Abstract
The most common wound care procedures (WPCs) performed on open wounds are dressing changes and wound cleansing. Dressing changes cause moderate to severe pain in 74% of patients, nearly half (36%) of whom experience severe pain (rated as 8 to 10 on a 10-point numeric rating scale). The purpose of this paper is to propose a model of clinically accessible factors that can be tested in order to develop a clinical tool to identify which patients are likely to experience high intensity pain during non-operative WCPs, such as dressing changes. Although multiple factors are known to be associated with pain, the factors selected for this model were limited to those that 1) are supported based on evidence and/or pain mechanisms and 2) are readily accessible to clinicians/practitioners and can be tested as a prediction tool to be used prior to WCPs. This model may be helpful to identify those likely to experience high intensity pain during WCPs. In this way, use of aggressive pain management strategies, including specialty dressings, pharmacologic analgesics, and/or non-pharmacological strategies, such as high intensity transcutaneous electrical stimulation.
Keywords: Wound, Pain, Wound Care Procedures, Conceptual Model
Introduction
The most common wound care procedures (WPPs) performed on open wounds are dressing changes and wound cleansing1. Dressing changes cause moderate to severe pain in 74% of patients, nearly half (36%) of whom experience severe pain (rated as 8 to 10 on a 10-point numeric rating scale) 1. High intensity pain causes substantial stress for both patient and clinicians. When WCPs cause high intensity pain, those changing the dressing may “hurry through the procedure” in an effort to stop the pain [personal communication, Linda Abbott, RN, CWOCN]. This may lead to incomplete cleansing and packing, an increased risk of infection, delayed healing, and higher costs. Moreover, one episode of pain changes nociceptive pain pathways (i.e., sensitization; 2) and elevates anticipatory pain 3, making future procedures even more painful. Thus, addressing wound care pain, an under-recognized and under-studied issue, could greatly impact healing and reduce the cost of wound care. The most effective approach is to prevent such episodes so the ability to predict who will experience high intensity pain during WCPs is a key aspect of prevention.
WCPs, such as dressing changes, involve the movement or manipulation of wound tissue and, therefore, provide a mechanical trigger that causes or increases pain beyond baseline or “resting” pain, similar to movement-evoked pain during walking or lifting. Pain experienced during dressing changes, therefore, is similar to pain caused by mechanical triggers used to assess pain sensitivity in humans, such as pressure pain thresholds or punctate pain intensities 4. If peripheral or central sensitivity is involved in the pain experience during WCPs, then interventions targeting this response may be effective.
To date, mainstay recommendations to prevent pain during WCPs have focused on either administration of preventive and procedural analgesia and/or use of non-adherent dressings (e.g., silicone, foam, hydrofibers, and alginate) but provide no guidance on which patients should be targeted for these expensive dressing materials, or other adjunctive therapies to address peripheral or central sensitivity 5–7. Although 74% of subjects in our study experienced moderate or severe pain during WCPs only 37% received analgesia and even fewer were treated with specialized dressings 1.
The purpose of this paper is to propose a model of clinically accessible factors that can be tested in order to develop a clinical tool to identify which patients are likely to experience high intensity pain during non-operative WCPs, such as dressing changes. Although multiple factors are known to be associated with pain, the factors selected for this model were limited to those that are readily accessible to clinicians/practitioners and could be incorporated into a prediction tool for identifying patients likely to experience high levels of pain during a dressing change. The accessible factors include both patient-level and wound-level factors and were selected on both empirical and inductive approaches based on evidence and/or pain mechanisms that suggest biologically plausible factors to include. A systematic literature search was conducted in the Cumulative Index to Nursing and Allied Health Literature, PubMed, and Embase using the keywords-wound* AND pain And (procedures or dressing changes) with research as a limiter. From the retrieved articles, we excluded those that focused on resting wound pain (i.e., non-procedure related pain) and debridement. The remaining articles provided very little evidence on factors associated with WCPs. Therefore, many of the factors were derived from the evidence on factors associated with movement pain, which we propose is the same mechanism that leads to pain with WCPs.
Accessible Patient Factors
Younger Age
Studies evaluating the relationship between age and pain intensity have consistently shown that younger age is associated with higher pain intensities. Stotts et al. (2004) 8 found that younger patients had greater pain than older patients before and after wound care procedures (r = −.252, p = .001; r = −.138, p = .006, respectively). Additionally, a systematic review identifying risk factors of post-surgical pain and analgesia consumption showed that age was commonly found to have a negative correlation with incisional pain intensity and analgesia consumption, suggesting that the younger the patient, the more pain and analgesia requirements 9.
