Abstract
BACKGROUND
To estimate the frequency and duration of detectable Zika virus (ZIKV) RNA in human body fluids, we prospectively assessed a cohort of newly infected participants in Puerto Rico.
METHODS
We evaluated samples obtained from 150 participants (including 55 men) in whom ZIKV RNA was detected on reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay in urine or blood in an enhanced arboviral clinical surveillance site. We collected serum, urine, saliva, semen, and vaginal secretions weekly for the first month and then at 2, 4, and 6 months. All specimens were tested by means of RT-PCR, and serum was tested with the use of anti–ZIKV IgM enzyme-linked immunosorbent assay. Among the participants with ZIKV RNA in any specimen at week 4, biweekly collection continued until all specimens tested negative. We used parametric Weibull regression models to estimate the time until the loss of ZIKV RNA detection in each body fluid and reported the findings in medians and 95th percentiles.
RESULTS
The medians and 95th percentiles for the time until the loss of ZIKV RNA detection were 14 days (95% confidence interval [CI], 11 to 17) and 54 days (95% CI, 43 to 64), respectively, in serum; 8 days (95% CI, 6 to 10) and 39 days (95% CI, 31 to 47) in urine; and 34 days (95% CI, 28 to 41) and 81 days (95% CI, 64 to 98) in semen. Few participants had detectable ZIKV RNA in saliva or vaginal secretions.
CONCLUSIONS
The prolonged time until ZIKV RNA clearance in serum in this study may have implications for the diagnosis and prevention of ZIKV infection. Current sexual-prevention guidelines recommend that men use condoms or abstain from sex for 6 months after ZIKV exposure; in 95% of the men in this study, ZIKV RNA was cleared from semen after about 3 months. (Funded by the Centers for Disease Control and Prevention.)
After its discovery in uganda in 1947, Zika virus (ZIKV) was identified in Brazil in 2015 and subsequently spread throughout the Americas.1 ZIKV is now recognized as a cause of congenital neurologic birth defects, notably microcephaly,2 and has been associated with potentially fatal complications.3,4
ZIKV infection can be diagnosed through detection of ZIKV RNA in blood, urine, and other body fluids on reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay.5 However, the frequency with which ZIKV RNA can be detected in various body fluids and the length of time that it remains detectable are not well understood. Similarly, ZIKV infection can also be diagnosed by the detection of anti–ZIKV IgM antibodies, although the kinetics of IgM antibody production have not been fully described.
Although most ZIKV infections are probably transmitted by infected mosquitoes, ZIKV transmission has been documented through sexual contact,6 blood transfusion,7 laboratory exposure,1 and both intrauterine and intrapartum transmission.8 ZIKV RNA has been detected in semen,9 urine,10 saliva,11 cerebrospinal fluid,12 vaginal or cervical secretions,13,14 and other body fluids.15-18 Most transmissions through sexual contact have been from men with symptomatic infection to their female partners.19-21 However, sexual transmission has also occurred from asymptomatic men,22,23 through male-to-male24 and female-to-male sex,25 and possibly through oral sex.9 Shedding in the female genital tract appears to be rare and of short duration.13 In contrast, there are reports of prolonged detection of ZIKV RNA in semen, with the longest reported duration of detection up to 188 days after onset.26,27 Infectious virus has been reported in semen up to 69 days.28
A detailed understanding of the dynamics of the early stages of ZIKV infection is needed to inform diagnostic testing algorithms and prevention interventions, since existing evidence is based on case reports and cross-sectional observations, primarily from returning travelers.29 To estimate the presence and duration of the detection of ZIKV RNA in body fluids and anti–ZIKV IgM antibody among participants with acute ZIKV infection, we established the ZIKV Persistence (ZiPer) cohort study in Puerto Rico, in which we prospectively evaluated multiple concurrently collected specimens from participants. Here, we report the results of the interim analyses to provide timely data that can inform recommendations.
METHODS
STUDY DESIGN AND OVERSIGHT
ZiPer is a prospective cohort study involving participants of all ages with ZIKV infection, as diagnosed by means of RT-PCR, with a target enrollment of 350 participants. Beginning in May 2016, participants were identified through the Sentinel Enhanced Dengue Surveillance System (SEDSS), a prospective surveillance of acute febrile illness among patients presenting to the emergency department of a tertiary care hospital or an outpatient clinic, both located in Ponce, Puerto Rico. Patients who presented with fever (temperature, ≥38.0°C [100.5°F]), rash, conjunctivitis, or arthralgia were offered participation in SEDSS. Among the participants who provided written informed consent, blood and urine specimens were tested for causative agents of acute febrile illness, including ZIKV. SEDSS participants who tested positive for ZIKV infection on RT-PCR (index participants) were systematically contacted by study staff and were offered enrollment in ZiPer. The household members of index participants were invited to participate and provide specimens, and those who tested positive on RT-PCR joined the prospective cohort study. Full details regarding the study design are provided in the protocol (available with the full text of this article at NEJM.org), which was reviewed and approved by the institutional review boards at the Centers for Disease Control and Prevention (CDC) and Ponce Health Sciences University.
