Abstract
We describe a case of cerebral trichomoniasis in a neonate who developed seizures and multi-organ failure during treatment for staphylococcal sepsis. Brain abscesses were identified on cranial sonography. Trichomonas vaginalis was isolated from cerebrospinal fluid. There was a fatal outcome despite metronidazole therapy. This is the first report of T. vaginalis brain abscess in a neonate.
Keywords: Trichomonas vaginalis, Brain abscess, Neonate
Case
A female infant born at 24 weeks gestation weighing 740 grams was transferred to our neonatal intensive care unit two hours after delivery in a nearby medical center. She was born to a 28-year-old gravida 8, para 5 (2 abortions, 5 living) woman who had limited prenatal care consisting of a single prenatal visit prior to 24 weeks. The infant’s mother had a history of heroin use with recurrent cellulitis at drug injection sites. Her physical exam upon presentation at the outside hospital showed cellulitis of the inner thighs. Her urine drug screen was positive for methamphetamines and marijuana. Prenatal screening labs indicated that she was hepatitis C antibody positive, hepatitis B surface antigen negative, reactive plasma reagin (RPR) negative, human immunodeficiency virus (HIV) antibody negative, and rubella immune. She was not tested for nor treated for trichomoniasis prenatally.
The infant was born via spontaneous, vaginal delivery precipitated by a partial placental abruption. She was intubated in the delivery room and transferred to our facility. Blood and cerebrospinal fluid (CSF) cultures were sent, and she was commenced on intravenous ampicillin and gentamicin for presumed sepsis. Both antibiotics were discontinued 96 hours later following negative blood and CSF cultures.
On hospital day 11, she developed oliguria and hemodynamic instability (bradycardia, desaturations and hypotension) necessitating increased ventilator support. Complete blood count showed a leukocyte count of 3.3 × 103/μL with 32% bands, 7% neutrophils, 36% lymphocytes, 20% monocytes and 3% eosinophils; hemoglobin of 9.3 g/dl, and thrombocytopenia with a platelet count of 88 × 103/μL. Oxacillin and gentamicin were commenced for presumed sepsis after obtaining a blood culture. Within 24 hours, blood culture showed growth of gram positive cocci in clusters prompting replacement of IV oxacillin with vancomycin. The isolate was subsequently identified as methicillin-resistant Staphylococcus aureus (MRSA). Despite antibiotics, the infant continued to have progressive hemodynamic instability requiring vasopressive therapy with dopamine and dobutamine and then hydrocortisone for pressor-resistant hypotension.
On that same day, the patient began to have seizures so a cranial ultrasound and electroencephalogram (EEG) were performed. The ultrasound showed non-specific, markedly decreased cerebral sulcation or smoothing which was attributed to prematurity and the EEG was inconclusive due to excessive artifact. She was started on levetiracetam and phenobarbital. She also developed abdominal distension, bloody gastric aspirates and pustules overlying a swollen right upper arm. Abdominal paracentesis yielded scant serous fluid that showed growth of MRSA. Culture of swabs of the cellulitic upper arm grew MRSA and Group B streptococcus (GBS).
Lumbar puncture (LP) was performed on day 13 which showed clear CSF with 2 total nucleated cells/μL, glucose of 138 mg/dl and protein of 535 mg/dl. The CSF gram stain and culture were negative. She remained unstable and numerous daily blood cultures in the following days drawn from central catheters and peripheral venipunctures remained positive for MRSA. Infectious disease consultation was requested and her antimicrobial regimen was changed to vancomycin, rifampin and cefotaxime. Her critically-ill condition and need for multiple infusions including vasopressors precluded removal of infected central lines. Echocardiogram did not reveal vegetations. No intra-abdominal fluid collections were noted on abdominal ultrasound and an X-ray of the right arm showed no evidence of osteomyelitis.