Female Sex
While Stotts et al. (2004) 8 found no differences between genders in pain during wound care procedures, the majority of research evaluating the relationship between sex and pain have found that females report higher pain intensities than males. Studies testing sex differences in post-surgical and acute whiplash pain have found that women report more intense pain than men 10,11 and exhibit greater sensitivity to noxious stimuli in the laboratory compared to men, particularly when they have a positive pain history 12. Finally, women are more likely than men to develop certain chronic pain disorders 13.
Non-white Race/Ethnicity
Racial and ethnic disparities exist in pain perception in various settings (e.g. postoperative, emergency room) and across all types of pain (e.g. acute, cancer, chronic, procedural, and experimental) 14. Stotts et al. (2004) 8 found that non-whites had greater pain intensity than whites during wound care procedures (t = −2.12, p = .034). Additionally, findings from clinical studies of postoperative pain 15,16 and studies with healthy adults. Edwards, Fillingim, and Keefe (2001) 17 suggest greater pain sensitivity among African-Americans and Latinos compared to non-Hispanic Caucasians.
Chronic Pain Conditions
Chronic pain conditions, particularly those associated with augmented central nervous system pain processing, such as fibromyalgia and irritable bowel syndrome, have been linked to higher pain during procedures such as surgery 18. Research has demonstrated that patients with chronic pain conditions have alterations in central neurotransmitters that lead to both augmented pain and sensory processing 19. While no study has evaluated the influence of chronic pain conditions on wound procedure pain, there is growing evidence demonstrating its effect on postoperative pain following a variety of surgeries, such as joint arthroplasty, pelvic floor surgery, etc. 18–20.
Opioid Tolerant/Amount
Repeated administration of opioids can elicit increased sensitivity to noxious stimuli (i.e., induce hyperalgesia) through activation of pain facilitory systems which increase the perception of pain 21. Numerous reports have demonstrated that opioid-induced hyperalgesia is observed both in animal and human experimental models 22. More recently, Wibbenmeyer et al. (2015) 23 investigated the relationship between opioid intake and postoperative pain in 130 patients with burn wounds. They found that the amount of opioids taken prior to surgery was significantly correlated to postoperative pain reports in these patients. Therefore, the amount of opioids taken prior to wound care procedures may influence the degree of pain experienced during WCP.
Anxiety and High Anticipatory Pain
The relationship between anxiety and pain intensity is well established. Anxiety promotes an overestimation of pain intensity. Level of anxiety before dressing change was significantly related to anticipatory pain (r = 0.66, p < 0.01), pain at dressing removal (r = 0.53, p < 0.01) and pain at cleansing (r = 0.44, p < 0.01) suggesting that assessment of anxiety be part of chronic wound pain evaluation and management 24. In a subsequent study, Woo (2015) 25 found that anxiety mediated the relationship between anticipation and pain perception during dressing change suggesting that anticipation of pain triggers anxiety that leads to increased pain 25. Upton (2014) 26 also found a significant, positive relationship between chronic stress and acute pain reported during dressing changes on chronic wounds, and suggests that stress due to anticipation of pain following previous bad experiences cause higher pain during dressing change 27. Anxiety has also been identified as a common predictor of incisional pain during the immediate postoperative period 9,28.
Depression
Depression and pain are common co-morbidities that share biological pathways and neurotransmitters 29,30. Dysfunction at the level of the serotonergic and noradrenergic neurons affects both ascending and descending pathways. This results in the psychological and somatic symptoms of depression and causes routine sensory input that is not normally felt to become interpreted as disagreeable or even painful 31. Converging lines of evidence suggest that four key neurotransmitters are involved in both pain and depression (serotonin, norepinephrine, substance P, and corticotrophin-releasing factor) 32. While no study has investigated the influence of depression on wound pain during dressing changes, meta-analyses evaluating psychological correlates of acute post-surgical pain have identified depression, along with anxiety and pain catastrophizing, as significantly associated 33.
