Abstract
Susceptibility to autoimmune diseases is dependent on multigenic inheritance, environmental factors, and stochastic events. Although there has been substantial progress in identifying predisposing genetic variants, a significant challenge facing autoimmune disease research is the identification of the specific events that trigger loss of tolerance, autoreactivity and ultimately autoimmune disease. Accordingly, studies have indicated that a wide range of extrinsic factors including drugs, chemicals, microbes, and other environmental factors can induce autoimmunity, particularly systemic autoimmune diseases such as lupus. This review describes a class of environmental factors, namely xenobiotics, epidemiologically linked to human autoimmunity. Mechanisms of xenobiotic autoimmune disease induction are discussed in terms of human and animal model studies with a focus on the role of inflammation and the innate immune response. We argue that localized tissue damage and chronic inflammation elicited by xenobiotic exposure leads to the release of self-antigens and damage-associated molecular patterns as well as the appearance of ectopic lymphoid structures and secondary lymphoid hypertrophy, which provide a milieu for the production of autoreactive B and T cells that contribute to the development and persistence of autoimmunity in predisposed individuals.
Keywords: xenobiotic, autoimmunity, animal model, inflammation, epidemiology
1. Introduction
In this review, we examine the development of autoimmunity elicited by exposure to environmental factors with emphasis on chemical agents that are often referred to as xenobiotics because they are foreign chemical substances that are not naturally produced by or present in organisms. Xenobiotics can be present in the air we breathe, the fluids we drink, the food we eat, synthetic and natural chemicals, and industrial by-products. Linking exposure to these agents with human autoimmune disease manifestations is difficult because of the inherent limitations of epidemiological studies to draw causal conclusions [1]. Furthermore, human populations are rarely exposed to a single agent over time, there can be a significant delay between exposure and disease onset, and it is often not possible to identify all of the toxicants to which a population may have been exposed [2]. Additionally, the magnitude of autoreactivity following human xenobiotic exposure can differ with some xenobiotics inducing clinical disease [1] while others trigger features of autoimmunity without evidence of overt disease [3]. Differences in autoreactivity can also reflect the degree of exposure [4] or the extent of genetic predisposition. Consequently, it is not surprising that only a few xenobiotic agents have been established to promote autoimmune disease. However, many of these limitations can often be overcome by studying experimental animal models, which have confirmed the autoimmune-promoting potential of certain xenobiotics and have also provided insights into pathogenic mechanisms. Therefore, this review draws from both human and animal research in describing the role that xenobiotic exposure plays in autoimmunity.
2. Autoimmunity
Autoimmunity is the reaction of cells or products of the immune system with constituents of the body’s own tissues leading to pathology and disease. Autoimmunity is responsible for a variety of clinical conditions with common features including expansion of self-reactive T and B cells, production of autoantibodies, and tissue damage. The most challenging aspect of autoimmunity is identifying the events that contribute to the initiation of the response. While many intrinsic factors including age, sex, and genetics contribute to autoimmunity, it is believed that extrinsic factors such as drugs, chemicals, and microbes can trigger the initiation of an autoimmune response [5].
3. Autoimmune diseases associated with environmental exposure
Autoreactivity has been argued to lead to three distinct yet overlapping clinical consequences reflecting the severity of self-reactivity [5]. A physiological level of self-reactivity is required for lymphocyte selection and immune homeostasis and is not associated with autoimmunity. Early loss of tolerance is thought to initially produce a pre-clinical form of autoimmunity limited to detectable autoantibodies and minor cellular infiltrates. Finally, autoreactivity can progress to pathogenic autoimmunity in which there is clinically significant cell or tissue damage. Notably, xenobiotic exposures can affect all three of these autoreactive states. Several xenobiotics were shown to impact immune system development [6], some exposures such as mercury can lead to predominantly preclinical features [3], while crystalline silica exposure can result in several clinical syndromes [1].
Xenobiotic exposure can promote autoimmunity by the de novo triggering of autoimmunity in healthy individuals, by exacerbating underlying idiopathic autoimmunity, or by inducing xenobiotic-specific autoimmunity against a backdrop of idiopathic disease [7–9]. Differentiating between these possibilities is challenging because of the lack of accepted criteria for the diagnosis or classification of environmentally associated autoimmunity [2]. Moreover, it is unclear to what extent idiopathic and induced diseases arise by common mechanisms [7, 10].
