-month treatment with fish oil (2.9% energy as EPA and DHA, 3 g/d) raised the content of those lipids in the right atrial appendage (removed at surgery) from 5.3% to 11.5%, and decreased the AA content from 21% to 16%.3 Interestingly, dietary supplementation with energy equivalent quantities of ALA or olive oil had no significant impact on cardiac lipid composition.3 Experimental studies also show that diets enriched with long-chain ω3 PUFA lead to ω3 PUFA incorporation into cardiac tissues.4,5
Epidemiological Data Show That Dietary Fatty Acids Affect Cardiovascular Health
Epidemiological studies suggest that the composition of dietary fatty acids (eg, saturated versus unsaturated; ω3 versus ω6, etc) has important consequences for cardiovascular health and cardiac arrhythmogenesis.6 Saturated and trans-fats increase cardiovascular risk.6 Both ω3 and ω6 PUFA have shown some evidence of cardiovascular benefit.7,8 Regional and ethnic differences in food availability and preference result in significant variations in dietary fatty acid composition. Hibbeln et al8 reported an inverse relationship between dietary ω3 PUFA intake and mortality resulting from cardiovascular disease, with the lowest mortality reported in countries such as Japan, Greenland, and Iceland, whose citizens have the highest proportion dietary lipid calories derived from ω3 PUFA. Interestingly, the proportion of dietary calories derived from fat was high in Greenland and relatively low in Japan, yet both countries showed decreased cardiovascular risk. Although dietary ALA is more readily available (from plant-based sources), evidence is stronger for a cardiovascular benefit of EPA and DHA than for ALA.9 Among the ω6 PUFA, a recent AHA advisory cites several epidemiological and prospective cohort studies showing that individuals with the highest tissue/blood levels of LA had the lowest cardiovascular risk.7
AHA Guidelines for Dietary Fats
The American Heart Association (AHA) recognizes that dietary fatty acids and cardiovascular disease risk are interrelated. Current AHA Dietary Guidelines recommend limiting total fat intake to <35% of daily calories, with saturated fat <7% of daily calories, and the remainder coming from monounsaturated and polyunsaturated fats.2 It is intriguing, however, that the Women’s Health Initiative reported that a low-fat diet did not significantly affect cardiovascular disease incidence and only modestly altered the risk factors for cardiovascular disease.10
Stress-Dependent Effects of Dietary Fats
The impact of dietary fatty acids on cardiovascular function under normal conditions may differ from that under conditions of hemodynamic, ischemic, or autonomic stress. Under normal conditions, fatty acids are used for many cellular processes; however, when lipid availability exceeds the capacity for utilization, fatty acids can alter mitochondrial structure11 and function, increasing lipid peroxidation,12 mitochondrial uncoupling, and reactive oxygen species production,13 eventually leading to cytochrome c release, caspase activation, DNA laddering, and apoptosis.14,15 Despite evidence showing that dietary fat can be cytotoxic, dietary fat appears cardioprotective in several animal models of left ventricular dysfunction. Studies in the Dahl salt-sensitive rat model of hypertension-induced cardiomyopathy,16 a mouse model of transverse aortic constriction,17 and a rat model of abdominal aortic banding18 have shown that 60% high saturated fat feeding did not exacerbate the hypertrophic response to injury. A 60% high saturated fat diet in a rat model of coronary artery ligation–induced heart failure also did not adversely affect myocardial contractile function but increased mitochondrial enzyme activities and oxidative phosphorylation.19,20 These alterations in mitochondrial function were not evident in sham animals fed high saturated fat,19 suggesting that the effects of a high fat diet represented responses to pathological stress. In a subsequent study, mitochondrial oxidative phosphorylation was unaltered in rats fed a high fat diet after ligation surgery but was decreased in sham animals fed the high fat diet.21 However, the proarrhythmic consequences of such a diet are notable; rats fed the same high fat diet before coronary artery ligation had an increased risk of sudden death early after myocardial infarction.20 These studies suggest that manipulation of dietary fat content and composition can have different effects under normal versus pathological conditions and that ischemic and hemodynamic stressors can modify the outcome.
Arrhythmogenic Mechanisms Affected by Dietary Fatty Acids
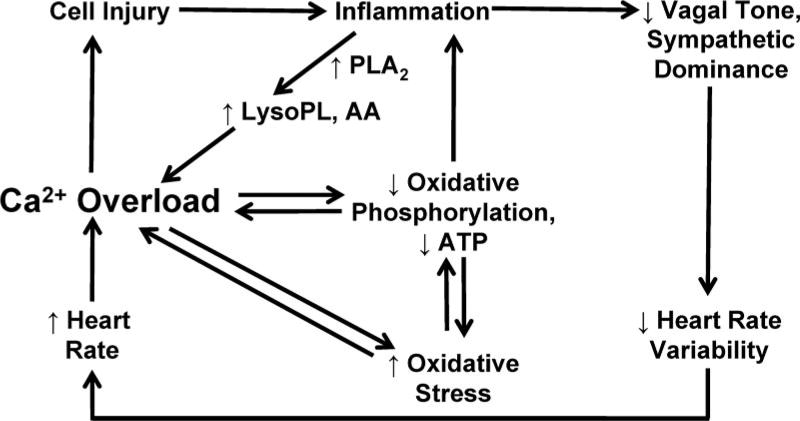
Dietary fatty acids can promote and/or prevent cardiac arrhythmia via several pathways (Figure 2), including (1) modulation of electrophysiological and metabolic heterogeneities secondary to atherosclerotic disease, (2) modulation of cardiac myocyte metabolic activity and cardiovascular oxidant stress, (3) direct modulation of ion channel and transporter activity, (4) indirect modulation of ion channel and transporter activity, via modulation of autonomic nervous system activity, and (5) modulation of inflammatory pathways that promote ectopic electric activity and abnormal conduction. These mechanisms are considered in the paragraphs below.
Figure 2.
Pathways underlying lipid modulation of cardiac arrhythmogenesis. Lipids can promote cell injury and inflammation, which can increase lysophospholipids (lysoPL) through enhanced phospholipase A2 (PLA2) activity. Dyslipidemia also leads to loss of vagal tone and sympathetic dominance, resulting in decreased heart rate variability and increased heart rate. These effects promote myocyte calcium overload, which amplifies cellular injury. Calcium overload also promotes oxidative stress and inhibition of mitochondrial oxidative phosphorylation.
1: Dietary Fatty Acids, Atherosclerosis, and Arrhythmogenesis
Elevated blood cholesterol and triglycerides are associated with increased risk for cardiovascular disease.1 Although dietary saturated fat increases cardiovascular risk,6 ω3 PUFA have been shown to decrease plasma triglyceride content16,22,23 and cardiovascular risk. One potential antiarrhythmic mechanism involves modulation of the extent of atherosclerosis and subsequent cardiac ischemia.