Pain Catastrophizing
Pain catastrophizing, broadly defined as a negative emotional and cognitive response to pain involving elements of magnification, helplessness, and pessimism, has been shown to magnify behavioral responses to pain and exaggerate pain intensity 34. In the above mentioned meta-analysis 33, pain catastrophizing was the most strongly linked psychological variable with acute, post-surgical pain. Additionally, Roth, Lowery, & Hamill (2004) 35 found a strong association between pain catastrophizing and pain intensity (r = 0.63, p < 0.05) in patients with chronic wounds.
Accessible Wound Factors
Recent injury/Tissue Loss
The process of wound healing starts at the time of injury and can be divided into four overlapping phases: 1) Hemostasis and Coagulation; 2) Inflammation; 3) Proliferation; and 4) Remodeling 36. At the time of injury, processes of hemostasis are initiated and completed in first few hours. During this phase, clotting mechanisms produce a clot filled with cytokines and growth factors. In addition, vasoconstriction in the first few hours decreases oxygen levels, increases glycolysis and lowers pH 36. Low wound oxygen levels 37 encourage anaerobic metabolism, lowering pH and elevating lactate levels in the wound, which sensitize nociceptors 38. Vasodilation follows this initial vasoconstriction with the influx of platelets and neutrophils, which release cytokines (IL-1a, IL-1b, IL-6 and TNF-a) which sensitize nociceptors leading to pain and hyperalgesia 39–43.
The inflammatory phase is characterized by additional neutrophil recruitment 36 which further increases the release of IL-1β IL-6, and TNF-α [44] thereby further sensitizing nociceptors. Therefore, when a wound is in the first two phases, WCPs are more likely to be painful. The inflammatory period is proposed to last 3 to 5 days in acute surgical wounds 36. It is unclear how long this phase may last in large acute or traumatic wounds healing by secondary intention or in chronic wounds. Although research is lacking, these biological mechanisms suggest that days from time of injury or tissue loss may be an accessible factor that may be associated with high pain intensity during WCPs.
Chronic Wound
Type of wound (acute vs. chronic) may also be associated with high levels of pain during WCPs because chronic wounds are in a prolonged state of inflammation and, therefore, have relatively higher levels of inflammatory cytokines than acute wounds 45,46. Price and colleagues (2008) 45 found that venous, mixed, and arterial ulcers were associated with more frequent pain at dressing changes (p< 0,001) than other types of wounds. Although evidence of differences in pain intensity during WCPs between acute and chronic wounds is limited, higher levels of wound cytokines suggest that type of wound may be an accessible factor that could be associated with pain intensity during WCPs.
Clinical Inflammation
Clinical inflammation, (i.e., erythema and heat) is positively correlated with higher pain during WCPs 1. In a small pilot study, these differences approached significance in this exploratory study with an alpha of 0.10. Although other studies are lacking to corroborate this relationship, the findings of this study suggest that clinical inflammation, which are signs of vasodilation in the early phases of wound healing, chronic inflammation, or high wound bioburden may associated with high pain intensity during WCPs. High wound bioburden 47 may lead to the recruitment of neutrophils to the wound and the release of inflammatory cytokines. One of the few studies that examined the relationship between wound pain and bioburden found that high microbial load (>105 organisms/ gram of tissue) predicted chronic wound pain (predictive value = 1.00 or 100% 48).
Resting Wound Pain
High resting wound pain is likely associated with high levels of WCP pain because it may be an indication of peripheral or primary nocioceptive sensitization. Postoperative resting and movement pain were significantly correlated in a study of incisional pain following total knee arthroplasty (Spearman rho r = .54, P < 0.0001) 28. Similar to movement pain, the mechanical manipulation during WCPs stimulates sensitized nocioceptors through mechanoreceptors in the wound bed resulting in higher pain.
Partial Thickness Wounds
Level of tissue loss may also be associated with high intensity pain during WCPs because nocioceptor fiber types and densities differ in various tissues. Although studies that examine wound depth with pain during WCPs are limited. shallow wounds or partial thickness wounds may be more painful than wounds that penetrate the muscle or viscera because the cutaneous tissues are more densely innervated with nociceptors (i.e. a-delta and c fibers) than deeper tissues 49.