For example, the same agent can induce different autoimmune disorders, e.g., silica [1], while multiple agents can produce a similar clinical picture, i.e., different drugs leading to similar lupus-like syndromes [8]. Other environmental exposures, including toxic oil syndrome and eosinophilia myalgia syndrome, exhibit unique cllnical features that have allowed the development of syndrome-specific classification criteria and which suggest exposure-specific disease mechanisms [11–13].
A recent analysis [1] examined the epidemiological evidence for a relationship between xenobiotic exposures and human autoimmune diseases, identifying classifications of “confident”, “likely” and “unlikely” (Table 1). Exposure-disease associations supported by multiple studies resulted in high confidence that certain exposures contribute to disease development. For example, occupational exposure to crystalline silica has been linked to several autoimmune diseases including rheumatoid arthritis (RA), systemic sclerosis (SSc), SLE and anti-neutrophil cytoplasmic antibody (ANCA)-related diseases. Solvents, or chemicals with similar structures, including vinyl chloride, epoxy resin, trichloroethylene (TCE), perchloroethylene, or mixed solvents were confidently associated with SSc. Other studies supported a likely association between solvent exposure and multiple sclerosis (MS). Many studies have identified smoking as a strong risk activity for RA particularly if autoantibody positive, while fewer studies identify smoking as a risk for other autoimmune diseases such as SLE, MS, and thyroid autoimmunity. A complicated association has been reported for smoking and inflammatory bowel disease, with smoking argued to contribute to Crohn’s disease, but to protect from ulcerative colitis. There is some evidence for an association of cosmetics with SLE, RA and primary biliary cholangitis (PBC) [1, 14]. Finally, the association of hair dye exposure with SLE was considered unlikely [1].
Table 1.
Environmental exposures and human autoimmune diseases.
| Environmental Agent+ | Autoimmune Disease | Sex bias | Exposure |
|---|---|---|---|
| Confident | |||
| Crystalline silica | RA#, SSc, SLE, ANCA | SLE, none SSc, male RA, none |
Occupational |
| Solvents | SSc | Male | Occupational Lifestyle |
| Smoking | Seropositive RA | Increased risk higher in males | |
| Likely | |||
| Solvents | MS | Increased risk for male and female dependent on sex biased occupation (insufficient data) | Occupational |
| Smoking | SLE, MS, Crohn’s disease, Hashimoto
thyroiditis, Grave’s disease Ulcerative colitis* |
Lifestyle | |
| Unlikely | |||
| Hair dyes | SLE | - | Lifestyle |
Does not include biological (virus, bacteria), dietary (food, supplements)
Protective
Abbreviations: RA, rheumatoid arthritis; SSc, systemic sclerosis; SLE, systemic lupus erythematosus; ANCA, anti-neutrophil cytoplasmic antibody; MS, multiple sclerosis.
Although criteria for the diagnosis of environmental-associated autoimmune disease remain to be established [2], there are several circumstances that might suggest an environmental cause. Evidence of prior exposure to agents linked to autoimmunity (Table 1) should raise the possibility of an environmental etiology especially if symptomology deviates from standard classification criteria for idiopathic autoimmune diseases [2]. Occupation and lifestyle are other factors that should be considered, as these are often associated with environmental exposures linked to autoimmunity (Table 1). Another, is an absence of the usual female predilection commonly present in most idiopathic autoimmune diseases [14], which could occur in male-dominated occupations associated with adverse environmental exposures [14, 15].
4. Experimental models
Much of our understanding of the cellular and genetic requirements of xenobiotic-induced autoimmunity has come from exposures to mercury and pristane [16, 17]. For these agents, induction of autoreactivity/autoimmunity is impacted for the most part by the same immune mediators that influence disease expression in spontaneous autoimmune-prone mice, including T and B cell co-stimulatory molecules, cytokines such as IFN-γ and IL-6, and complement regulatory factor CD55 [16–19]. However, some differences have been described such as in the requirement for the inflammasome or type I IFN for disease [20–22]. These differences may reflect pathways unique to xenobiotic-induced autoimmunity or the many pathways that constitute the spectrum of idiopathic autoimmune disease pathogenesis.
Surprisingly few studies have successfully used animal models to investigate mechanisms underlying the environmental agent/autoimmune disease associations listed in Table 1. A small number of studies have investigated autoimmunity induced by crystalline silica [23, 24] although these were restricted to lupus-like disease. The literature for smoking-induced autoimmunity has focused primarily on RA with exposure to cigarette smoke having opposite effects depending on the study [9, 25–27]. Several studies have investigated the induction of autoimmunity by TCE in mice [9], but they focused on lupus–like disease rather than SSc.