The impact of dietary fatty acid composition on the development of atherosclerosis was recently evaluated in 3 different populations: Japanese men living in Japan, American men, and men of Japanese origin living in the United States.24 The Japanese men living in Japan consumed a diet more enriched in ω3 PUFA than the American diet and had less atherosclerosis, with a significant inverse relationship between serum ω3 PUFA levels and carotid intima-medial thickness.24 Japanese men living in the United States had more atherosclerosis than either the native Japanese or American men, suggesting that genetic factors do not underlie this relationship.24 This and other studies suggest that consumption of a diet enriched in ω3 PUFA is antiatherogenic. The GISSI Prevenzione trial reported that consumption of a Mediterranean diet supplemented with 1 g per day of ω3 PUFA (but not vitamin E) was associated with a 45% reduction in the incidence of sudden cardiac death.25 Animal studies also have shown that dietary manipulation of lipid composition profoundly affects cardiac arrhythmogenesis. Pepe et al26 reported that animals fed a diet enriched with saturated fat had increased susceptibility to ventricular fibrillation and tachycardia after ischemia and reperfusion; fish oil supplementation reversed these effects. Although atherosclerosis-induced ischemia is an important element of arrhythmogenesis, it is not the only factor affected by dietary fatty acids.
2: Impact of Dietary Lipids on Cardiac Metabolism and Arrhythmogenesis
Arrhythmias frequently occur in the metabolically challenged heart, consistent with the hypothesis that metabolic instability underlies electric instability.27 Possible mediators include insufficient ATP for contractile and ion cycling requirements, lack of oxygen, lack of substrate availability, or impaired enzymatic activity.27 Fatty acids are the primary energy substrate in the healthy heart. With the development and progression of ventricular dysfunction, expression of the primary transcriptional regulator of fatty acid metabolism in the heart, peroxisome proliferator activated receptor-α (PPARα), and enzymes involved in fatty acid oxidation are downregulated.28–30
Studies in human31 and animal heart failure models have reported abnormalities in mitochondrial morphology,32 damage to the phosphorylation apparatus, and decreased mitochondrial respiration33–35 and electron transport chain activities.36,37 Atrial tissues from patients with AF show evidence of abnormal mitochondrial morphology,38 deletion of mitochondrial DNA segments,39 decreased oxidative phosphorylation,38 and increased proton leak.40 Changes in atrial mitochondrial structure similar to those that occur in heart failure seem likely to contribute to the progression of AF; however, alterations in atrial energetics during the progression of AF are currently less well characterized than in the failing ventricle.
Although the role of metabolic alterations in arrhythmogenesis is not well understood, there is a clear association between dietary lipids and metabolism. Specific actions of fatty acids can vary, depending on the composition of the fatty acid (saturation, chain length, etc). For example, fatty acids are natural ligands for PPARα, but long-chain unsaturated fatty acids are more effective ligands than long-chain saturated and short-chain fatty acids.41,42 Dietary PUFAs lower plasma16,22,23 and tissue triglycerides43; however, dietary ω3 PUFAs (EPA, DHA, and ALA) decrease serum triglycerides and phospholipids more effectively than LA (an ω6 PUFA).43 Supplementation of an ALA-enriched diet with EPA/DHA has been reported to further increase the expression of genes involved in mitochondrial biogenesis and fatty acid oxidation.44 It seems plausible that diets enriched in protective fatty acids (eg, LA, ALA, EPA, and DHA) could decrease metabolic stress and reduce the incidence of metabolically induced arrhythmias.
Oxidant Stress in Heart Failure and Arrhythmia
Failing hearts frequently show signs of oxidant stress,45,46 including lipid peroxidation, protein nitration, and other post-translational modifications induced by the interaction of reactive oxygen and nitrogen species with cellular proteins and lipids. Our group was the first to show evidence of oxidative stress in the atria of patients with AF,47 with increased nitrotyrosine abundance (a marker of peroxynitrite formation) in atria from AF patients. Others have shown that the redox state is more oxidized and that markers of oxidant stress are elevated in the plasma of patients with AF.48
Mitochondria are a major source of oxidant generation and an important target for oxidative damage. Cardiolipin, a phospholipid unique to the mitochondrial inner membrane, is susceptible to oxidative modification because of its highly unsaturated structure and its proximity to the electron transport chain.49 Because cardiolipin plays an essential role in the structure and activity of electron transport chain complexes, alterations in cardiolipin content have serious implications for mitochondrial energy production. The resulting inhibition of the electron transport chain can promote generation of reactive oxygen species at complexes I36 and III.37 Oxidant production also can result in modification of mitochondrial proteins. Reactive nitrogen species inhibit the activity of mitochondrial enzymes aconitase, catalase, and glutathione peroxidase, as well as various components of the electron transport chain.50
Oxidant stress is increased in patients after cardiac surgery. In a small, case-controlled study, serum total peroxide levels and right atrial protein oxidation at 6 hours after cardiac surgery were greater in patients who later had postoperative AF than in those who did not.51 A proteomic analysis of atrial tissues from surgical patients reported that patients who had postoperative AF also showed evidence of metabolic alterations and depletion of the antioxidant protein peroxiredoxin.52 Preservation of cardiac mitochondrial function, therefore, could be an important step toward preventing disease progression.
Mitochondrial oxidant generation is sensitive to dietary lipid composition. Experimentally, a cholesterol-rich diet promoted increased superoxide production, nitrotyrosine abundance, and cardiac dysfunction53; however, expression of cardiac antioxidants Mn-superoxide dismutase and glutathione peroxidase was enhanced in rats fed an ω3 PUFA-enriched (EPA and DHA) diet compared with those fed a saturated fat diet.54 Additionally, ω3 PUFA supplementation of a diet rich in saturated fats increased the efficiency of oxygen utilization and inhibited arrhythmias associated with ischemia and reperfusion.26 Together, these studies provide evidence that dietary ω3 PUFA enrichment may attenuate arrhythmia risk, in part by preserving mitochondrial function.
3: Modulation of Ion Channel and Transporter Activity
Intracellular sodium and calcium homeostasis is a critical determinant of arrhythmogenesis, and levels of these ions are coregulated by the activity of the sodium-calcium exchanger (NCX). NCX normally provides a brief period of calcium influx during the peak of the action potential and facilitates calcium extrusion during the action potential plateau. Increased intracellular sodium levels resulting from rapid heart rate or altered sodium channel inactivation impede NCX-mediated calcium extrusion. Although elevated cytosolic calcium can have a positive inotropic effect, calcium overload has metabolic,55 arrhythmogenic,56,57 and contractile58 consequences. In patients with AF, atrial NCX protein expression was increased by 67% relative to control patients with no history of AF.59 NCX current is sensitive to the lipid composition of the membrane and to plasma lipids. In porcine ventricular myocytes, dietary fish oil supplementation prevented calcium overload and reduced the incidence of triggered activity in response to norepinephrine exposure.60 Fatty acid block of NCX is isoform specific; whereas NCX1.1 is only blocked by ω3 PUFAs (EPA, DHA), multiple fatty acids can inhibit NCX1.3 currents.61 Modulation of calcium influx is an important element underlying the beneficial effects of ω3 PUFAs on cardiac electric activity.