PROCEDURES
All the participants completed an interviewer-administered questionnaire, which included reporting the number of days that had elapsed since the onset of ZIKV symptoms; household contacts of the participants reported such data at the time of enrollment. Participants were defined as being symptomatic if they reported having had signs or symptoms of ZIKV infection (fever, conjunctivitis, rash, or arthralgia) during the 30 days before the interview. Serum, urine, saliva, semen, and vaginal secretions (the last two in adults only) were collected weekly for the first month and at 2, 4, and 6 months thereafter. Among the participants in whom ZIKV RNA was detected in any specimen at week 4, biweekly collection continued until all the specimens tested negative. (Details are provided in the Methods section in the Supplementary Appendix, available at NEJM.org.) All the participants received a $50 reimbursement per visit.
LABORATORY TESTING
Specimens were tested by means of the Trioplex RT-PCR assay, as recommended by the CDC for the detection of dengue, chikungunya, and ZIKV RNA.30 In addition, we performed validation analyses for the use of the Trioplex RT-PCR assay in semen (see the Supplementary Appendix). Specimens were considered to be positive if target amplification was detected within 38 threshold cycles. The RNA extraction and real-time RT-PCR process were considered to be valid if the human RNase P reaction was positive. Intermittent RNA detection was defined as the detection of viral RNA that was followed by a lack of detection and then subsequent detection, regardless of the interval between specimen collections. Serum was tested by means of anti–ZIKV IgM antibody capture enzyme-linked immunosorbent assay.31 ZIKV isolation was attempted through culture in a subset of semen and serum specimens (see the Supplementary Appendix).
STATISTICAL ANALYSIS
We summarized the demographic and clinical characteristics of the participants, along with details regarding the detection of ZIKV RNA in fluids and IgM antibody according to the number of days that had elapsed since the onset of ZIKV symptoms. We used the kappa statistic to assess the beyond-chance agreement in RNA detection in paired samples of semen and serum and in paired samples of semen and urine obtained from male participants. Since all the participants had positive results on testing of serum or urine at enrollment, we could not independently assess the serum–urine agreement. The time until the loss of RNA detection in each fluid was defined as the number of days between the onset of ZIKV symptoms and the first negative RT-PCR result. To estimate model-derived percentiles for the time until virus clearance at the population level, we assumed that all infected participants had ZIKV RNA in all specimens at symptom onset. For the participants who had intermittent shedding of ZIKV, we used the first negative result after the final recorded test result that was positive on RT-PCR; data were censored for the participants who still had positive results on RT-PCR at the time of the analysis.
The time until the detection of IgM antibody was defined as the number of days between the onset of ZIKV symptoms and the first IgM-positive result; data were censored for the participants in whom the results were still IgM-negative at the time of the analysis. We used the Kaplan–Meier method to estimate survival functions for these outcomes, along with the non-parametric maximum-likelihood Turnbull estimator and parametric Weibull regression models. (Details about these models are provided in the Supplementary Appendix.) The Turnbull method and Weibull models accounted for interval censoring (since the loss of detection of ZIKV RNA occurred within an interval between visits instead of being observed on an exact date). From the Weibull models, we estimated survival functions and their 95% confidence intervals, as well as medians and 95th percentiles. In supplementary analyses, we estimated models for the time until the loss of detection that were restricted to the participants with any ZIKV RNA in a given fluid and to index participants. Model-derived medians were not estimated for saliva and vaginal secretions because of the few positive results. All statistical analyses were performed with the use of SAS software, version 9.3.
RESULTS
As of September 21, 2016, we had contacted 414 of the 1258 index participants with symptomatic ZIKV infection, as confirmed on RT-PCR assay. Of these participants, 127 were enrolled in the study. The percentage of index participants who were adults (≥18 years of age) was higher among those who were enrolled in the study than among those who were not enrolled (92% vs. 74%), and more were male (59% vs. 45%). Of the 195 household contacts of the index participants who were screened, 23 (12%) tested positive for ZIKV RNA, for a total of 150 prospective participants. All the participants remained under prospective observation, with 493 of 549 visits (90%) attended, except for 1 participant who withdrew and 2 who were administratively discontinued.
The mean age of participants was 38 years; 44% were female, including 5 who were pregnant (Table 1). Four household contacts with positive results were asymptomatic at enrollment. Among the 146 participants with signs or symptoms of ZIKV infection at enrollment, 92% were enrolled within 1 week after the onset of illness.
Table 1.