The common theme running through these animal model studies is the use of autoimmune-prone strains as susceptible genotypes to determine if xenobiotic exposure can exacerbate disease [9]. This is particularly true of the lupus-prone strains, i.e., MRL, BXSB, NZB/W, where disease features, especially autoantibodies, are readily exacerbated by xenobiotic exposure, e.g., HgCl2, silica, TCE [9]. Although this experimental approach has yielded useful information, there is an urgent need for animal models that recapitulate exposure-disease associations relevant to human studies (Table 1).
5. Mechanisms
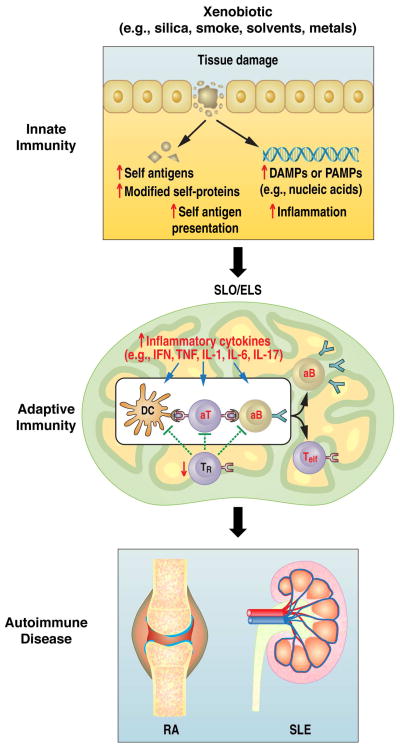
Exposure to xenobiotic agents has been linked to a variety of autoimmune diseases [1], however, the mechanisms by which exposure leads to autoimmunity remain unclear [28]. Analysis of data from many studies suggests a common pathway in which exposure results in the activation of the innate immune response leading to inflammation, the release and in some cases modification of self-antigens, and then recruitment and activation of T and B cells of the adaptive immune system. In autoimmune-susceptible individuals, this response can advance to humoral and cellular autoreactivity and ultimately disease pathology (Figure 1).
Figure 1.
The pathway to xenobiotic-induced autoimmunity. We propose that xenobiotic tissue damage can lead to the availability of nucleic acids and other damage associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs), the release of self- and modified self-antigens, the presentation of self-antigens to non-tolerant lymphocytes, and the induction of inflammation. Such effects lead to the engagement of TLRs and other innate sensors, the production of inflammatory cytokines, the contraction of T regulatory cell populations, the expansion of autoreactive B and T effector-cell populations, and the production of autoantibodies. These events can occur at the site of exposure such as the lungs where they occur in ELS or in SLO. In either case the expansion of autoreactive cells and their migration to target tissues such as the kidney in SLE results in autoimmune disease. SLO, secondary lymphoid organ; ELS, ectopic lymphoid structures; IFN, interferons; TNF, tumor necrosis factor; IL, interleukin; Teff, effector T cell; TR, T regulatory cell; aT, autoreactive T cell; aB, autoreactive B cell; DC, dendritic cell.
5.1 Site of exposure and inflammation
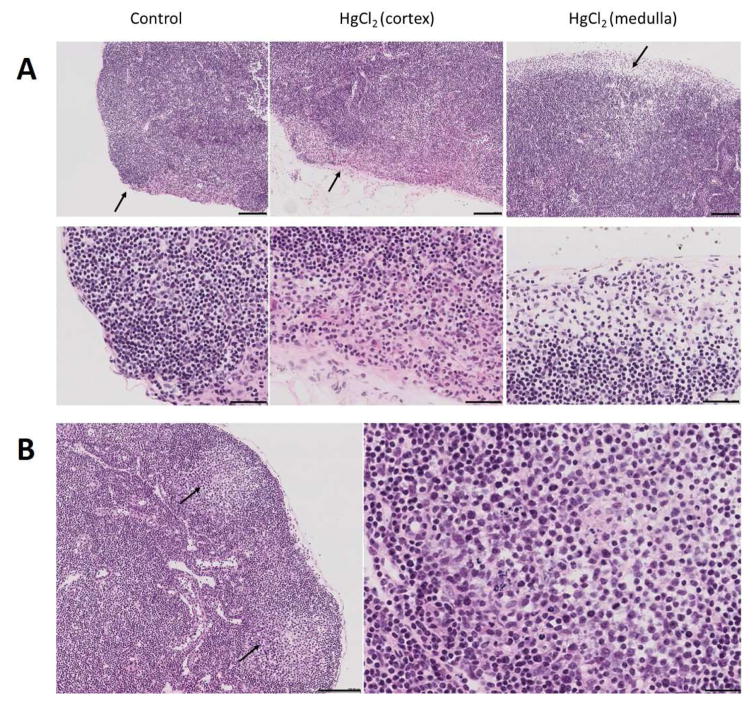
Xenobiotic exposure occurs via inhalation (lungs), ingestion (alimentary canal), adsorption (skin), and injection (muscle/circulation) and often results in a localized inflammatory response that can have significant effects on the subsequent development of autoimmunity [24, 29–31]. For example, lung exposure from smoking is a significant environmental risk factor associated with RA and exacerbation of SLE [32], silica inhalation is strongly