Exposure of myocytes to oxidant stress is reported to increase reverse-mode NCX activity58 and protein expression,62 to delay the inactivation of the sodium current,63 and to modulate NCX current,64 in part secondary to the increased sodium load resulting from oxidant modified sodium channels.63 Delayed sodium channel inactivation, often referred to as “late” sodium current, has been documented in myocytes from failing hearts65 and in human atrial myocytes.66 Sodium entry via late sodium current can prolong the action potential plateau, resulting in increased intracellular sodium load, increasing risk of early afterdepolarizations and triggered and ectopic electric activity.67 Fish oil– derived ω3 PUFAs (EPA and DHA) suppress late sodium current in cells expressing recombinant human cardiac sodium channels.68
In the setting of ischemia, lipid metabolism is a critical modulator of cardiac electric activity. Under ischemic conditions, phospholipase A2 is activated, promoting the release of fatty acids such as AA (ω6 PUFA) and lysophospholipids from the cell membrane. In regional ischemia, AA and lysophospholipid release contribute to the development of proarrhythmic heterogeneities of conduction velocity and repolarization. AA can uncouple gap junctions,69 leading to conduction slowing, and modify the activity of voltage-dependent sodium, calcium, and potassium channels. AA metabolites can modify cardiac ion channel activity (isoketals70) and activate G-protein– coupled receptors (EP, FP71) that promote ectopic electric activity. Lysophospholipids also modulate ion channel and mitochondrial activity.72,73
The quantity of AA released during ischemia is dependent on its abundance in the cell membrane, which is sensitive to dietary fatty acid composition. In a canine acute infarction model, pretreatment with a diet enriched with ω3 PUFA attenuated the arrhythmogenic response to ischemia.74 Similarly, patients who had ventricular fibrillation during a first myocardial infarction were reported to have lower levels of ω3 incorporated into cell membranes than those who did not.75 Thus, the type of dietary fatty acids incorporated into cardiac membranes is an important determinant of the electrophysiological response of the heart to ischemia.
Antiarrhythmic Effects of Infusion/Superfusion Versus Dietary Incorporation of Fatty Acids
To probe the therapeutic benefit of modifying lipid composition on cardiac electric activity, several studies have evaluated the effects of acute infusion of lipid emulsions on experimentally induced arrhythmias. In a canine model of ischemia during exercise after myocardial infarction, Billman et al76 showed that infusion of an ω3 PUFA-enriched emulsion protected animals from sudden death caused by lethal ventricular fibrillation. Emulsions containing individual ω3 PUFAs (EPA, DHA, or ALA) were similarly protective.77 Antiarrhythmic efficacy was associated with a slower heart rate, shorter Q-T interval (corresponding to effects on ventricular action potential duration), reduced left ventricular systolic pressure, and prolonged atrial-ventricular conduction time (P-R interval of the ECG).76 In normal dogs given an acute infusion of either ω3 PUFA or ω6 PUFA, neither PUFA affected atrial effective refractory period (aERP), R-R interval, P-wave duration, P-Q interval, QRS duration, QT or QTc interval over a 6-hour period.78 However, after 6 hours of rapid atrial pacing, infusion with the ω3 PUFA but not the ω6 PUFA attenuated the characteristic pacing-induced abbreviation of aERP.75,78
Lipid infusion/superfusion may not accurately predict the cellular response to dietary fatty acids. In cultured neonatal cardiac myocytes, acute superfusion with DHA (ω3) but not AA (ω6) also slowed spontaneous beating rate, decreased calcium influx via L-type calcium channels, and attenuated the response of the calcium channel to the dihydropyridine agonist Bay K 8644.79 Dietary changes in ω3 PUFA consumption have a more subtle electrophysiological impact than the acute effects of lipid infusion or superfusion. Whereas superfusion of isolated myocytes with ω3 PUFAs (EPA) acutely suppressed sodium current,80 electrophysiological studies of ventricular myocytes isolated from pigs administered an ω3 PUFA–enriched diet for 8 weeks showed no evidence of altered sodium current density or voltage-dependent channel activation.81 Nonetheless, dietary administration of ω3 PUFA is associated with alterations in ion channel/exchanger activity. Consistent with the acute superfusion studies, a hyperpolarizing shift in sodium channel steady-state inactivation was observed in pigs fed an ω3 PUFA–enriched diet.81 Ventricular myocytes from these animals had attenuated NCX currents, an abbreviated action potential duration, and an ≈20% reduction in peak L-type calcium current density, with no change in voltage-dependent activation or inactivation parameters.81 Diastolic calcium levels and calcium transient amplitude were not altered in animals receiving the ω3 PUFA–enriched diet, but decay of the calcium transient was accelerated.81 Inward rectifier K+ current (IK1) and slow delayed rectifier K+ current (IKs) densities were increased.81 Overall, this study suggests that dietary ω3 PUFA supplementation shortens ventricular action potential duration, simultaneously decreasing the occurrence of early afterdepolarizations and triggered arrhythmic activity via altered action potential duration and changes in cytosolic calcium handling.
4: Autonomic Modulation of Ion Channel and Transporter Activity
Heart rate is controlled by parasympathetic (vagal) and sympathetic (β-adrenergic) nerves innervating the sinoatrial and atrioventricular nodes. Excessive stimulation of either parasympathetic or sympathetic nerves promotes arrhythmogenic responses,82 due either to atrial action potential shortening (strong vagal stimulation) or excessive calcium influx (calcium channel phosphorylation due to adrenergic phosphorylation). In the (small) subset of young, athletic individuals with AF, increased vagal tone and slow heart rate may contribute to the onset of AF.
In the majority of individuals with senile AF, the patients have elements of the metabolic syndrome (obesity, dyslipidemia, insulin resistance, hypertension). Vagal withdrawal occurs in patients with metabolic syndrome and heart failure, leading to sympathetic dominance, abnormal heart rate variability, 83,84 and elevated resting heart rate85 (Figure 2). Although sympathetic stimulation can provide for acute increases in calcium influx, contractility, and energy production, persistent sympathetic activation promotes ectopic electric activity and initiation of the apoptotic cascade.86 Interventions that improve vagal tone, including exercise and dietary ω3 PUFA supplementation, favorably affect mechanisms of cardiac arrhythmogenesis, potentially due to vagal modulation of heart rate and calcium cycling.87 Vagal activity also has anti-inflammatory effects, protecting the heart from the deleterious effects of excessive cytokine stimulation.88
Several clinical studies have reported decreased heart rate after increased dietary fish intake89 and administration of fish oil capsules.90–92 A modest improvement in heart rate variability was reported in individuals with high fish consumption,93 consistent with improved vagal tone. However, in a small study of patients after myocardial infarction, 1g/d ω3 PUFAs did not affect heart rate variability.94 Similarly, a study by Geelen et al95 reported no change in heart rate variability or baroreceptor sensitivity in healthy subjects after fish oil supplementation. Factors influencing the response of heart rate and heart rate variability to dietary ω3 PUFAs may include (1) the baseline plasma and tissue lipid composition, (2) the baseline systemic inflammatory and autonomic state, (3) the dose and duration of ω3 PUFA supplementation, and (4) the specific composition of ω3 PUFAs in the diet (as ALA, EPA, and DHA have distinct effects5,90).