On hospital day 21, the patient developed increased seizure activity despite anti-epileptic medications. A cranial ultrasound demonstrated increased ventricular size, new grade one sub-ependymal hemorrhage and a complex, bilateral, extra-axial fluid collection. These changes were worse in the left fronto-parietal region described as a “chaotic appearance”. In this region, there was a complex fluid collection with uniform internal echoes and a thick echogenic rim with small amounts of air. Numerous foci of air were also seen within the periventricular white matter. These findings were radiographically interpreted as suggestive of a brain abscess with a gas-forming organism or infarction with necrotizing encephalitis. A repeat LP was performed showing bloody CSF with 1 total nucleated cell/μL, 3361 red blood cells/μL, glucose of 33 mg/dl and protein of 1,245 mg/dl. Several motile protozoa were noted on CSF microscopy, suspicious for trichomonads. Metronidazole was started at 15 mg/kg/dose intravenously every 12 hours. Her condition further worsened, with multiple episodes of bradycardia and severe hypotension despite maximum ventilator and vasopressor support. She became significantly edematous and anuric. The patient’s mother consented to withdrawal of care. She expired on hospital day 22. A request for an autopsy was declined.
The CSF sample obtained on day 21 was sent to the Reference Diagnostics Laboratory of the Centers for Disease Control (CDC). Here, the organism was morphologically identified as a T. vaginalis based upon the distinctive elongate nucleus, large axostyle and four anterior flagella (figure 1). DNA was extracted and subjected to a PCR using primers developed at the CDC for amplifying the ITS1/ITS2 region of the Trichomonas mitochondrial genome (TriITS1F; 5′-TTATCTAGAGGAAGGAGAAGT CG-3′ and TriITS2R; 5′-CTTCAGTTCAGCGGGTCTTC-3′). The product revealed 100% sequence homology to T. vaginalis by BLAST.
Figure 1.
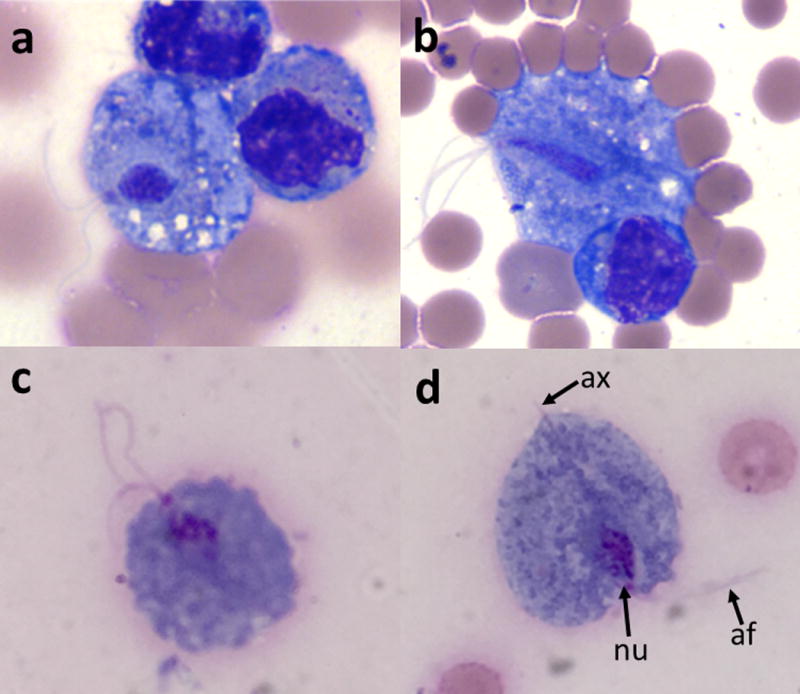
Trophozoite of Trichomonas vaginalis in CSF, adjacent to a lymphocyte (Wright’s stain, ×1000 magnification).
af: anterior flagella; nu: nucleus; ax: axostyle
Discussion
Trichomonas vaginalis is a flagellated protozoan commonly implicated in genitourinary (GU) tract infections in sexually-active individuals. Infections outside of the GU tract are uncommon. Three other species of trichomonads are known to colonize humans; Trichomonas tenax in the oral cavity and Pentatrichomonas hominis and Dientamoeba fragilis in the gastrointestinal tract [1]. Lower respiratory tract and intra-abdominal infections secondary to T. tenax, T. vaginalis and other unidentified or non-human trichomonads have been described in adults and neonates [1–6]. A case of T. vaginalis causing perinephric abscess has been reported [6].