associated with several systemic autoimmune diseases including lupus [1], and particulate exposure from air pollution is linked to SLE [33]. Accordingly, exposure to chemically unrelated xenobiotics at a common site, such as lungs, often leads to similar autoimmune diseases [1] and early inflammatory events suggest a shared pathology [34]. Thus, inhalation of cigarette smoke or silica results in epithelial cell and macrophage activation, neutrophil recruitment, the release of inflammatory mediators, such as cytokines and chemokines, and growth factors to stimulate tissue repair and fibrosis [34–36]. Animal models have also revealed that localized inflammation induced by injection of mercury [29, 30] or pristane [17] is required for the development of systemic autoimmunity. The process involves early inflammatory events, followed by lymphoid cell accumulations typical of germinal centers (Figure 2).
Figure 2.
Development of germinal centers in draining lymph node following HgCl2 exposure. A, Popliteal lymph node 3 hours after footpad injection of HgCl2 showing marked enlargement of the subcapsular sinus and large numbers of polymorphonuclear leukocytes (arrows). Similar areas were selected for the control and HgCl2 (cortex) images. Notice that the subcapsular sinus in the control is barely visible below the single cell layer capsule. B, Popliteal lymph node 7 days after footpad injection of HgCl2 showing germinal centers (GC) (left panel, arrows). An enlargement of the GC area is shown in the right panel. Bars in A are 100 μm in top panel and 50 μm in the bottom panel. In B bar on the left is 100 μm and 25 μm on the right.
5.2 Contribution of innate immunity
Early inflammatory steps, following xenobiotic exposure, likely arise as a result of tissue damage and the release of damage-associated molecular pattern molecules (DAMPs) and, in the presence of mucosal barrier disruption, pathogen-associated molecular pattern molecules (PAMPs) (Figure 1). Given the nature of xenobiotic exposures, it is difficult to exclude the possibility of concomitant micro-organism exposure, nonetheless, the end result is exposure of cellular debris to a variety of molecular sensors in and on a variety of cells of the innate immune system [5]. These sensors, which include Toll-like receptors (TLRs), are not only essential for the development of immune responses against foreign antigens, but also for autoimmune diseases [21, 37] because of their critical role in initiating early inflammation and in inducing and amplifying the adaptive immune response [37, 38]. Although it is unclear how xenobiotic exposure manipulates these processes toward autoreactivity, the persistence of the xenobiotic (silica [35] and particulates from smoking [33] in the lung, or pristane [17] and mercury [39] in the peritoneum) and the consequent chronic irritation and tissue damage appears to be a common factor [5].