5: Modulation of Inflammatory Pathways That Lead to Changes in Cardiac Conduction
Eicosanoids have physiological and pathological effects on the heart, affecting both heart rate and the structural responses to hemodynamic stress.96 Dietary fatty acids can affect cardiovascular function by modulating systemic inflammatory pathways. Activation of leukocytes (especially monocytes and macrophages) promotes the release of AA, which is then metabolized into chemotactic compounds (eg, leukotriene B4, LTB4) that recruit inflammatory cells (neutrophils, monocytes) to injured tissues. The corresponding ω3 PUFA metabolite LTB5 is much less effective as a chemokine.97 Slow and heterogeneous conduction is prominent in areas with increased inflammatory cell infiltration98; however, the cellular basis for inflammatory arrhythmias is not well defined.99 In studies based on receptor knockout mice, thromboxane A2 and prostaglandin F2α (both AA metabolites) were implicated as mediators of inflammatory tachycardias.100 Increased ω3 PUFA consumption decreases the availability of AA and subsequently may modulate the production of prostaglandin E2 and other inflammatory eicosanoids.101,102 Duda et al102 reported that serum levels of tumor necrosis factor-α (TNF-α), as well as urinary thromboxane B2 and 6-keto prostaglandin F1, were elevated in a rat model of abdominal aortic banding. Dietary EPA/DHA supplementation blunted this effect and attenuated the left ventricular remodeling and systolic dysfunction that is characteristic of the abdominal aortic banding model.102 Cardiac-specific deletion of cyclooxygenase-2 (COX-2) expression eliminates the ability to synthesize COX-2–dependent eicosanoids; these mice have a slower heart rate and increased fibrosis after aortic banding.96 Together, these studies suggest that eicosanoids are important modulators of cardiac function and arrhythmogenesis. The balance of dietary ω3 and ω6 PUFAs modulates the distribution of eicosanoids produced, thus affecting heart rate, ectopic activity, and cardiac conduction patterns.
Impact of Dietary Fatty Acids on Cardiac Fibroblasts and Interstitial Fibrosis
Arrhythmias require an initiating trigger and a substrate to become persistent. Structural remodeling, including reactive and replacement fibrosis, often underlies reentrant arrhythmias. Fibroblast expression is normally low in the healthy heart but increases in response to inflammatory stimuli and with advanced age, hypertension, hemodynamic overload, valve dysfunction, and heart failure.103 Multiple signaling pathways regulate the development of interstitial fibrosis, with prominent roles evident for the renin-angiotensin system,104 aldosterone,105 and cytokines, including platelet-derived growth factor (PDGF),106 transforming growth factor-β (TGF-β),107 TNF-α,108 and AA metabolites.109 Fish oil (ω3 PUFA) has been shown to suppress endothelial PDGF formation.110 As fibrosis is an important determinant of arrhythmia persistence, it is not surprising that antifibrotic agents demonstrate antiarrhythmic efficacy (eg, angiotensin-converting enzyme inhibitors,104 statins,111 and aldosterone antagonists105,112).
Fibroblast proliferation and extracellular matrix accumulation is a normal and important element of wound healing.113 In conditions such as heart failure and myocardial infarction, fibroblasts elaborate extracellular matrix components (primarily collagen) that can provide stiffness to injured myocardium. In heart failure, fibroblast proliferation and matrix accumulation occur more rapidly in the atrial than ventricular myocardium, contributing to an increase in AF vulnerability.114 Myocyte interactions with myofibroblasts can promote heterogeneous conduction,115,116 because myofibroblasts typically have a less negative (more depolarized) resting potential than cardiac myocytes. Miragoli et al116 showed that myofibroblasts can modulate conduction velocity and ectopic activity115 of cardiac tissues, primarily via gap junction–mediated electrotonic interactions. In a canine model of heart failure subsequent to rapid ventricular pacing, development of atrial interstitial fibrosis has also been shown to be a critical determinant of AF episode duration.117 In this model, aERP was prolonged, and, after the development of atrial fibrosis, arrhythmia episode duration became independent of the electric remodeling status.118
Fibrosis, Dietary Lipids, and Arrhythmogenesis
Dietary lipids are implicated in the development of cardiac fibrosis and modulate arrhythmias that are fibrosis-dependent. Aubin et al119 reported that rats fed a high-fat diet (42% by calories versus 12.5% in control rats) for 8 weeks became hypertensive and had evidence of reactive fibrosis; unfortunately, the composition of dietary lipids was not reported.119 In a comparison of 2 AF models, Sakabe et al120 reported that oral administration of ω3 PUFA (EPA/DHA) suppressed AF inducibility and duration in a canine model of ventricular pacing–induced heart failure; in contrast, it did not modify aERP changes resulting from 1 week of rapid atrial pacing. This result contrasts with the effects of ω3 PUFA infusion on acute aERP changes after rapid atrial pacing.78 In the same canine ventricular pacing–induced heart failure model, dietary supplementation with ω3 PUFAs attenuated the development of atrial fibrosis121 and prevented vagally induced AF.121
A recent clinical trial suggests that supplemental ω3 PUFA therapy can help to prevent perioperative AF.122 After coronary artery bypass graft surgery, 15% of patients randomly assigned to receive the ω3 PUFA supplement had AF, compared with 33% of the control patients.122 Patients who received ω3 PUFAs also had a shorter length of hospital stay.122 Because of the promising preclinical and clinical evidence, several randomized trials of supplemental ω3 PUFA for prevention of postoperative AF or recurrent AF are underway, seeking to confirm and extend the encouraging preclinical and clinical observations.
Summary and Conclusions
The specific composition of dietary lipids, the daily caloric intake, and the fraction of calories consumed as lipids are quite variable around the world. Epidemiological data suggest that the typical Western diet is not optimal from the perspective of cardiovascular health or longevity. The Western diet is frequently excessive with respect to total calories consumed, calories derived from sugar, and calories derived from saturated or trans-fatty acids. In contrast, the Western diet is often deficient with respect to ω3 PUFA content. The prevalence of cardiovascular disease and death caused by arrhythmia is increased in the United States, relative to populations consuming Mediterranean diets or those regions with greater ω3 PUFA content.
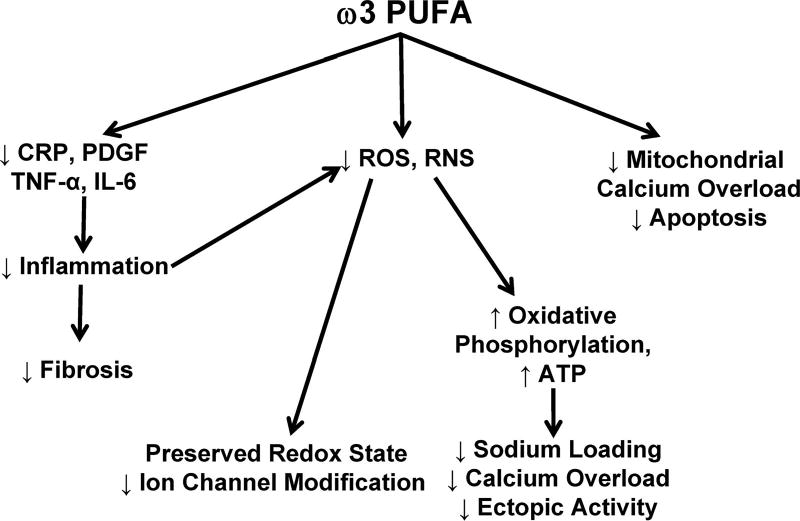
Dietary lipids can promote the development of atherosclerosis1,6 and activation of inflammatory cells.100 In the setting of ischemia, fatty acid metabolites can exacerbate vasoconstriction, spontaneous electric activity, and heterogeneities of repolarization and conduction (by modulating voltage-gated ion channels, gap junctions, intracellular calcium homeostasis, and ectopic electric activity).69–73 These alterations promote arrhythmogenesis. However, dietary fatty acids also can have numerous beneficial effects (Figure 3). ω3 PUFAs decrease the inflammatory response to injury101,102 and the development of fibrosis in the setting of heart failure.120,121 In addition, ω3 PUFAs may preserve mitochondrial function by decreasing oxidant stress and subsequent inhibition of the electron transport chain. In the setting of inflammation or failure, vagal tone is preserved or enhanced, and heart rate is slowed. These effects of ω3 PUFA are anticipated to promote the maintenance of normal cardiac rhythm.