Conceptual Framework of Accessible Factors Associated with High Intensity Pain During Wound Care Procedures
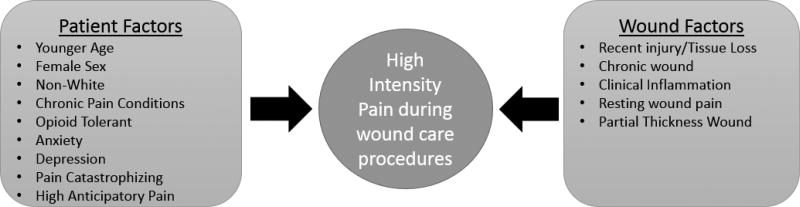
Based on the evidence to date, we propose that clinical accessible patient and wound factors may be useful in a prediction model to determine which patients are likely to experience high intensity pain during WCPs (see Figure 1). To develop the model and a scoring system by which to classify or identify patient’s likely to have high intensity pain during wound care procedures, we will develop a predictive model of pain during WCPs based on accessible wound and patient factors, with pain during WCPs treated as a dichotomous variable: low/moderate (≤7/10) vs. severe (≥8/10) and also as low (≤ 3) vs moderate/severe (≥4). Using logistic regression we will regress pain during WCPs on the 14 factors depicted in Figure 1, while controlling for dressing factors, analgesic intake, and practitioner performing the WCP, if needed. Since the predictors are readily available, all will be left in the model because our interest is in obtaining a good prediction model, not in evaluating the effects of individual factors, and model-reduction procedures (e.g., stepwise) that eliminate statistically insignificant factors typically do not generate prediction models that are as good as those resulting from leaving all factors in the model 50. The performance of the fitted model will be evaluated by the c-statistic (equivalent to the area under the ROC curve or AUC), which can be interpreted as the probability that the estimated prediction index will accurately discriminate between low/moderate versus severe pain subjects. We will also describe the performance of the model by an ROC curve, which is a plot of the true-positive rate (sensitivity) vs. the false-positive rate (1-specificity) corresponding to each possible threshold of the estimated prediction index. Bootstrap methods 50 will be used to account for “optimism” in the c-statistic estimate, because the prediction index arises from the same data from which the c-statistic was calculated. This eliminates the need for a separate “test” sample.
Figure 1.
Factors associated with high intensity pain during wound care procedures model
Nonetheless, the model, if shown to be predictive, will need to be tested in a large, independent sample and refined as needed. In this way, use of aggressive pain management strategies, including specialty dressings, pharmacologic analgesics, and/or non-pharmacological strategies, such as high intensity transcutaneous electrical stimulation 1, can be targeted in a cost-effective and personalized manner. The annual market for wound care products was estimated at $15.3 billion, reflecting the prevalence and economic burden of wounds 50. When used appropriately, these interventions will decrease the pain associated with WCPs leading to less nociceptive sensitization, more thorough, complete wound care, better wound outcomes and lowered costs.
Acknowledgments
Funding: NIH/NINR R01NR015642
References
- 1.Gardner SE, Blodgett NP, Hillis SL, Borhart E, Malloy L, Abbott L, et al. HI-TENS reduces moderate-to-severe pain associated with most wound care procedures: a pilot study. [cited 2016 Oct 4];Biol Res Nurs [Internet] 2014 Jul;16(3):310–9. doi: 10.1177/1099800413498639. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23956353. [DOI] [PubMed] [Google Scholar]
- 2.Bullitt E. Induction of c-fos-like protein within the lumbar spinal cord and thalamus of the rat following peripheral stimulation. [cited 2016 Oct 4];Brain Res [Internet] 1989 Jul 31;493(2):391–7. doi: 10.1016/0006-8993(89)91177-3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2504439. [DOI] [PubMed] [Google Scholar]
- 3.Woo KY. Meeting the challenges of wound-associated pain: anticipatory pain, anxiety, stress, and wound healing. Ostomy Wound Manag. 2008;54(9):10–2. [PubMed] [Google Scholar]
- 4.Rakel BA, Blodgett NP, Bridget Zimmerman M, Logsden-Sackett N, Clark C, Noiseux N, et al. Predictors of postoperative movement and resting pain following total knee replacement. [cited 2016 Oct 4];Pain [Internet] 2012 Nov;153(11):2192–203. doi: 10.1016/j.pain.2012.06.021. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22840570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Union of Wound Healing Societies’ Initiative. A consensus document. London: MEP Ltd; 2004. [cited 2016 Oct 4]. Principles of Best Practice: Minimising pain at wound dressing-related procedures. Available from: http://www.woundsinternational.com/media/issues/79/files/content_39.pdf. [Google Scholar]
- 6.World Union of Wound Healing Societies’ Initiative. Principles of Best Practice: Minimising pain at wound dressing-related procedures, A consensus document. Toronto, Ontario, Canada: WoundPedia, Inc; 2007. [cited 2016 Oct 4]. Available from: http://www.woundpedia.com/pdf/PainMolnlyckeSupplement.pdf. [Google Scholar]
- 7.Wounds UK. [cited 2010 Aug 11];Best Practice Statement: Minimising Trauma and Pain in Wound Management [Internet] 2004 Available from: http://www.wounds-uk.com/best-practice-statements/best-practice-statement-minimising-trauma-and-pain-in-wound-management-1.