5.3 Contribution of adaptive immunity
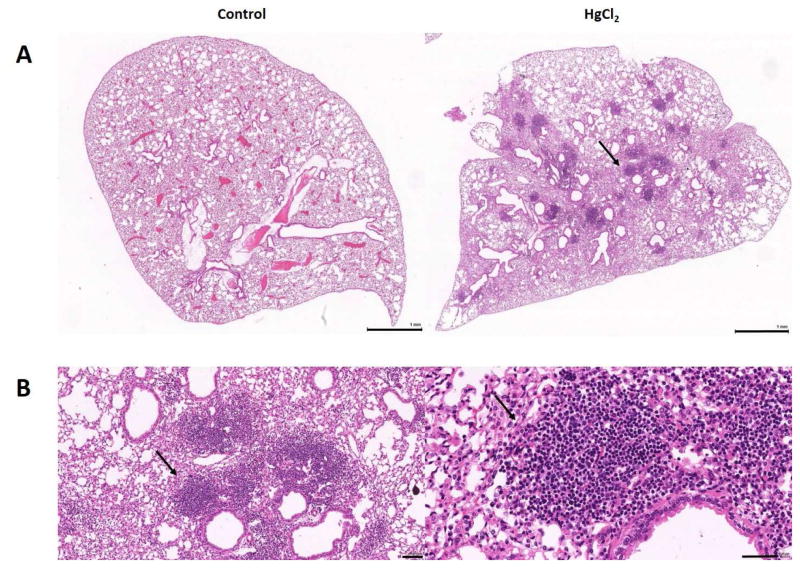
Chronic inflammatory responses at the site of xenobiotic exposure can lead to secondary lymphoid organ hypertrophy as well as the formation of transient organized aggregates of lymphoid cells called ectopic lymphoid structures (ELS) or tertiary lymphoid structures (TLS) [40, 41]. ELS-like accumulations have been described in the lungs of lupus-prone NZBWF1 mice following silica exposure [24] and the gut of mice with pristane-induced lupus [31], and we have observed them in the lungs of mercury-exposed mice (Figure 3). The relationship between smoking and RA has identified the lung as a potential site for the initiation of seropositive RA [42, 43]. ELS can allow affinity maturation of B cells and the expression of disease-specific autoantibodies [44] and have been found in target organs of various autoimmune diseases [40, 41, 45]. Although it is unclear how the presence of ELS at the site of xenobiotic exposure leads to the development of systemic autoimmunity, it is becoming clear that diagnostically relevant autoantibodies occur years before the clinical diagnosis of autoimmune disease [46–50]. This suggests that other factors may contribute to the spreading of pathology to distant tissues, such as the joints in RA or the kidneys in SLE. Possibilities include the suggestion that autoantibodies arising in ELS are non-pathogenic and only gain arthritogenic or nephritogenic potential following epitope spreading, or a second hit scenario whereby trauma or minor infection in the target tissues allows appropriate epitopes to be expressed [5, 32, 51]. An alternative scenario for xenobiotic-induced autoimmunity could be the distribution of the xenobiotic from the site of exposure to other tissues, such as the kidney following silica exposure [52], leading to persistence of the xenobiotic autoreactive response.
Figure 3.
Mercury-induced ectopic lymphoid structures (ELS) in the lung. A, Lungs from mice given PBS (Control) or HgCl2 showing the presence of lymphoid cell accumulations (arrow) after mercury exposure for 4 weeks. B, Enlarged views of the area indicated by the arrow in A showing the dense accumulation of lymphoid cells. Bars in A are 1 mm and in B 100 μm on the left and 50 μm on the right.
6. Future directions
Although the identification of xenobiotics associated with autoimmunity and the mechanisms by which they perturb the immune system has grown in importance in recent years, there are significant gaps in our understanding. Greater emphasis needs to be placed on identifying xenobiotic-induced autoimmunity in human populations with emphasis on ascertaining the role of genetic predisposition. Thought should be given to development of databases containing clinical, serologic, genetic, epigenetic and other laboratory features of cohorts with suspected or known exposures, especially those where there is high confidence of a causal relationship with autoimmunity (Table 1). Such studies should help identify phenotypic and genotypic criteria for diagnosing xenobiotic-induced autoimmunity, and help determine if human xenobiotic-induced autoimmunity arises de novo, requires genetic predisposition or exacerbates pre-existing autoreactivity.
Mechanistic discoveries are most likely to come from experimental animal studies. Therefore, such studies should focus on those exposures relevant to human autoimmunity rather than those that may only impact a given animal model. The wealth of studies on inflammatory responses elicited by xenobiotic exposure associated with autoimmune diseases, i.e., smoking and silica [34–36], may provide insight into molecular and cellular targets required for chronic inflammation that may precede autoreactivity [53]. Linking such studies with insights coming from human or animal idiopathic autoimmunity should help speed understanding of mechanisms of xenobiotic disease development. Because of the genetic heterogeneity of human populations consideration should be given to the use of outbred or genetically heterogeneous animal species to model xenobiotic exposures leading to autoimmunity. Such animal cohorts are likely to give rise to novel approaches for investigating autoreactivity elicited by xenobiotic exposure, and to accelerate understanding of the contribution of genetic factors.
Highlights.
Extrinsic factors such as drugs, chemicals, microbes, and other environmental/xenobiotic factors can induce autoimmunity, particularly systemic autoimmune diseases.
Xenobiotics may promote autoimmunity by de novo triggering of autoimmunity in healthy individuals, exacerbation of underlying idiopathic autoimmunity, or by induction of xenobiotic-specific autoimmunity against a background of idiopathic disease.