Genital tract infection with T. vaginalis in pregnant women has been associated with preterm labor and premature rupture of membranes [7]. In most cases, infants born to infected mothers are asymptomatic but may have low birth weight or experience self-limiting vaginitis. Asymptomatic urinary tract colonization is also common. Occasionally, vaginitis requiring antimicrobial therapy has been observed in infants of mothers with T. vaginalis vaginitis. [8–11]
Neuroinvasive disease caused by Trichomonas spp. is exceedingly rare and has only been described in two adults with cancer [12, 13]. Masur, et al. [12] reported a 69-year-old man with esophageal carcinoma who developed fever and meningeal irritation signs following radiation therapy and esophago-gastrostomy. Trichomonads were identified on Giemsa stain and culture of spinal fluid. The patient also had multiple enteric organisms identified in the CSF. Surgical exploration revealed a fistulous tract between the prevertebral space and esophago-gastrostomy. The patient was treated with metronidazole but ultimately succumbed. Okamoto, et al. [13] also described a case of Trichomonas foetus meningoencephalitis and epididymitis in a 34-year-old man who had undergone an allogeneic stem cell transplant for acute myelogenous leukemia. Symptoms were fever, seizures, meningeal irritation signs, loss of consciousness and scrotal swelling. Computed tomography scans of the brain showed hydrocephalus. Numerous trichomonads were detected on CSF staining and based on morphology, these were identified as T. foetus, a bovine trichomonad. The outcome was also fatality despite metronidazole treatment. Severe meningoencephalitis, ventriculitis and epididymitis were observed on autopsy and trichomonads found in the meninges on histopathologic examination.
Thus, to our knowledge, this is the first reported case of T. vaginalis encephalitis and brain abscess in a neonate. Our patient was an extremely premature infant born to a mother who had limited prenatal care. We surmise that our patient’s mother likely had T. vaginalis vaginitis which may have contributed to preterm labor and premature rupture of membranes; and subsequent infection of her infant. The timing and route of infection was likely peripartum, during passage through the birth canal. However, the pathway of nervous system invasion is unclear. Hematogenous spread and seeding via the blood-brain-barrier is plausible although parasitemia following T. vaginalis infection has not been documented. In the aforementioned case by Masur et al [12], a fistulous tract was probably the portal of entry. In the patient reported by Okamoto et al [13], the GU tract was the presumed source because of the presence of epididymitis and prostatitis but the pathway of brain invasion was also unclear. An autopsy could not be performed on our patient to determine if fistulous connections existed. We were unable to test other clinical specimens because death ensued shortly after diagnosis. The clinical picture in our patient was similar to the adult cases. Symptoms indicative of neurologic involvement were present such as seizures and altered mentation. It is noteworthy that our patient differed from the adult cases in the absence of a spinal fluid pleocytosis. Hydrocephalus and severe parenchymal damage on neuroimaging were similarly noted in our patient.
Nitroimidazoles (metronidazole and tinidazole) are the drugs of choice for T. vaginalis GU tract infections. Our patient, like the other two reported invasive Trichomonas cases, had a fatal outcome. However, all three cases had significant co-morbidities suggesting a role as an opportunistic pathogen [12–14]. Although MRSA brain abscesses have been described in preterm neonates with MRSA sepsis, we theorize that the extensive brain damage noted in our patient may not be solely attributable to MRSA. In the case reported by Masur et al [12], meningitis resulted from a polymicrobial infection and it was determined that death resulted from an intra-abdominal hemorrhage. Giemsa stains of the paraspinal tissue and meninges during autopsy of their patient did not reveal trichomonads suggesting microbiologic clearance. Although spontaneous recovery has been noted in untreated T. vaginalis respiratory tract infections [3] calling to question its pathogenic potential [9, 14], treatment with systemic metronidazole is typically instituted. In the patient reported by Okamoto et al [13], death occurred a day after treatment with metronidazole was started, although delayed diagnosis and immune compromise likely contributed to the poor outcome. Whether metronidazole is effective therapy for T. vaginalis brain abscesses remains unanswered. Metronidazole has reliable CNS penetration and is widely used in the antibiotic regimen for treating brain abscesses. As care was withdrawn soon after therapy was begun in our patient, its impact on our patient’s clinical course could not be determined.