Characteristics of the Participants at Baseline.
| Characteristic | Participants (N = 150) |
|---|---|
| Age | |
| Mean (range) — yr | 37.8 (<1 to 83) |
| Age group — no. (%) | |
| 0–17 yr | 17 (11.3) |
| 18–64 yr | 124 (82.7) |
| ≥65 yr | 9 (6.0) |
| Female sex — no. (%) | 66 (44.0) |
| Pregnancy — no. (%) | 5 (3.3) |
| Presence of signs or symptoms of Zika virus infection at enrollment — no. (%) | |
| No* | 4 (2.7) |
| Yes | 146 (97.3) |
| Days after symptom onset at enrollment — no./total no. (%) | |
| 0–2 days | 66/146 (45.2) |
| 3–5 days | 63/146 (43.2) |
| 6–7 days | 5/146 (3.4) |
| 8–14 days | 4/146 (2.7) |
| ≥15 days | 8/146 (5.5) |
| Signs or symptoms at enrollment — no./total no. (%)† | |
| Fever | 115/146 (78.8) |
| Red eyes or eye pain | 119/146 (81.5) |
| Rash | 135/144 (93.8) |
| Pruritus | 117/145 (80.7) |
| Photophobia | 59/144 (41.0) |
| Edema | 92/145 (63.4) |
| Arthralgia | 120/139 (86.3) |
| Myalgia | 102/125 (81.6) |
| Headache | 115/145 (79.3) |
| Abdominal pain | 73/145 (50.3) |
| Lymphadenopathy | 50/144 (34.7) |
| Diarrhea | 62/145 (42.8) |
| Nausea | 63/145 (43.4) |
| Vomiting | 17/145 (11.7) |
| Pelvic pain | 25/139 (18.0) |
| Dysuria | 25/145 (17.2) |
| Other‡ | 129/144 (89.6) |
| Laboratory findings | |
| Median white-cell count (range) per mm3 | 5200 (2100 to 40,000) |
| Median platelet count (range) per mm3 | 216,000 (80,000 to 373,000) |
| Median hematocrit (range) — % | 42.2 (30.9 to 51.9) |
This category includes two participants who were asymptomatic at baseline but in whom signs or symptoms developed within 7 days after specimen collection.
The median duration of fever was 2 days (range, 1 to 22); red eyes, 3 days (range, 1 to 7); and rash, 5 days (range, 1 to 25).
Other signs or symptoms included cough (in 33.1% of the participants), yellow eyes or skin (4.8%), difficulty urinating (7.8%), blood in urine (5.5%), painful ejaculation (6.7% of men), and penile discharge (2.7% of men).
ANTIBODY RESPONSE
Anti–ZIKV IgM antibody was detected in at least one specimen obtained from 140 of 143 participants (97.9%). Only one specimen was collected from each of the 3 IgM-negative participants on days 4, 7, and 30 after the onset of symptoms. Details regarding the detection of IgM antibody are provided in Figure S1 in the Supplementary Appendix.
ZIKV RNA IN SERUM
A total of 132 of 150 participants (88.0%) had detectable ZIKV RNA in at least one serum specimen (Table 2). Of the 132 participants, 42 (31.8%) had detectable ZIKV RNA more than once. (Values for threshold cycle are provided in Fig. S8A in the Supplementary Appendix.) The median time until the loss of RNA detection was 14 days (95% confidence interval [CI], 11 to 17), and the 95th percentile of time was 54 days (95% CI, 43 to 64) on the basis of the Weibull model (Fig. 1A). Results that were obtained with the use of the Turnbull model were similar to those obtained with the Weibull model (Fig. S2A in the Supplementary Appendix). The number of days after the onset of symptoms at enrollment did not influence the time until the loss of ZIKV RNA detection. When the analyses were restricted to the 50% of participants who were enrolled within 2 days after symptom onset, the median time until the loss of detection was 13 days (95% CI, 10 to 17). Among the 5 pregnant women, 3 had detectable RNA at 46 days after symptom onset. At the interim analysis, 20 of 150 (13.3%) had detectable RNA at their last visit and were still being followed. The maximum duration of detection was 80 days after symptom onset in a pregnant participant; an estimated 2% had detectable RNA at this time (Fig. S3A in the Supplementary Appendix).
Table 2.