Figure 3.
Antiarrhythmic effects of dietary ω3 PUFA. ω3 PUFAs decrease fibrosis by inhibiting cytokine production and systemic inflammation (PDGF, platelet-derived growth factor; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; and IL-6, interleukin-6). Inhibition of cytokine production and antioxidant effects minimize reactive oxygen (ROS) and reactive nitrogen (RNS) production, resulting in decreased post-translational modification of ion channels and preservation of a reduced redox state. Decreased ROS production improves ATP production by oxidative phosphorylation, limiting sodium loading, calcium overload, and ectopic activity. Finally, ω3 PUFA can prevent apoptosis induced by mitochondrial calcium overload
Diet affects autonomic tone. In young athletic individuals with AF, increased vagal tone may contribute to the etiology of AF; in such individuals, increased dietary ω3 fatty acid intake might not be advisable. In contrast, for individuals with elements of the metabolic syndrome, changes in dietary lipid composition may lower the risk of cardiovascular disease and cardiac arrhythmia. Preventative measures, including changes such as increased ω3 PUFA consumption, in combination with lifestyle changes (increased activity) may help to achieve this goal. Development of practical and effective guidelines will require additional research to determine the nature and extent of changes required and to identify optimal dietary sources of ω3 PUFA.
Acknowledgments
We acknowledge the helpful discussions with and inspiration of William E.M. Lands, PhD, a pioneer in studies of dietary fatty acid metabolism.
Sources of Funding
Funding was provided by the Atrial Fibrillation Innovation Center, an Ohio Wright Center Initiative, and by the Fondation Leducq European-North American Atrial Fibrillation Research Alliance (network grant 07/CVD-03).
Footnotes
Disclosures
None.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De SG, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics: 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van HL, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 3.Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, Stuklis R, Roberts-Thomson K, Young GD, Cleland LG. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr. 2007;85:1222–1228. doi: 10.1093/ajcn/85.5.1222. [DOI] [PubMed] [Google Scholar]
- 4.Lands WE, Morris A, Libelt B. Quantitative effects of dietary polyunsaturated fats on the composition of fatty acids in rat tissues. Lipids. 1990;25:505–516. doi: 10.1007/BF02537156. [DOI] [PubMed] [Google Scholar]
- 5.Brochot A, Guinot M, Auchere D, Macaire JP, Weill P, Grynberg A, Rousseau-Ralliard D. Effects of alpha-linolenic acid vs docosahexaenoic acid supply on the distribution of fatty acids among the rat cardiac subcellular membranes after a short- or long-term dietary exposure. Nutr Metab (Lond) 2009;6:14. doi: 10.1186/1743-7075-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erkkila A, de M V, Riserus U, Laaksonen DE. Dietary fatty acids and cardiovascular disease: an epidemiological approach. Prog Lipid Res. 2008;47:172–187. doi: 10.1016/j.plipres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 8.Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr. 2004;134:919–922. doi: 10.1093/jn/134.4.919. [DOI] [PubMed] [Google Scholar]
- 10.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 11.Ouwens DM, Boer C, Fodor M, de Galan P, Heine RJ, Maassen JA, Diamant M. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia. 2005;48:1229–1237. doi: 10.1007/s00125-005-1755-x. [DOI] [PubMed] [Google Scholar]
- 12.Diniz YS, Cicogna AC, Padovani CR, Santana LS, Faine LA, Novelli EL. Diets rich in saturated and polyunsaturated fatty acids: metabolic shifting and cardiac health. Nutrition. 2004;20:230–234. doi: 10.1016/j.nut.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Cocco T, Di Paola M, Papa S, Lorusso M. Arachidonic acid interaction with the mitochondrial electron transport chain promotes reactive oxygen species generation. Free Radic Biol Med. 1999;27:51–59. doi: 10.1016/s0891-5849(99)00034-9. [DOI] [PubMed] [Google Scholar]
- 14.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2000;279:H2124–H2132. doi: 10.1152/ajpheart.2000.279.5.H2124. [DOI] [PubMed] [Google Scholar]
- 16.Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, Tserng KY, Hoit BD, Ernsberger P, Young ME, Stanley WC. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–H44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 17.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Effects of a high saturated fat diet on cardiac hypertrophy and dysfunction in response to pressure overload. J Card Fail. 2008;14:82–88. doi: 10.1016/j.cardfail.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duda MK, O’Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Low-carbohydrate/high-fat diet attenuates pressure overload-induced ventricular remodeling and dysfunction. J Card Fail. 2008;14:327–335. doi: 10.1016/j.cardfail.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennison JH, McElfresh TA, Okere IC, Patel HV, Foster AB, Patel KK, Stoll MS, Minkler PE, Fujioka H, Hoit BD, Young ME, Hoppel CL, Chandler MP. Enhanced acyl-CoA dehydrogenase activity is associated with improved mitochondrial and contractile function in heart failure. Cardiovasc Res. 2008;79:331–340. doi: 10.1093/cvr/cvn066. [DOI] [PubMed] [Google Scholar]
- 20.Rennison JH, McElfresh TA, Okere IC, Vazquez EJ, Patel HV, Foster AB, Patel KK, Chen Q, Hoit BD, Tserng KY, Hassan MO, Hoppel CL, Chandler MP. High-fat diet postinfarction enhances mitochondrial function and does not exacerbate left ventricular dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1498–H1506. doi: 10.1152/ajpheart.01021.2006. [DOI] [PubMed] [Google Scholar]
- 21.Rennison JH, McElfresh TA, Chen X, Anand VR, Hoit BD, Hoppel CL, Chandler MP. Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol. 2009;46:883–890. doi: 10.1016/j.yjmcc.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duda MK, O’Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007;76:303–310. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madsen L, Rustan AC, Vaagenes H, Berge K, Dyroy E, Berge RK. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids. 1999;34:951–963. doi: 10.1007/s11745-999-0445-x. [DOI] [PubMed] [Google Scholar]
- 24.Sekikawa A, Curb JD, Ueshima H, El-Saed A, Kadowaki T, Abbott RD, Evans RW, Rodriguez BL, Okamura T, Sutton-Tyrrell K, Nakamura Y, Masaki K, Edmundowicz D, Kashiwagi A, Willcox BJ, Takamiya T, Mitsunami K, Seto TB, Murata K, White RL, Kuller LH. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J Am Coll Cardiol. 2008;52:417–424. doi: 10.1016/j.jacc.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchioli R, Levantesi G, Macchia A, Maggioni AP, Marfisi RM, Silletta MG, Tavazzi L, Tognoni G, Valagussa F. Antiarrhythmic mechanisms of n-3 PUFA and the results of the GISSI-Prevenzione trial. J Membr Biol. 2005;206:117–128. doi: 10.1007/s00232-005-0788-x. [DOI] [PubMed] [Google Scholar]