- 8.Stotts NA, Puntillo K, Bonham Morris A, Stanik-Hutt J, Thompson CL, White C, et al. Wound care pain in hospitalized adult patients. Hear Lung J Acute Crit Care. 2004;33(5):321–32. doi: 10.1016/j.hrtlng.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Ip HYV, Abrishami A, Peng PWH, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. [cited 2016 Oct 11];Anesthesiology [Internet] 2009 Sep;111(3):657–77. doi: 10.1097/ALN.0b013e3181aae87a. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19672167. [DOI] [PubMed] [Google Scholar]
- 10.Morin C, Lund JP, Villarroel T, Clokie CML, Feine JS. Differences between the sexes in post-surgical pain. Pain. 2000;85(1–2):79–85. doi: 10.1016/s0304-3959(99)00248-1. [DOI] [PubMed] [Google Scholar]
- 11.Koren L, Peled E, Trogan R, Norman D, Berkovich Y, Israelit S. Gender, age and ethnicity influence on pain levels and analgesic use in the acute whiplash injury. [cited 2016 Oct 11];Eur J trauma Emerg Surg [Internet] 2015 Jun;41(3):287–91. doi: 10.1007/s00068-014-0419-2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26037975. [DOI] [PubMed] [Google Scholar]
- 12.Fillingim RB, Edwards RR, Powell T. Sex-dependent effects of reported familial pain history on recent pain complaints and experimental pain responses. [cited 2016 Oct 11];Pain [Internet] 2000 May;86(1–2):87–94. doi: 10.1016/s0304-3959(00)00239-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10779665. [DOI] [PubMed] [Google Scholar]
- 13.Lourenço S, Costa L, Rodrigues AM, Carnide F, Lucas R. Gender and psychosocial context as determinants of fibromyalgia symptoms (fibromyalgia research criteria) in young adults from the general population. Rheumatol (United Kingdom) 2015;54:1806–15. doi: 10.1093/rheumatology/kev110. [DOI] [PubMed] [Google Scholar]
- 14.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. [cited 2016 Oct 11];Pain Med [Internet] 2003 Sep;4(3):277–94. doi: 10.1046/j.1526-4637.2003.03034.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12974827. [DOI] [PubMed] [Google Scholar]
- 15.Faucett J, Gordon N, Levine J. Differences in Postoperative Pain Severity Among Four Ethnic Groups. J Pain Symptom Manage. 1994;9(4):383–9. doi: 10.1016/0885-3924(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 16.White SF, Asher MA, Lai SM, Burton DC. Patients’ perceptions of overall function, pain, and appearance after primary posterior instrumentation and fusion for idiopathic scoliosis. [cited 2016 Oct 4];Spine (Phila Pa 1976) [Internet] 1999 Aug 15;24(16) doi: 10.1097/00007632-199908150-00011. 1693-9-700. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10472104. [DOI] [PubMed] [Google Scholar]
- 17.Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. [cited 2016 Oct 11];Pain [Internet] 2001 Nov;94(2):133–7. doi: 10.1016/S0304-3959(01)00408-0. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11690726. [DOI] [PubMed] [Google Scholar]
- 18.Butrick CW. Persistent Postoperative Pain. [cited 2016 Oct 4];Female Pelvic Med Reconstr Surg [Internet] 2016 22(5):390–6. doi: 10.1097/SPV.0000000000000298. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=01436319-201609000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, et al. Characteristics of Fibromyalgia Independently Predict Poorer Long-Term Analgesic Outcomes Following Total Knee and Hip Arthroplasty. [cited 2016 Oct 4];Arthritis Rheumatol [Internet] 2015 May;67(5):1386–94. doi: 10.1002/art.39051. Available from: http://doi.wiley.com/10.1002/art.39051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brummett CM, Janda AM, Schueller CM, Tsodikov A, Morris M, Williams DA, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. [cited 2016 Oct 4];Anesthesiology [Internet] 2013 Dec;119(6):1434–43. doi: 10.1097/ALN.0b013e3182a8eb1f. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24343289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang D, Shi X, Qiao Y, Angst MS, Yeomans DC, Clark JD. Chronic morphine administration enhances nociceptive sensitivity and local cytokine production after incision. [cited 2016 Oct 11];Mol Pain [Internet] 2008 4(7) doi: 10.1186/1744-8069-4-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18294378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koppert W, Schmelz M. The impact of opioid-induced hyperalgesia for postoperative pain. [cited 2016 Oct 11];Best Pract Res Clin Anaesthesiol [Internet] 2007 Mar;21(1):65–83. doi: 10.1016/j.bpa.2006.12.004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17489220. [DOI] [PubMed] [Google Scholar]