Detection of ZIKV RNA in Body Fluids and Anti–ZIKV IgM Antibody in Serum, According to Subgroup.*
| Subgroup | ZIKV RNA | Anti–ZIKV IgM Antibody | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Serum | Urine | Saliva | Vaginal Secretions | Semen | Serum | |
| number/total number (percent) | ||||||
| All participants | 132/150 (88.0) | 92/149 (61.7) | 15/147 (10.2) | 1/50 (2.0) | 31/55 (56.4) | 140/143 (97.9) |
|
| ||||||
| Age (yr) | ||||||
|
| ||||||
| 0–17 | 15/17 (88.2) | 9/16 (56.3) | 1/16 (6.3) | NA | NA | 17/17 (100) |
|
| ||||||
| 18–64 | 109/123 (88.6) | 73/123 (59.3) | 14/122 (11.5) | 1/47 (2.1) | 30/54 (55.6) | 114/117 (97.4) |
|
| ||||||
| ≥65 | 7/9 (77.8) | 9/9 (100) | 0/9 | 0/3 | 1/1 (100) | 9/9 (100) |
|
| ||||||
| Sex | ||||||
|
| ||||||
| Male | 72/83 (86.8) | 54/83 (65.1) | 9/83 (10.8) | NA | 31/55 (56.4) | 78/80 (97.5) |
|
| ||||||
| Female | 59/66 (89.4) | 37/65 (56.9) | 6/64 (9.4) | 1/50 (2.0) | NA | 62/63 (98.4) |
|
| ||||||
| Pregnancy | 5/5 (100) | 0/5 | 0/5 | 0/5 | NA | 5/5 (100) |
Of the 150 index participants, 127 were recruited into the study after a visit to the Sentinel Enhanced Dengue Surveillance System (SEDSS). Saliva, vaginal secretions, and semen are not collected as part of SEDSS. Therefore, few specimens of these types were available within 7 days after the onset of symptoms. NA denotes not applicable.
Figure 1. Time until the Clearance of Zika Virus RNA in Serum, Urine, and Semen.
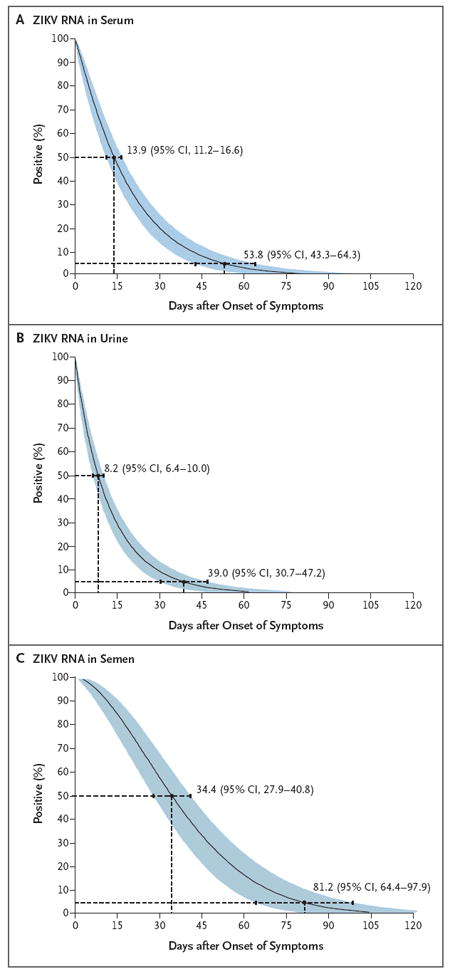
Shown are models of the time until the loss of Zika virus (ZIKV) RNA detection after the onset of symptoms in serum (Panel A), urine (Panel B), and semen (Panel C) obtained from 150 study participants, as estimated with the use of Weibull regression. To estimate model-derived percentiles for the time until virus clearance at the population level, we assumed that all infected participants had ZIKV RNA in all specimens at symptom onset. Also shown are medians and 95th percentiles of the time until the loss of detection, the key values that were reported in this preliminary study. Blue shading denotes 95% confidence intervals. Data for 4 participants who were asymptomatic at the time of enrollment were excluded from the estimates of the time until the loss of RNA detection, since the number of days after the onset of symptoms could not be determined.
ZIKV RNA IN URINE
ZIKV RNA was detected in at least one urine specimen in 92 of 149 participants (61.7%) (Table 2). Overall, 15 (10.1%) had detectable RNA in urine but not in serum, whereas 55 (36.7%) had RNA in serum but not urine. The model-based median time until the loss of detection was 8 days (95% CI, 6 to 10), and the 95th percentile of time was 39 days (95% CI, 31 to 47) (Fig. 1B).
ZIKV RNA IN SALIVA AND VAGINAL SECRETIONS
Among the 147 participants who were tested, 15 (10.2%) had ZIKV RNA in at least one saliva specimen (Table 2). The rate of positivity in these samples was lower than the rate in serum and urine at any number of days after symptom onset (Table 3). Similarly, only 1 in 50 women (2%) had ZIKV RNA in vaginal secretions (at 3 days after symptom onset). All the samples were positive in the RNase P control reaction.
Table 3.