- 26.Pepe S, McLennan PL. (n-3) Long chain PUFA dose-dependently increase oxygen utilization efficiency and inhibit arrhythmias after saturated fat feeding in rats. J Nutr. 2007;137:2377–2383. doi: 10.1093/jn/137.11.2377. [DOI] [PubMed] [Google Scholar]
- 27.Cha YM, Dzeja PP, Shen WK, Jahangir A, Hart CY, Terzic A, Redfield MM. Failing atrial myocardium: energetic deficits accompany structural remodeling and electrical instability. Am J Physiol Heart Circ Physiol. 2003;284:H1313–H1320. doi: 10.1152/ajpheart.00337.2002. [DOI] [PubMed] [Google Scholar]
- 28.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–578. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 30.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 31.Thijssen VL, Ausma J, Liu GS, Allessie MA, van Eys GJ, Borgers M. Structural changes of atrial myocardium during chronic atrial fibrillation. Cardiovasc Pathol. 2000;9:17–28. doi: 10.1016/s1054-8807(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 32.Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1992;24:1333–1347. doi: 10.1016/0022-2828(92)93098-5. [DOI] [PubMed] [Google Scholar]
- 33.Sanbe A, Tanonaka K, Kobayasi R, Takeo S. Effects of long-term therapy with ACE inhibitors, captopril, enalapril and trandolapril, on myocardial energy metabolism in rats with heart failure following myocardial infarction. J Mol Cell Cardiol. 1995;27:2209–2222. doi: 10.1016/s0022-2828(95)91551-6. [DOI] [PubMed] [Google Scholar]
- 34.Hoppel CL, Tandler B, Parland W, Turkaly JS, Albers LD. Hamster cardiomyopathy. a defect in oxidative phosphorylation in the cardiac interfibrillar mitochondria. J Biol Chem. 1982;257:1540–1548. [PubMed] [Google Scholar]
- 35.Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–2367. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- 36.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 37.Marin-Garcia J, Goldenthal MJ, Moe GW. Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc Res. 2001;52:103–110. doi: 10.1016/s0008-6363(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 38.Bukowska A, Schild L, Keilhoff G, Hirte D, Neumann M, Gardemann A, Neumann KH, Huth C, Rohl FW, Goette A, Lendeckel U. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Exp Biol Med (Maywood) 2008;233:558–574. doi: 10.3181/0706-RM-155. [DOI] [PubMed] [Google Scholar]
- 39.Tsuboi M, Hisatome I, Morisaki T, Tanaka M, Tomikura Y, Takeda S, Shimoyama M, Ohtahara A, Ogino K, Igawa O, Shigemasa C, Ohgi S, Nanba E. Mitochondrial DNA deletion associated with the reduction of adenine nucleotides in human atrium and atrial fibrillation. Eur J Clin Invest. 2001;31:489–496. doi: 10.1046/j.1365-2362.2001.00844.x. [DOI] [PubMed] [Google Scholar]
- 40.Seppet E, Eimre M, Peet N, Paju K, Orlova E, Ress M, Kovask S, Piirsoo A, Saks VA, Gellerich FN, Zierz S, Seppet EK. Compartmentation of energy metabolism in atrial myocardium of patients undergoing cardiac surgery. Mol Cell Biochem. 2005;270:49–61. doi: 10.1007/s11010-005-3780-y. [DOI] [PubMed] [Google Scholar]
- 41.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krey G, Braissant O, L’Horset F, Kalkhoven E, Perroud M, Parker MG, Wahli W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda I, Cha JY, Yanagita T, Nakatani N, Oogami K, Imaizumi K, Yazawa K. Effects of dietary alpha-linolenic, eicosapentaenoic and docosahexaenoic acids on hepatic lipogenesis and beta-oxidation in rats. Biosci Biotechnol Biochem. 1998;62:675–680. doi: 10.1271/bbb.62.675. [DOI] [PubMed] [Google Scholar]
- 44.Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van HN, Ruzickova J, Sponarova J, Drahota Z, Vlcek C, Keijer J, Houstek J, Kopecky J. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia. 2005;48:2365–2375. doi: 10.1007/s00125-005-1944-7. [DOI] [PubMed] [Google Scholar]
- 45.Choudhary G, Dudley SC., Jr Heart failure, oxidative stress, and ion channel modulation. Congest Heart Fail. 2002;8:148–155. doi: 10.1111/j.1527-5299.2002.00716.x. [DOI] [PubMed] [Google Scholar]
- 46.Hare JM. Oxidative stress and apoptosis in heart failure progression. Circ Res. 2001;89:198–200. [PubMed] [Google Scholar]
- 47.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 48.Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, Dudley SC., Jr Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–1657. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Brown GC, Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res. 2007;75:283–290. doi: 10.1016/j.cardiores.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 51.Ramlawi B, Otu H, Mieno S, Boodhwani M, Sodha NR, Clements RT, Bianchi C, Sellke FW. Oxidative stress and atrial fibrillation after cardiac surgery: a case-control study. Ann Thorac Surg. 2007;84:1166–1172. doi: 10.1016/j.athoracsur.2007.04.126. [DOI] [PubMed] [Google Scholar]
- 52.Mayr M, Yusuf S, Weir G, Chung YL, Mayr U, Yin X, Ladroue C, Madhu B, Roberts N, De SA, Fredericks S, Stubbs M, Griffiths JR, Jahangiri M, Xu Q, Camm AJ. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol. 2008;51:585–594. doi: 10.1016/j.jacc.2007.09.055. [DOI] [PubMed] [Google Scholar]
- 53.Csont T, Bereczki E, Bencsik P, Fodor G, Gorbe A, Zvara A, Csonka C, Puskas LG, Santha M, Ferdinandy P. Hypercholesterolemia increases myocardial oxidative and nitrosative stress thereby leading to cardiac dysfunction in apoB-100 transgenic mice. Cardiovasc Res. 2007;76:100–109. doi: 10.1016/j.cardiores.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Jahangiri A, Leifert WR, Kind KL, McMurchie EJ. Dietary fish oil alters cardiomyocyte Ca2+ dynamics and antioxidant status. Free Radic Biol Med. 2006;40:1592–1602. doi: 10.1016/j.freeradbiomed.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 55.Anmann T, Eimre M, Kuznetsov AV, Andrienko T, Kaambre T, Sikk P, Seppet E, Tiivel T, Vendelin M, Seppet E, Saks VA. Calcium-induced contraction of sarcomeres changes the regulation of mitochondrial respiration in permeabilized cardiac cells. FEBS J. 2005;272:3145–3161. doi: 10.1111/j.1742-4658.2005.04734.x. [DOI] [PubMed] [Google Scholar]
- 56.Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation: time course and mechanisms. Circulation. 1996;94:2968–2974. doi: 10.1161/01.cir.94.11.2968. [DOI] [PubMed] [Google Scholar]
- 57.Tieleman RG, Van Gelder IC, Crijns HJ, De KP, Van Den Berg MP, Haaksma J, Van Der Woude HJ, Allessie MA. Early recurrences of atrial fibrillation after electrical cardioversion: a result of fibrillation-induced electrical remodeling of the atria? J Am Coll Cardiol. 1998;31:167–173. doi: 10.1016/s0735-1097(97)00455-5. [DOI] [PubMed] [Google Scholar]