In conclusion, we report a rare case of T. vaginalis brain abscess in a preterm neonate with concomitant MRSA sepsis, born to a mother with limited prenatal care. This case illustrates the potential of T. vaginalis to cause severe disease outside the GU tract and underscores the importance of identifying and adequately treating pregnant women with T. vaginalis infection. It also highlights the need to reassess the traditional expectations of spontaneous resolution of T. vaginalis vaginitis in infants.
Acknowledgments
We wish to show our appreciation to Dr. Pamela McMahon and the Institutional Review Board of Woman’s Hospital, Baton Rouge, Louisiana for their guidance in obtaining approval and consent. We thank Drs. Steven Spedale, Cynthia Voelker, and Kimberly Stewart, and the staff of the Neonatology department of Woman’s Hospital, for their assistance with providing access to medical records used for manuscript preparation.
Funding
No funding was used for the development of this case report.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Martinez-Giron R, Esteban JG, Ribas A, Doganci L. Protozoa in respiratory pathology: a review. Eur Respir J. 2008;32(5):1354–70. doi: 10.1183/09031936.00022008. [DOI] [PubMed] [Google Scholar]
- 2.Hiemstra I, Van Bel F, Berger HM. Can Trichomonas vaginalis cause pneumonia in newborn babies? Brit Med J. 1984;289:355–356. doi: 10.1136/bmj.289.6441.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter JE, Whithaus KC. Neonatal respiratory tract involvement by Trichomonas vaginalis: a case report and review of the literature. Am J Trop Med Hyg. 2008;78(1):17–9. [PubMed] [Google Scholar]
- 4.Hersh SM. Pulmonary trichomoniasis and Trichomonas tenax. J Med Microbiol. 1985;20(1):1–10. doi: 10.1099/00222615-20-1-1. [DOI] [PubMed] [Google Scholar]
- 5.Zalonis CA, Pillay A, Secor W, Humburg B. Rare Case of Trichomonal Peritonitis. Emerg Infect Dis. 2011;17(7):1312–1313. doi: 10.3201/eid1707.100892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suriyanon V, Nelson KE, Choomsai NA, Ayudhya V. Trichomonas vaginalis in a perinephric abscess. A case report. Am J Trop Med Hyg. 1975;24:776–780. doi: 10.4269/ajtmh.1975.24.776. [DOI] [PubMed] [Google Scholar]
- 7.Silver BJ, Guy RJ, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and metaanalysis. Sex Transm Dis. 2014;41(6):369–76. doi: 10.1097/OLQ.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 8.Al-Salihi FL, Curran JP, Wang JS. Neonatal Trichomonas Vaginalis: Report of Three Cases and Review of the Literature. Pediatrics. 1974;74(2):196–200. [PubMed] [Google Scholar]
- 9.Trinitis J, Epie N, Boss R, Riedel S. Neonatal Trichomonas vaginalis infection: a case report and review of literature. Int J STD AIDS. 2010;21(8):606–7. doi: 10.1258/ijsa.2010.010174. [DOI] [PubMed] [Google Scholar]
- 10.Danesh IS, Stephen JM, Gorbach J. Neonatal Trichomonas vaginalis infection. J Emerg Med. 1995;13(1):51–4. doi: 10.1016/0736-4679(94)00112-x. [DOI] [PubMed] [Google Scholar]
- 11.Smith LM, Wang M, Zangwill K, Yeh S. Trichomonas vaginalis infection in a premature newborn. Journal Perinatol. 2002;22:502–502. doi: 10.1038/sj.jp.7210714. [DOI] [PubMed] [Google Scholar]
- 12.Masur H, Hook E, Armstrong D. A Trichomonas species in a mixed microbial meningitis. JAMA. 1976;236:1978–1979. [PubMed] [Google Scholar]
- 13.Okamoto S, Wakui M, Kobayashi H, Sato N, Ishida A, Tanabe M, Takeuchi T, Fukushima S, Yamada T, Ikeda Y. Trichomonas foetus meningoencephalitis after allogenic peripheral blood stem cell transplantation. Bone Marrow Transplant. 1998;21(1):89–91. doi: 10.1038/sj.bmt.1701032. [DOI] [PubMed] [Google Scholar]
- 14.Maritz JM, Land KM, Carlton JM, Hint RP. What is the importance of zoonotic trichomonads for human health? Trends Parasitol. 2014;30(7):333–41. doi: 10.1016/j.pt.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