Detection of ZIKV RNA in Body Fluids and Anti–ZIKV IgM Antibody in Serum, According to the Number of Days after Symptom Onset.*
| Positivity and Days after Symptom Onset | ZIKV RNA† | Anti–ZIKV IgM Antibody‡ | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Serum | Urine | Saliva | Vaginal Secretions | Semen | Serum | |
| number/total number (percent) | ||||||
|
Participant analyses
| ||||||
| Any interval after symptom onset | 128/146 (87.7) | 90/145 (62.1) | 13/143 (9.1) | 1/49 (2.0) | 31/55 (56.4) | 137/139 (98.6) |
|
| ||||||
| 0–7 days | 118/134 (88.1) | 77/129 (59.7) | 3/6 (50.0) | 1/1 (100) | 0/1 | 17/50 (34.0) |
|
| ||||||
| 8–15 days | 10/28 (35.7) | 12/29 (41.4) | 1/25 (4.0) | 0/6 | 5/8 (62.5) | 28/28 (100) |
|
| ||||||
| 16–30 days | 27/129 (20.9) | 21/125 (16.8) | 5/127 (3.9) | 0/39 | 20/40 (50.0) | 120/121 (99.2) |
|
| ||||||
| 31–45 days | 14/126 (11.1) | 6/119 (5.0) | 4/125 (3.2) | 0/42 | 20/46 (43.5) | 108/111 (97.3) |
|
| ||||||
| 46–60 days | 6/67 (9.0) | 1/65 (1.5) | 1/64 (1.6) | 0/21 | 6/25 (24.0) | 58/60 (96.7) |
|
| ||||||
| >60 days | 3/79 (3.8) | 0/71 | 1/80 (1.3) | 0/30 | 3/23 (13.0) | 52/60 (86.7) |
|
| ||||||
|
Specimen analyses
| ||||||
| Any interval after symptom onset | 190/805 (23.6) | 120/750 (16.0) | 17/647 (2.6) | 1/219 (0.5) | 76/216 (35.2) | 563/622 (90.5) |
|
| ||||||
| 0–7 days | 119/135 (88.2) | 77/129 (59.7) | 4/7 (57.1) | 1/1 (100) | 0/1 | 17/50 (34.0) |
|
| ||||||
| 8–15 days | 10/28 (35.7) | 12/29 (41.4) | 1/25 (4.0) | 0/6 | 5/8 (62.5) | 28/28 (100) |
|
| ||||||
| 16–30 days | 34/227 (15.0) | 24/214 (11.2) | 6/216 (2.8) | 0/66 | 29/65 (44.6) | 205/207 (99.0) |
|
| ||||||
| 31–45 days | 16/211 (7.6) | 6/197 (3.0) | 4/203 (2.0) | 0/73 | 29/76 (38.2) | 176/180 (97.8) |
|
| ||||||
| 46–60 days | 7/79 (8.9) | 1/77 (1.3) | 1/75 (1.3) | 0/30 | 6/28 (21.4) | 69/71 (97.2) |
|
| ||||||
| >60 days | 4/125 (3.2) | 0/104 | 1/121 (0.8) | 0/43 | 7/38 (18.4) | 68/86 (79.1) |
The number of participants and specimens that were evaluated at each interval after the onset of symptoms varies because participants were enrolled as they presented for surveillance or tested positive as household contacts. Data for the four household contacts who were asymptomatic at the time of enrollment were excluded from this analysis, since there was no known date of symptom onset. For these participants, the maximum duration of detection after enrollment was 65 days for serum and 15 days for urine; none of these participants contributed semen.
Among the participants who had positive results for ZIKV RNA at the last study visit and are still being followed are 20 participants with ongoing serum analysis (maximum duration at last analysis, 80 days after symptom onset); 10 participants with ongoing urine analysis (maximum duration, 50 days after symptom onset); and 11 participants with ongoing semen analysis (maximum duration, 125 days after symptom onset).
Three participants who had negative results on testing for IgM antibody are still being followed, since the last visit for each occurred at 4, 7, and 30 days after symptom onset.
ZIKV RNA IN SEMEN
Of 68 eligible male participants, 55 (81%) provided at least one semen specimen. ZIKV RNA was present in at least one specimen in 31 participants (56%). Chance-corrected agreement of RNA detection was low in paired samples of semen and serum (κ = 0.05; 95% CI, −0.05 to 0.16) and samples of semen and urine (κ = 0.11; 95% CI, 0.01 to 0.22). The model-derived median time until the loss of RNA detection was 34 days (95% CI, 28 to 41), and the 95th percentile of time was 81 days (95% CI, 64 to 98) (Fig. 1C). However, 11 of 55 participants had ZIKV RNA in semen at their last visit and are still being followed. The maximum duration of RNA detection was 125 days after symptom onset; an estimated 4% continued to have detectable RNA at that time (Fig. S3C in the Supplementary Appendix).