- 23.Wibbenmeyer L, Eid A, Kluesner K, Heard J, Zimmerman B, Kealey GP, et al. An Evaluation of Factors Related to Postoperative Pain Control in Burn Patients. [cited 2016 Oct 11];J Burn Care Res [Internet] 2015 36(5):580–6. doi: 10.1097/BCR.0000000000000199. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26335109. [DOI] [PubMed] [Google Scholar]
- 24.Woo KY, Sadavoy J, Sidani S, Maunder R, Sibbald RG. The relationship between anxiety, anticipatory pain, and pain during dressing change in the older population. Ostomy Wound Manag. 2008;54(4):77. [Google Scholar]
- 25.Woo KY. Unravelling nocebo effect: the mediating effect of anxiety between anticipation and pain at wound dressing change. J Clin Nurs. 2015;24(13–14):1975–84. doi: 10.1111/jocn.12858. [DOI] [PubMed] [Google Scholar]
- 26.Upton D. Psychological aspects of wound care: implications for clinical practice. J Community Nurs. 2014;28(2):52–7. [Google Scholar]
- 27.Upton D, Solowiej K, Hender C, Woo KY. Stress and Pain associated with dressing change in patients with chronic wounds. J Wound Care. 2012;21(2):53–4. 56, 58 passim. doi: 10.12968/jowc.2012.21.2.53. [DOI] [PubMed] [Google Scholar]
- 28.Rakel BA, Zimmerman MB, Geasland K, Embree J, Clark CR, Noiseux NO, et al. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: A randomized, blinded, placebo-controlled trial. [cited 2016 Oct 11];Pain [Internet] 2014 Dec;155(12):2599–611. doi: 10.1016/j.pain.2014.09.025. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25270585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. [cited 2016 Oct 11];Arch Intern Med [Internet] 2003 Nov 10;163(20):2433–45. doi: 10.1001/archinte.163.20.2433. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14609780. [DOI] [PubMed] [Google Scholar]
- 30.Robinson MJ, Edwards SE, Iyengar S, Bymaster F, Clark M, Katon W. Depression and pain. [cited 2016 Oct 11];Front Biosci (Landmark Ed [Internet] 2009 14:5031–51. doi: 10.2741/3585. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19482603. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan MJ, Weinshenker B, Mikail S, Edgley K. Depression before and after diagnosis of multiple sclerosis. [cited 2016 Oct 11];Mult Scler [Internet] 1995 Jun;1(2):104–8. doi: 10.1177/135245859500100208. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9345460. [DOI] [PubMed] [Google Scholar]
- 32.Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. [cited 2016 Oct 11];Biol Psychiatry [Internet] 2003 Aug 1;54(3):399–409. doi: 10.1016/s0006-3223(03)00545-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12893114. [DOI] [PubMed] [Google Scholar]
- 33.Sobol-Kwapinska M, Bąbel P, Plotek W, Stelcer B. Psychological correlates of acute postsurgical pain: A systematic review and meta-analysis. [cited 2016 Oct 11];Eur J Pain [Internet] 2016 May 2;:1–14. doi: 10.1002/ejp.886. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27136510. [DOI] [PubMed]
- 34.Richardson C. An introduction to the biopsychosocial complexities of managing wound pain. [cited 2016 Oct 11];J Wound Care [Internet] 2012 Jun;21(6):267–8. 270–3. doi: 10.12968/jowc.2012.21.6.267. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22886291. [DOI] [PubMed] [Google Scholar]
- 35.Roth RS, Lowery JC, Hamill JB. Assessing Persistent Pain and Its Relation to Affective Distress, Depressive Symptoms, and Pain Catastrophizing in Patients with Chronic Wounds. [cited 2016 Oct 11];Am J Phys Med Rehabil [Internet] 2004 Nov;83(11):827–34. doi: 10.1097/01.phm.0000140800.83146.fa. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15502735. [DOI] [PubMed] [Google Scholar]