- 58.Zeitz O, Maass AE, Van Nguyen P, Hensmann G, Kogler H, Moller K, Hasenfuss G, Janssen PM. Hydroxyl radical-induced acute diastolic dysfunction is due to calcium overload via reverse-mode Na+-Ca2+ exchange. Circ Res. 2002;90:988–995. doi: 10.1161/01.res.0000018625.25212.1e. [DOI] [PubMed] [Google Scholar]
- 59.Schotten U, Greiser M, Benke D, Buerkel K, Ehrenteidt B, Stellbrink C, Vazquez-Jimenez JF, Schoendube F, Hanrath P, Allessie M. Atrial fibrillation-induced atrial contractile dysfunction: a tachycardiomyopathy of a different sort. Cardiovasc Res. 2002;53:192–201. doi: 10.1016/s0008-6363(01)00453-9. [DOI] [PubMed] [Google Scholar]
- 60.Berecki G, Den Ruijter HM, Verkerk AO, Schumacher CA, Baartscheer A, Bakker D, Boukens BJ, van Ginneken AC, Fiolet JW, Opthof T, Coronel R. Dietary fish oil reduces the incidence of triggered arrhythmias in pig ventricular myocytes. Heart Rhythm. 2007;4:1452–1460. doi: 10.1016/j.hrthm.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 61.Ander BP, Hurtado C, Raposo CS, Maddaford TG, Deniset JF, Hryshko LV, Pierce GN, Lukas A. Differential sensitivities of the NCX1.1 and NCX1.3 isoforms of the Na(+)-Ca(2+) exchanger to alpha-linolenic acid. Cardiovasc Res. 2007;73:395–403. doi: 10.1016/j.cardiores.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Luo J, Xuan YT, Gu Y, Prabhu SD. Prolonged oxidative stress inverts the cardiac force-frequency relation: role of altered calcium handling and myofilament calcium responsiveness. J Mol Cell Cardiol. 2006;40:64–75. doi: 10.1016/j.yjmcc.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 63.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 64.Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol. 1996;271:H823–H833. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 65.Maltsev VA, Sabbah HN, Higgins RS, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98:2545–2552. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- 66.Le Grand B, Coulombe A, John GW. Late sodium current inhibition in human isolated cardiomyocytes by R 56865. J Cardiovasc Pharmacol. 1998;31:800–804. doi: 10.1097/00005344-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 67.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 4):iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pignier C, Revenaz C, Rauly-Lestienne I, Cussac D, Delhon A, Gardette J, Le Grand B. Direct protective effects of poly-unsaturated fatty acids, DHA and EPA, against activation of cardiac late sodium current: a mechanism for ischemia selectivity. Basic Res Cardiol. 2007;102:553–564. doi: 10.1007/s00395-007-0676-x. [DOI] [PubMed] [Google Scholar]
- 69.Schmilinsky-Fluri G, Valiunas V, Willi M, Weingart R. Modulation of cardiac gap junctions: the mode of action of arachidonic acid. J Mol Cell Cardiol. 1997;29:1703–1713. doi: 10.1006/jmcc.1997.0409. [DOI] [PubMed] [Google Scholar]
- 70.Fukuda K, Davies SS, Nakajima T, Ong BH, Kupershmidt S, Fessel J, Amarnath V, Anderson ME, Boyden PA, Viswanathan PC, Roberts LJ, Balser JR. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res. 2005;97:1262–1269. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- 71.Jovanovic N, Pavlovic M, Mircevski V, Du Q, Jovanovic A. An unexpected negative inotropic effect of prostaglandin F2alpha in the rat heart. Prostaglandins Other Lipid Mediat. 2006;80:110–119. doi: 10.1016/j.prostaglandins.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 72.Corr PB, Yamada KA. Selected metabolic alterations in the ischemic heart and their contributions to arrhythmogenesis. Herz. 1995;20:156–168. [PubMed] [Google Scholar]
- 73.Fearon IM. OxLDL enhances L-type Ca2+ currents via lysophosphatidylcholine-induced mitochondrial reactive oxygen species (ROS) production. Cardiovasc Res. 2006;69:855–864. doi: 10.1016/j.cardiores.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 74.Culp BR, Lands WE, Lucches BR, Pitt B, Romson J. The effect of dietary supplementation of fish oil on experimental myocardial infarction. Prostaglandins. 1980;20:1021–1031. doi: 10.1016/0090-6980(80)90056-8. [DOI] [PubMed] [Google Scholar]
- 75.Aarsetoy H, Ponitz V, Nilsen OB, Grundt H, Harris WS, Nilsen DW. Low levels of cellular omega-3 increase the risk of ventricular fibrillation during the acute ischaemic phase of a myocardial infarction. Resuscitation. 2008;78:258–264. doi: 10.1016/j.resuscitation.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Billman GE, Kang JX, Leaf A. Prevention of ischemia-induced cardiac sudden death by n-3 polyunsaturated fatty acids in dogs. Lipids. 1997;32:1161–1168. doi: 10.1007/s11745-997-0149-2. [DOI] [PubMed] [Google Scholar]
- 77.Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- 78.da Cunha DN, Hamlin RL, Billman GE, Carnes CA. n-3 (omega-3) polyunsaturated fatty acids prevent acute atrial electrophysiological remodeling. Br J Pharmacol. 2007;150:281–285. doi: 10.1038/sj.bjp.0706977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pepe S, Bogdanov K, Hallaq H, Spurgeon H, Leaf A, Lakatta E. Omega 3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2+, and contraction in adult rat cardiac myocytes. Proc Natl Acad Sci U S A. 1994;91:8832–8836. doi: 10.1073/pnas.91.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiao YF, Ke Q, Wang SY, Auktor K, Yang Y, Wang GK, Morgan JP, Leaf A. Single point mutations affect fatty acid block of human myocardial sodium channel alpha subunit Na+ channels. Proc Natl Acad Sci U S A. 2001;98:3606–3611. doi: 10.1073/pnas.061003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verkerk AO, van Ginneken AC, Berecki G, Den Ruijter HM, Schumacher CA, Veldkamp MW, Baartscheer A, Casini S, Opthof T, Hovenier R, Fiolet JW, Zock PL, Coronel R. Incorporated sarcolemmal fish oil fatty acids shorten pig ventricular action potentials. Cardiovasc Res. 2006;70:509–520. doi: 10.1016/j.cardiores.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 82.Coumel P. Autonomic influences in atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 1996;7:999–1007. doi: 10.1111/j.1540-8167.1996.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 83.Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol. 1991;18:464–472. doi: 10.1016/0735-1097(91)90602-6. [DOI] [PubMed] [Google Scholar]
- 84.Stein PK, Barzilay JI, Domitrovich PP, Chaves PM, Gottdiener JS, Heckbert SR, Kronmal RA. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study. Diabet Med. 2007;24:855–863. doi: 10.1111/j.1464-5491.2007.02163.x. [DOI] [PubMed] [Google Scholar]
- 85.Palatini P. Elevated heart rate as a predictor of increased cardiovascular morbidity. J Hypertens Suppl. 1999;17:S3–S10. [PubMed] [Google Scholar]
- 86.Singh K, Communal C, Sawyer DB, Colucci WS. Adrenergic regulation of myocardial apoptosis. Cardiovasc Res. 2000;45:713–719. doi: 10.1016/s0008-6363(99)00370-3. [DOI] [PubMed] [Google Scholar]