TIME UNTIL THE LOSS OF DETECTABLE ZIKV RNA
We also estimated the time until the loss of RNA detection among the participants with any ZIKV RNA in a given fluid during follow-up. Among this subset, the model-derived estimated percentiles of time until the loss of RNA detection were longer because the analyses were limited to participants with continued viral shedding (Figs. S4, S5, and S6 in the Supplementary Appendix).
ANALYSES LIMITED TO INDEX PARTICIPANTS
Index participants were more likely to be male than were the 19 symptomatic household contacts (59% vs. 42%), to be at least 18 years of age (92% vs. 68%), and to have been recruited within 1 week after symptom onset (99% vs. 42%). When the model was limited to index participants, the estimated median time until the loss of detection of ZIKV RNA decreased by 1 day in serum and urine and increased by 2 days in semen (Fig. S7 in the Supplementary Appendix).
INTERMITTENT RNA DETECTION
We observed intermittent ZIKV RNA detection in serum obtained from 15 participants, in urine samples obtained from 5 participants, and in semen samples obtained from 3 participants (Fig. S9 in the Supplementary Appendix). The range of days between positive specimens was 14 to 62 in serum, 14 to 35 in urine, and 21 to 36 in semen.
ISOLATION OF ZIKV
ZIKV isolation was attempted in 20 semen specimens with threshold-cycle values ranging from 19 to 37 and in 20 serum specimens with threshold-cycle values ranging from 22 to 37. Virus isolation was successful in 6 of 20 semen specimens (30%) with threshold-cycle values ranging from 19 to 27 and in 1 of 20 serum specimens (5%) with a threshold-cycle value of 22.
DISCUSSION
In this preliminary analysis, we obtained data on how long it takes for ZIKV RNA to clear among the participants with acute ZIKV infection and detectable ZIKV RNA at enrollment. The recruitment of participants was based on an ongoing surveillance platform that enabled 90% of participants to enroll within the first week after the onset of symptoms, which provided increased resolution for ZIKV RNA detection starting soon after symptom onset. In our study, half of the participants had detectable viral RNA in urine for at least 1 week after symptom onset, in serum for 2 weeks, and in semen for more than 1 month, whereas 5% or less had detectable viral RNA in urine for 6 weeks, in serum for 8 weeks, and in semen for 3 months. Conversely, ZIKV RNA was infrequently detected in saliva and vaginal secretions.
The CDC recommends RT-PCR testing of serum and urine samples obtained from symptomatic participants less than 14 days after symptom onset.5 Our results contrast with the findings of other studies,10,29 which showed more frequent detection of ZIKV RNA in urine than in serum. However, the cited studies had small sample sizes that limit generalizability. The discrepant results may also be explained by differences in the population that was included in the analyses. A previous study showed frequent ZIKV RNA detection in saliva within 5 days after symptom onset.11 We detected ZIKV RNA infrequently in saliva; however, we did not have enough specimens to determine RNA detection early after onset.
IgM antibody was detected in almost all ZIKV-infected participants in this study. This finding may reflect a primary immune response to ZIKV. However, given the high prevalence of previous flavivirus infection in Puerto Rico,32 these results may not be generalizable to a population that has not been extensively exposed to flavivirus. The usefulness with regard to the specificity of IgM testing for diagnosis of ZIKV in geographic areas with discrepant exposure to flavivirus requires further study.
In our study, the observed duration of ZIKV RNA in serum was longer than detection times reported for dengue virus. More than 90% of the patients who are infected with any of the four dengue viruses clear RNA within 10 days after the onset of symptoms.33 Studies involving asymptomatic blood donors with the use of transcription-mediated amplification (a technique that is more sensitive than RT-PCR) showed that the median time until RNA clearance for West Nile virus was 13 days (95% CI, 12 to 15), an interval that is similar to what we observed for ZIKV.34 Since the cross-reactivity of antibodies between flaviviruses limits the use of serologic analysis, we recruited participants who had detectable RNA at enrollment, a factor that could have contributed to increased times until RNA clearance. Although we were able to isolate ZIKV in serum and semen specimens with low threshold-cycle values, further study is required to determine whether the extended duration of ZIKV RNA in serum correlates with infectivity. The minimal time that persons who have potential exposure to ZIKV should avoid donating blood is currently 120 days, which covers the maximum duration of RNA detection that we observed in our study.35
The CDC recommends that women who have been infected or exposed to ZIKV wait at least 8 weeks from symptom onset or last exposure before attempting conception.36 In our study, 95% of the participants no longer had detectable ZIKV RNA in serum at 8 weeks. Although these data suggest that the risk of intrauterine transmission among ZIKV-infected women who are trying to conceive toward the end of an 8-week period after symptom onset is small, we will continue to monitor women of reproductive age to inform evaluations of these recommendations.