- 36.Reinke JM, Sorg H. Wound repair and regeneration. [cited 2016 Oct 11];Eur Surg Res [Internet] 2012 49(1):35–43. doi: 10.1159/000339613. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22797712. [DOI] [PubMed] [Google Scholar]
- 37.Krasner DL, Shapshak D, Hopf HW. Managing Wound Pain. In: Bryant Ruth A, Nix Denise P., editors. Acute and Chronic Wounds: Current Management Concepts [Internet] 3. St. Louis, Missouri: Mosby Elsevier; 2007. Available from: https://www.amazon.com/Acute-Chronic-Wounds-Management-Concepts/dp/0323069436. [Google Scholar]
- 38.Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. [cited 2016 Oct 11];J Pain [Internet] 2007 Jan;8(1):59–66. doi: 10.1016/j.jpain.2006.06.003. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16949881. [DOI] [PubMed] [Google Scholar]
- 39.Czeschik JC, Hagenacker T, Schäfers M, Büsselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. [cited 2016 Oct 11];Neurosci Lett [Internet] 2008 Apr 4;434(3):293–8. doi: 10.1016/j.neulet.2008.01.070. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18314270. [DOI] [PubMed] [Google Scholar]
- 40.Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res. 1994 Sep;657(1–2):133–40. doi: 10.1016/0006-8993(94)90960-1. [DOI] [PubMed] [Google Scholar]
- 41.Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000 Mar;85(1–2):145–51. doi: 10.1016/s0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki Y, Zhang L, Cheng J-K, Ji R-R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. [cited 2016 Oct 11];J Neurosci [Internet] 2008 May 14;28(20):5189–94. doi: 10.1523/JNEUROSCI.3338-07.2008. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18480275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. [cited 2016 Oct 11];Neuroscience [Internet] 1997 Nov;81(1):255–62. doi: 10.1016/s0306-4522(97)00147-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9300418. [DOI] [PubMed] [Google Scholar]
- 44.Meaume S, Teot L, Lazareth I, Martini J, Bohbot S. The importance of pain reduction through dressing selection in routine wound management: the MAPP study. J Wound Care. 2004 Nov;13(10):409–13. doi: 10.12968/jowc.2004.13.10.27268. [DOI] [PubMed] [Google Scholar]
- 45.Price PE, Fagervik-Morton H, Mudge EJ, Beele H, Ruiz JC, Nystrøm TH, et al. Dressing-related pain in patients with chronic wounds: an international patient perspective. [cited 2016 Oct 11];Int Wound J [Internet] 2008 Jun;5(2):159–71. doi: 10.1111/j.1742-481X.2008.00471.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18494622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiegand C, Schönfelder U, Abel M, Ruth P, Kaatz M, Hipler U-C. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. [cited 2016 Oct 11];Arch Dermatol Res [Internet] 2010 Aug;302(6):419–28. doi: 10.1007/s00403-009-1011-1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20033193. [DOI] [PubMed] [Google Scholar]
- 47.Tengvall OM, Bjornhagen VC, Lindholm C, Jonsson C-E, Wengstrom Y. Differences in pain patterns for infected and noninfected patients with burn injuries. Pain Manag Nurs. 2006 Dec;7(4):176–82. doi: 10.1016/j.pmn.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen. 2001;9(3):178–86. doi: 10.1046/j.1524-475x.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- 49.Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. [cited 2016 Oct 11];Neuroscience [Internet] 2016 Jun 9; doi: 10.1016/j.neuroscience.2016.06.006. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27291641. [DOI] [PMC free article] [PubMed]
- 50.Harrell FE., Jr . Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 51.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. [cited 2016 Oct 11];Wound Repair Regen [Internet] 2009 17(6):763–71. doi: 10.1111/j.1524-475X.2009.00543.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19903300. [DOI] [PMC free article] [PubMed] [Google Scholar]