Tissue damage and chronic inflammation at the site of xenobiotic exposure can promote the appearance of ectopic lymphoid structures and secondary lymphoid hypertrophy, which provide a milieu for the production of autoreactive lymphoid cells essential to the development and persistence of autoimmunity.
Future studies need to place greater emphasis on identifying xenobiotic-induced autoimmunity in human populations, the role of genetic predisposition, and development of relevant experimental models.
Development of databases containing clinical, serologic, genetic, epigenetic and other laboratory features of cohorts with suspected or known exposures will help identify phenotypic and genotypic criteria for diagnosing xenobiotic-induced autoimmunity.
Acknowledgments
This work was supported by the National Institute of Health [grants ES007511, ES021464, and ES022625] to KMP and [HL114408] to DHK. This is manuscript #29569 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, De Roos AJ. Epidemiology of environmental exposures and human autoimmune diseases: Findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. 2012;39(4):259–271. doi: 10.1016/j.jaut.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller FW, Pollard KM, Parks CG, Germolec DR, Leung PS, Selmi C, Humble MC, Rose NR. Criteria for environmentally associated autoimmune diseases. J Autoimmun. 2012;39(4):253–258. doi: 10.1016/j.jaut.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowe W, Allsopp PJ, Watson GE, Magee PJ, Strain JJ, Armstrong DJ, Ball E, McSorley EM. Mercury as an environmental stimulus in the development of autoimmunity - A systematic review. Autoimmun Rev. 2017;16(1):72–80. doi: 10.1016/j.autrev.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999;107(Suppl 5):793–802. doi: 10.1289/ehp.99107s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol. 2017;18(7):716–724. doi: 10.1038/ni.3731. Excellent review of the current understanding of mechanisms leading to expression of autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreitinger JM, Beamer CA, Shepherd DM. Environmental Immunology: Lessons Learned from Exposure to a Select Panel of Immunotoxicants. J Immunol. 2016;196(8):3217–3225. doi: 10.4049/jimmunol.1502149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollard KM, Hultman P, Kono DH. Toxicology of autoimmune diseases. Chem Res Toxicol. 2010;23(3):455–466. doi: 10.1021/tx9003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin RL. Drug-induced lupus. Toxicology. 2005;209(2):135–147. doi: 10.1016/j.tox.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 9•.Germolec D, Kono DH, Pfau JC, Pollard KM. Animal models used to examine the role of the environment in the development of autoimmune disease: Findings from an NIEHS Expert Panel Workshop. J Autoimmun. 2012;39(4):285–293. doi: 10.1016/j.jaut.2012.05.020. Synthesis if the literature pertainint to animal models responses to environmental factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gualtierotti R, Biggioggero M, Penatti AE, Meroni PL. Updating on the pathogenesis of systemic lupus erythematosus. Autoimmun Rev. 2010;10(1):3–7. doi: 10.1016/j.autrev.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Chang C, Gershwin ME. Drug-induced lupus erythematosus: incidence, management and prevention. Drug Saf. 2011;34(5):357–374. doi: 10.2165/11588500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Gelpi E, de la Paz MP, Terracini B, Abaitua I, de la Camara AG, Kilbourne EM, Lahoz C, Nemery B, Philen RM, Soldevilla L, et al. The Spanish toxic oil syndrome 20 years after its onset: a multidisciplinary review of scientific knowledge. Environ Health Perspect. 2002;110(5):457–464. doi: 10.1289/ehp.110-1240833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertzman PA, Clauw DJ, Kaufman LD, Varga J, Silver RM, Thacker HL, Mease P, Espinoza LR, Pincus T. The eosinophilia-myalgia syndrome: status of 205 patients and results of treatment 2 years after onset. Ann Intern Med. 1995;122(11):851–855. doi: 10.7326/0003-4819-122-11-199506010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Pollard KM. Gender differences in autoimmunity associated with exposure to environmental factors. J Autoimmun. 2012;38(2–3):J177–186. doi: 10.1016/j.jaut.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiniakou E, Costenbader KH, Kriegel MA. Sex-specific environmental influences on the development of autoimmune diseases. Clin Immunol. 2013;149(2):182–191. doi: 10.1016/j.clim.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiraldi M, Monestier M. How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity. Trends Immunol. 2009;30(10):502–509. doi: 10.1016/j.it.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30(9):455–464. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. Interferon-gamma and Systemic Autoimmunity. Discov Med. 2013;16(87):123–131. [PMC free article] [PubMed] [Google Scholar]