Despite model-based estimates suggesting that sexual transmission contributes only modestly to epidemic propagation,37,38 sexual transmission could complicate efforts to prevent the transmission of ZIKV. The CDC recommends that men with possible ZIKV exposure, regardless of symptom status, should use condoms or abstain from sex for at least 6 months.36 Although two case reports detected RNA in semen more than 180 days after symptom onset,26,27 such late detection seems infrequent. Our study documented that few men have detectable ZIKV RNA past 3 months, and the maximum time that has been observed in our study thus far was 125 days.
Our study has several limitations. By enrolling only participants with positive results for ZIKV RNA in urine or serum on RT-PCR assay at baseline and excluding those who were IgM-positive only, we may have biased our findings by recruiting persons who have a longer duration of ZIKV RNA in serum or urine. However, when our analyses were limited to participants who had enrolled within 2 days after symptom onset, our duration estimates were similar to those in the overall sample. The detection of ZIKV RNA does not necessarily correlate with having infectious virus, a factor that we are studying in additional virus-isolation assays. We determined the limit of detection of ZIKV RNA in semen, but we were unable to evaluate the sensitivity of the test for saliva and vaginal secretions with a similar validation study. However, all semen, saliva, and vaginal swabs tested positive for the RNase P internal control reaction, which suggests that the RNA extraction and conditions of the assay are probably not reasons for the failure to detect ZIKV RNA in saliva and vaginal secretions. Nonetheless, without knowing the limit of the detection of the Trioplex RT-PCR assay in these specimen types, negative results should be interpreted with caution. To estimate model-derived percentiles for the time until virus clearance at the population level, we assumed that all infected participants had ZIKV RNA in all specimens at symptom onset. This assumption resulted in shorter median and 95th percentile estimates than if we had limited our analyses only to participants with detectable ZIKV RNA. Data that were obtained from symptomatic participants may not be generalizable to all persons infected with ZIKV.
In conclusion, our study provides a longitudinal assessment of multiple body fluids to describe the persistence of ZIKV among infected participants. The results provide preliminary evidence that ZIKV is present in serum for a longer period than expected for other flaviviruses (e.g., dengue), a finding that may have implications for diagnostic recommendations and prevention of transmission.
Supplementary Material
Acknowledgments
Supported by the Centers for Disease Control and Prevention. Staffing support was provided by the Center for AIDS Research at Emory University (NIH P30AI050409).
We thank the study participants for their time and support of this project; and Dania Rodriguez-Vargas, Brad Biggerstaff, John T. Brooks, Laura Youngblood, Jennifer Read, Matt Lozier, Laura Adams, Candimar Colon, Tyree Staple, Olga Lorenzi, and ZiPer study staff members for their contributions to the study.
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Petersen LR, Jamieson DJ, Honein MA. Zika virus. N Engl J Med. 2016;375:294–5. doi: 10.1056/NEJMc1606769. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects — reviewing the evidence for causality. N Engl J Med. 2016;374:1981–7. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 3.Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–9. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharp TM, Muñoz-Jordán J, Perez-Padilla J, et al. Zika virus infection associated with severe thrombocytopenia. Clin Infect Dis. 2016;63:1198–201. doi: 10.1093/cid/ciw476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidance for U S laboratories testing for Zika virus infection. Atlanta: Centers for Disease Control and Prevention; 2016. https://www.cdc.gov/zika/laboratories/lab-guidance.html. [Google Scholar]
- 6.Brooks JT, Friedman A, Kachur RE, LaFlam M, Peters PJ, Jamieson DJ. Update: interim guidance for prevention of sexual transmission of Zika virus — United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65:745–7. doi: 10.15585/mmwr.mm6529e2. [DOI] [PubMed] [Google Scholar]
- 7.Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014;19(14) doi: 10.2807/1560-7917.es2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- 8.Perez S, Tato R, Cabrera JJ, et al. Confirmed case of Zika virus congenital infection, Spain, March 2016. Euro Surveill. 2016;21(24) doi: 10.2807/1560-7917.ES.2016.21.24.30261. [DOI] [PubMed] [Google Scholar]
- 9.D’Ortenzio E, Matheron S, Lamballerie X, et al. Evidence of sexual transmission of Zika virus. N Engl J Med. 2016;374:2195–8. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- 10.Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84–6. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol. 2015;68:53–5. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Rozé B, Najioullah F, Signate A, et al. Zika virus detection in cerebrospinal fluid from two patients with encephalopathy, Martinique, February 2016. Euro Surveill. 2016;21(16) doi: 10.2807/1560-7917.ES.2016.21.16.30205. [DOI] [PubMed] [Google Scholar]