- 87.Wang YG, Huser J, Blatter LA, Lipsius SL. Withdrawal of acetylcholine elicits Ca2+-induced delayed afterdepolarizations in cat atrial myocytes. Circulation. 1997;96:1275–1281. doi: 10.1161/01.cir.96.4.1275. [DOI] [PubMed] [Google Scholar]
- 88.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bao DQ, Mori TA, Burke V, Puddey IB, Beilin LJ. Effects of dietary fish and weight reduction on ambulatory blood pressure in overweight hypertensives. Hypertension. 1998;32:710–717. doi: 10.1161/01.hyp.32.4.710. [DOI] [PubMed] [Google Scholar]
- 90.Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253–260. doi: 10.1161/01.hyp.34.2.253. [DOI] [PubMed] [Google Scholar]
- 91.Geelen A, Brouwer IA, Schouten EG, Maan AC, Katan MB, Zock PL. Effects of n-3 fatty acids from fish on premature ventricular complexes and heart rate in humans. Am J Clin Nutr. 2005;81:416–420. doi: 10.1093/ajcn.81.2.416. [DOI] [PubMed] [Google Scholar]
- 92.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 93.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117:1130–1137. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 94.Hamaad A, Kaeng LW, Lip GY, MacFadyen RJ. Oral omega n3-PUFA therapy (Omacor) has no impact on indices of heart rate variability in stable post myocardial infarction patients. Cardiovasc Drugs Ther. 2006;20:359–364. doi: 10.1007/s10557-006-0295-z. [DOI] [PubMed] [Google Scholar]
- 95.Geelen A, Zock PL, Swenne CA, Brouwer IA, Schouten EG, Katan MB. Effect of n-3 fatty acids on heart rate variability and baroreflex sensitivity in middle-aged subjects. Am Heart J. 2003;146:E4. doi: 10.1016/S0002-8703(03)00441-1. [DOI] [PubMed] [Google Scholar]
- 96.Wang D, Patel VV, Ricciotti E, Zhou R, Levin MD, Gao E, Yu Z, Ferrari VA, Lu MM, Xu J, Zhang H, Hui Y, Cheng Y, Petrenko N, Yu Y, Fitzgerald GA. Cardiomyocyte cyclooxygenase-2 influences cardiac rhythm and function. Proc Natl Acad Sci U S A. 2009;106:7548–7552. doi: 10.1073/pnas.0805806106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 98.Ishii Y, Schuessler RB, Gaynor SL, Yamada K, Fu AS, Boineau JP, Damiano RJ., Jr Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 99.Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–313. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- 100.Takayama K, Yuhki K, Ono K, Fujino T, Hara A, Yamada T, Kuriyama S, Karibe H, Okada Y, Takahata O, Taniguchi T, Iijima T, Iwasaki H, Narumiya S, Ushikubi F. Thromboxane A2 and prostaglandin F2alpha mediate inflammatory tachycardia. Nat Med. 2005;11:562–566. doi: 10.1038/nm1231. [DOI] [PubMed] [Google Scholar]
- 101.Calder PC. Polyunsaturated fatty acids and inflammation. Biochem Soc Trans. 2005;33:423–427. doi: 10.1042/BST0330423. [DOI] [PubMed] [Google Scholar]
- 102.Duda MK, O’Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG, Sabbah HN, Stanley WC. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi Y, Li D, Tardif JC, Nattel S. Enalapril effects on atrial remodeling and atrial fibrillation in experimental congestive heart failure. Cardiovasc Res. 2002;54:456–461. doi: 10.1016/s0008-6363(02)00243-2. [DOI] [PubMed] [Google Scholar]
- 105.Milliez P, Deangelis N, Rucker-Martin C, Leenhardt A, Vicaut E, Robidel E, Beaufils P, Delcayre C, Hatem SN. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur Heart J. 2005;26:2193–2199. doi: 10.1093/eurheartj/ehi478. [DOI] [PubMed] [Google Scholar]
- 106.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–1641. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 107.Verheule S, Sato T, Everett T, Engle SK, Otten D, Rubart-von der LM, Nakajima HO, Nakajima H, Field LJ, Olgin JE. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–1465. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saba S, Janczewski AM, Baker LC, Shusterman V, Gursoy EC, Feldman AM, Salama G, McTiernan CF, London B. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol. 2005;289:H1456–H1467. doi: 10.1152/ajpheart.00733.2004. [DOI] [PubMed] [Google Scholar]
- 109.Levick SP, Loch DC, Taylor SM, Janicki JS. Arachidonic acid metabolism as a potential mediator of cardiac fibrosis associated with inflammation. J Immunol. 2007;178:641–646. doi: 10.4049/jimmunol.178.2.641. [DOI] [PubMed] [Google Scholar]
- 110.Fox PL, DiCorleto PE. Fish oils inhibit endothelial cell production of platelet-derived growth factor-like protein. Science. 1988;241:453–456. doi: 10.1126/science.3393911. [DOI] [PubMed] [Google Scholar]
- 111.Shiroshita-Takeshita A, Brundel BJ, Burstein B, Leung TK, Mitamura H, Ogawa S, Nattel S. Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc Res. 2007;74:75–84. doi: 10.1016/j.cardiores.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 112.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES): Rales Investigators. Circulation. 2000;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 113.Manso AM, Kang SM, Plotnikov SV, Thievessen I, Oh J, Beggs HE, Ross RS. Cardiac fibroblasts require focal adhesion kinase for normal proliferation and migration. Am J Physiol Heart Circ Physiol. 2009;296:H627–H638. doi: 10.1152/ajpheart.00444.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004;63:236–244. doi: 10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 115.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 116.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98:801–810. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 117.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs : atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 118.Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK, Nattel S. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004;109:412–418. doi: 10.1161/01.CIR.0000109501.47603.0C. [DOI] [PubMed] [Google Scholar]
- 119.Aubin MC, Lajoie C, Clement R, Gosselin H, Calderone A, Perrault LP. Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: therapeutic potential of resveratrol. J Pharmacol Exp Ther. 2008;325:961–968. doi: 10.1124/jpet.107.135061. [DOI] [PubMed] [Google Scholar]
- 120.Sakabe M, Shiroshita-Takeshita A, Maguy A, Dumesnil C, Nigam A, Leung TK, Nattel S. Omega-3 polyunsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation. 2007;116:2101–2109. doi: 10.1161/CIRCULATIONAHA.107.704759. [DOI] [PubMed] [Google Scholar]
- 121.Sarrazin J-F, Comeau G, Daleau P, Kingma J, Plante I, Fournier D, Molin F. Reduced incidence of vagally induced atrial fibrillation and expression levels of connexins by n-3 polyunsaturated fatty acids in dogs. J Am Coll Cardiol. 2007;50:1505–1512. doi: 10.1016/j.jacc.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 122.Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, Meo A, Pandozi C, Staibano M, Santini M. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]