- 19.Toomey CB, Cauvi DM, Pollard KM. The Role of Decay Accelerating Factor in Environmentally Induced and Idiopathic Systemic Autoimmune Disease. Autoimmune Dis. 2014;2014:452853. doi: 10.1155/2014/452853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollard KM, Hultman P, Toomey CB, Cauvi DM, Hoffman HM, Hamel JC, Kono DH. Definition of IFN-gamma-related pathways critical for chemically-induced systemic autoimmunity. J Autoimmun. 2012;39(4):323–331. doi: 10.1016/j.jaut.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollard KM, Kono DH. Requirements for innate immune pathways in environmentally induced autoimmunity. BMC medicine. 2013;11:100. doi: 10.1186/1741-7015-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollard KM, Escalante GM, Huang H, Haraldsson KM, Hultman P, Christy JM, Pawar RD, Mayeux JM, Gonzalez-Quintial R, Baccala R, et al. Induction of systemic autoimmunity by xenobiotic requires endosomal TLR trafficking and signaling from the late endosome/endolysosome but not type I IFN. J Immunol. doi: 10.4049/jimmunol.1700332. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayeux JM, Pawar RD, Pollard KM. Silicates and Autoimmunity. In: Otsuki A, Yoshioka Y, Holian A, editors. Biological Effects of Fibrous and Particulate Substances. Japan: Springer; 2016. pp. 163–180. [Google Scholar]
- 24.Bates MA, Brandenberger C, Langohr I, Kumagai K, Harkema JR, Holian A, Pestka JJ. Silica Triggers Inflammation and Ectopic Lymphoid Neogenesis in the Lungs in Parallel with Accelerated Onset of Systemic Autoimmunity and Glomerulonephritis in the Lupus-Prone NZBWF1 Mouse. PLoS One. 2015;10(5):e0125481. doi: 10.1371/journal.pone.0125481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindblad SS, Mydel P, Jonsson IM, Senior RM, Tarkowski A, Bokarewa M. Smoking and nicotine exposure delay development of collagen-induced arthritis in mice. Arthritis Res Ther. 2009;11(3):R88. doi: 10.1186/ar2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto S, Adachi M, Chujo S, Yamada K, Akita K, Itoh S, Takii T, Hayakawa K, Onozaki K. Etiological role of cigarette smoking in rheumatoid arthritis: Nasal exposure to cigarette smoke condensate extracts augments the development of collagen-induced arthritis in mice. Biochem Biophys Res Commun. 2011;404(4):1088–1092. doi: 10.1016/j.bbrc.2010.12.118. [DOI] [PubMed] [Google Scholar]
- 27•.Vassallo R, Luckey D, Behrens M, Madden B, Luthra H, David C, Taneja V. Cellular and humoral immunity in arthritis are profoundly influenced by the interaction between cigarette smoke effects and host HLA-DR and DQ genes. Clin Immunol. 2014;152(1–2):25–35. doi: 10.1016/j.clim.2014.02.002. Demonstration that DQ molecules can present citrullinated peptides more efficiently than native peptides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selmi C, Leung PS, Sherr DH, Diaz M, Nyland JF, Monestier M, Rose NR, Gershwin ME. Mechanisms of environmental influence on human autoimmunity: a National Institute of Environmental Health Sciences expert panel workshop. J Autoimmun. 2012;39(4):272–284. doi: 10.1016/j.jaut.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Cauvi DM, Cauvi G, Toomey CB, Jacquinet E, Pollard KM. Interplay between IFN-gamma and IL-6 impacts the inflammatory response and expression of interferon-regulated genes in environmental-induced autoimmunity. Toxicol Sci. 2017;158(1):227–239. doi: 10.1093/toxsci/kfx083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toomey CB, Cauvi DM, Hamel JC, Ramirez AE, Pollard KM. Cathepsin B regulates the appearance and severity of mercury-induced inflammation and autoimmunity. Toxicol Sci. 2014;142(2):339–349. doi: 10.1093/toxsci/kfu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nacionales DC, Kelly KM, Lee PY, Zhuang H, Li Y, Weinstein JS, Sobel E, Kuroda Y, Akaogi J, Satoh M, et al. Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (pristane) Am J Pathol. 2006;168(4):1227–1240. doi: 10.2353/ajpath.2006.050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catrina AI, Deane KD, Scher JU. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology (Oxford) 2016;55(3):391–402. doi: 10.1093/rheumatology/keu469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernatsky S, Fournier M, Pineau CA, Clarke AE, Vinet E, Smargiassi A. Associations between ambient fine particulate levels and disease activity in patients with systemic lupus erythematosus (SLE) Environ Health Perspect. 2011;119(1):45–49. doi: 10.1289/ehp.1002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong J, Magun BE, Wood LJ. Lung inflammation caused by inhaled toxicants: a review. Int J Chron Obstruct Pulmon Dis. 2016;11:1391–1401. doi: 10.2147/COPD.S106009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki H. A mechanistic review of silica-induced inhalation toxicity. Inhal Toxicol. 2015;27(8):363–377. doi: 10.3109/08958378.2015.1066905. [DOI] [PubMed] [Google Scholar]
- 36.Crotty Alexander LE, Shin S, Hwang JH. Inflammatory Diseases of the Lung Induced by Conventional Cigarette Smoke: A Review. Chest. 2015;148(5):1307–1322. doi: 10.1378/chest.15-0409. [DOI] [PubMed] [Google Scholar]
- 37••.Kono DH, Baccala R, Theofilopoulos AN. TLRs and interferons: a central paradigm in autoimmunity. Curr Opin Immunol. 2013;25(6):720–727. doi: 10.1016/j.coi.2013.10.006. Important contribution showing that, unlike idiopathic autoimmunity, xenobiotic-induced autoimmunity does not require type I interferon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh YT, Scatizzi JC, Gahan JD, Lawson BR, Baccala R, Pollard KM, Beutler BA, Theofilopoulos AN, Kono DH. Role of nucleic acid-sensing TLRs in diverse autoantibody specificities and anti-nuclear antibody-producing B cells. J Immunol. 2013;190(10):4982–4990. doi: 10.4049/jimmunol.1202986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hultman P, Johansson U, Turley SJ, Lindh U, Enestrom S, Pollard KM. Adverse immunological effects and autoimmunity induced by dental amalgam and alloy in mice. Faseb J. 1994;8(14):1183–1190. doi: 10.1096/fasebj.8.14.7958626. [DOI] [PubMed] [Google Scholar]
- 40.Jones GW, Jones SA. Ectopic lymphoid follicles: inducible centres for generating antigen-specific immune responses within tissues. Immunology. 2016;147(2):141–151. doi: 10.1111/imm.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 42.Sparks JA, Karlson EW. The Roles of Cigarette Smoking and the Lung in the Transitions Between Phases of Preclinical Rheumatoid Arthritis. Curr Rheumatol Rep. 2016;18(3):15. doi: 10.1007/s11926-016-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatzidionisyou A, Catrina AI. The lung in rheumatoid arthritis, cause or consequence? Curr Opin Rheumatol. 2016;28(1):76–82. doi: 10.1097/BOR.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 44•.Corsiero E, Nerviani A, Bombardieri M, Pitzalis C. Ectopic Lymphoid Structures: Powerhouse of Autoimmunity. Frontiers in immunology. 2016;7:430. doi: 10.3389/fimmu.2016.00430. Review of the role of Ectopic lymphoid structures (ELS) in autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vu Van D, Beier KC, Pietzke LJ, Al Baz MS, Feist RK, Gurka S, Hamelmann E, Kroczek RA, Hutloff A. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nature communications. 2016;7:10875. doi: 10.1038/ncomms10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–1533. doi: 10.1056/NEJMoa021933. Important contribution documenting the predictable appearance of autoantibodies prior to diagnosis of autoimmune disease. [DOI] [PubMed] [Google Scholar]
- 47.Theander E, Jonsson R, Sjostrom B, Brokstad K, Olsson P, Henriksson G. Prediction of Sjogren’s Syndrome Years Before Diagnosis and Identification of Patients With Early Onset and Severe Disease Course by Autoantibody Profiling. Arthritis & rheumatology. 2015;67(9):2427–2436. doi: 10.1002/art.39214. [DOI] [PubMed] [Google Scholar]
- 48.Steen VD, Powell DL, Medsger TA., Jr Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;31(2):196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- 49.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol. 2012;8(10):573–586. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- 50.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catrina AI, Svensson CI, Malmstrom V, Schett G, Klareskog L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(2):79–86. doi: 10.1038/nrrheum.2016.200. [DOI] [PubMed] [Google Scholar]
- 52.Ghahramani N. Silica nephropathy. The international journal of occupational and environmental medicine. 2010;1(3):108–115. [PubMed] [Google Scholar]
- 53.Pollard KM. Silica, Silicosis, and Autoimmunity. Frontiers in immunology. 2016;7:97. doi: 10.3389/fimmu.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]