- 13.Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000–1. doi: 10.1016/S1473-3099(16)30193-1. [DOI] [PubMed] [Google Scholar]
- 14.Nicastri E, Castilletti C, Balestra P, Galgani S, Ippolito G. Zika virus infection in the central nervous system and female genital tract. Emerg Infect Dis. 2016;22:2228–30. doi: 10.3201/eid2212.161280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonseca K, Meatherall B, Zarra D, et al. First case of Zika virus infection in a returning Canadian traveler. Am J Trop Med Hyg. 2014;91:1035–8. doi: 10.4269/ajtmh.14-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont-Rouzeyrol M, Biron A, O’Connor O, Huguon E, Descloux E. Infectious Zika viral particles in breastmilk. Lancet. 2016;387:1051. doi: 10.1016/S0140-6736(16)00624-3. [DOI] [PubMed] [Google Scholar]
- 17.Furtado JM, Espósito DL, Klein TM, Teixeira-Pinto T, da Fonseca BA. Uveitis associated with Zika virus infection. N Engl J Med. 2016;375:394–6. doi: 10.1056/NEJMc1603618. [DOI] [PubMed] [Google Scholar]
- 18.Calvet G, Aguiar RS, Melo AS, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653–60. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 19.Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17:880–2. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission — continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:215–6. doi: 10.15585/mmwr.mm6508e2. [DOI] [PubMed] [Google Scholar]
- 21.Venturi G, Zammarchi L, Fortuna C, et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill. 2016;21(8) doi: 10.2807/1560-7917.ES.2016.21.8.30148. [DOI] [PubMed] [Google Scholar]
- 22.Fréour T, Mirallié S, Hubert B, et al. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill. 2016;21(23) doi: 10.2807/1560-7917.ES.2016.21.23.30254. [DOI] [PubMed] [Google Scholar]
- 23.Brooks RB, Carlos MP, Myers RA, et al. Likely sexual transmission of Zika virus from a man with no symptoms of infection — Maryland, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:915–6. doi: 10.15585/mmwr.mm6534e2. [DOI] [PubMed] [Google Scholar]
- 24.Deckard DT, Chung WM, Brooks JT, et al. Male-to-male sexual transmission of Zika virus — Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:372–4. doi: 10.15585/mmwr.mm6514a3. [DOI] [PubMed] [Google Scholar]
- 25.Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika Virus — New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:716–7. doi: 10.15585/mmwr.mm6528e2. [DOI] [PubMed] [Google Scholar]
- 26.Barzon L, Pacenti M, Franchin E, et al. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill. 2016;21(32) doi: 10.2807/1560-7917.ES.2016.21.32.30316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill. 2016;21(32) doi: 10.2807/1560-7917.ES.2016.21.32.30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arsuaga M, Bujalance SG, Díaz-Menéndez M, Vázquez A, Arribas JR. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect Dis. 2016;16:1107. doi: 10.1016/S1473-3099(16)30320-6. [DOI] [PubMed] [Google Scholar]
- 29.Bingham AM, Cone M, Mock V, et al. Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease — Florida, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:475–8. doi: 10.15585/mmwr.mm6518e2. [DOI] [PubMed] [Google Scholar]
- 30.Zika virus emergency use authorization; emergency use authorizations; Trioplex real-time RT-PCR assay. Silver Spring, MD: Food and Drug Administration; 2016. http://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm#zika. [Google Scholar]
- 31.Zika virus emergency use authorization; emergency use authorizations; Zika MAC-ELISA. Silver Spring, MD: Food and Drug Administration; 2016. http://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm#zika. [Google Scholar]
- 32.Mohammed H, Tomashek KM, Stramer SL, Hunsperger E. Prevalence of anti-dengue immunoglobulin G antibodies among American Red Cross blood donors in Puerto Rico, 2006. Transfusion. 2012;52:1652–6. doi: 10.1111/j.1537-2995.2011.03492.x. [DOI] [PubMed] [Google Scholar]
- 33.Hunsperger EA, Muñoz-Jordán J, Beltran M, et al. Performance of dengue diagnostic tests in a single-specimen diagnostic algorithm. J Infect Dis. 2016;214:836–44. doi: 10.1093/infdis/jiw103. [DOI] [PubMed] [Google Scholar]
- 34.Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis. 2008;198:984–93. doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]
- 35.Guidance for industry: revised recommendations for reducing the risk of Zika virus transmission by blood and blood components. Silver Spring, MD: Food and Drug Administration; 2016. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM518213.pdf. [Google Scholar]
- 36.Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure — United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:315–22. doi: 10.15585/mmwr.mm6512e2. [DOI] [PubMed] [Google Scholar]
- 37.Gao D, Lou Y, He D, et al. Prevention and control of Zika as a mosquito-borne and sexually transmitted disease: a mathematical modeling analysis. Sci Rep. 2016;6:28070. doi: 10.1038/srep28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yakob L, Kucharski A, Hue S, Edmunds WJ. Low risk of a sexually-transmitted Zika virus outbreak. Lancet Infect Dis. 2016;16:1100–2. doi: 10.1016/S1473-3099(16)30324-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


